Abstract
The release rhythm of volatiles is an important physiological characteristic of plants, because the timing of release can affect the function of each particular volatile compound. However, most studies on volatiles release rhythms have been conducted using model plants, rather than crop plants. Here, we analyzed the variations in volatile compounds released from healthy and leafminer (Liriomyza huidobrensis)-infested kidney bean (Phaseolus vulgaris), an important legume crop plant, over a 24 h period. The constituents of the volatiles mixture released from plants were analyzed every 3 h starting from 08:00. The collected volatiles were identified and quantified by gas chromatography–mass spectrometry. Undamaged kidney bean plants released trace amounts of volatiles, with no obvious release rhythms. However, leafminer-damaged plants released large amounts of volatiles, in two main peaks. The main peak of emission was from 17:00 to 20:00, while the secondary peak was in the early morning. The terpene volatiles and (Z)-3-hexenyl acetate showed similar rhythms as that of total volatiles. However, the green leaf volatile (Z)-3-hexen-ol was emitted during the night with peak emission in the early morning. These results give us a clear picture of the volatiles release rhythms of kidney bean plants damaged by leafminer.
Keywords : green leaves volatiles, Liriomyza huidobrensis, rhythm, terpene, (Z)-3-hexen-ol
Introduction
During their long-term interaction with insects, plants have evolved complicated and effective defense systems. One of the important indirect defense strategies of plants is to attract natural enemies of their predators by releasing volatile organic compounds (VOC).1,2 Plants can release various VOCs, which make up the special volatile spectrum of that plant.3-5 Some VOCs are released continuously,6 but many others are only released in large volumes when plants are wounded by insects or mechanical damage. These volatiles, known as herbivore-induced plant volatiles, often attract the natural enemies of the insect pest and play important roles in indirect defense.7
In most organisms, behaviors form a biological rhythm under an oscillatory environment of a roughly 24 h cycle. Several previous studies indicated that plants release volatiles according to a particular rhythm. This phenomenon has attracted much attention recently. For example, Loughrin et al. found that volatiles were released according to a particular rhythm by Spodoptera exigua (Hubner)-infested Gossypium hirsutum.8 Arimura et al. analyzed a complex mixture of volatiles released by plants, and found that different components of the mixture showed different release rhythms.9 As an attractant of natural enemies, different VOCs may play different roles in the plant defense process.2,10,11 Consequently, the rhythmic release of volatiles can affect their functions over time. For example, our previous work indicated that the volatiles released by lima bean (Phaseolus lunatus) infested by leafminer (Liriomyza huidobrensis) could be divided into two categories according to their release rhythms: the peak emission of most terpenes was at 14:00–17:00, while the peak of (Z)-3-hexenol release was at dawn. Interestingly, terpenes attracted the natural enemy parasitoids of leafminer, and strongly affected their ovipositioning, which also peaked in the afternoon.12 (Z)-3-Hexenol attracted naive parasitoids, whose eclosion behavior peaked at dawn.12 These phenomena indicate that the release rhythms of plant volatiles from lima bean and the activities of its predators’ natural enemies co-evolved during their long-term interaction, and formed an effective defense net for the plant. However, further evidence is required to determine whether the release patterns of different plant volatiles are universal among different plants. Most research on plant volatiles release rhythms has been conducted using model plants; few studies have studied this phenomenon in important economic crop species. This restricts the exploitation of plant volatile rhythms to control pests more effectively.
In this study, we identified volatile compounds released over a 24 h period by kidney bean (Phaseolus vulgaris), an important economic crop, in response to damage by leafminer. We compared the release rhythms of different chemicals in the volatiles mixture. This will further our understanding of the patterns of plant volatiles release, and may allow better control of leafminer.
Results
Amounts of different volatiles released from healthy and leafminer-damaged kidney beans
We detected only 4 kinds of VOCs from the healthy kidney bean plants; (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), (Z)-3-hexenyl acetate, butyl aldoxime,3-methyl-,syn-, and butyl aldoxime,3-methyl-,anti-. These 4 compounds were released in very small quantities (Fig. 1). After leafminer attack, the amounts and types of VOCs released increased dramatically. All 4 kinds of VOCs detected from the healthy kidney bean plants were released continuously after leafminer damage, and the amounts of DMNT, (Z)-3-hexenyl acetate, and butyl aldoxime,3-methyl-,syn- increased significantly (Fig. 1). Seven new chemicals were detected; therefore, the mixture of VOCs comprised 11 chemicals in total. The most abundant components of the mixture were (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), (Z)-3-hexen-ol, (Z)-3-hexenyl acetate, the latter making up almost half of the (49.06%) of the total VOCs mixture. The next most abundant constituents were six types of -oximes, followed by linalool and caryophyllene (Fig. 1).
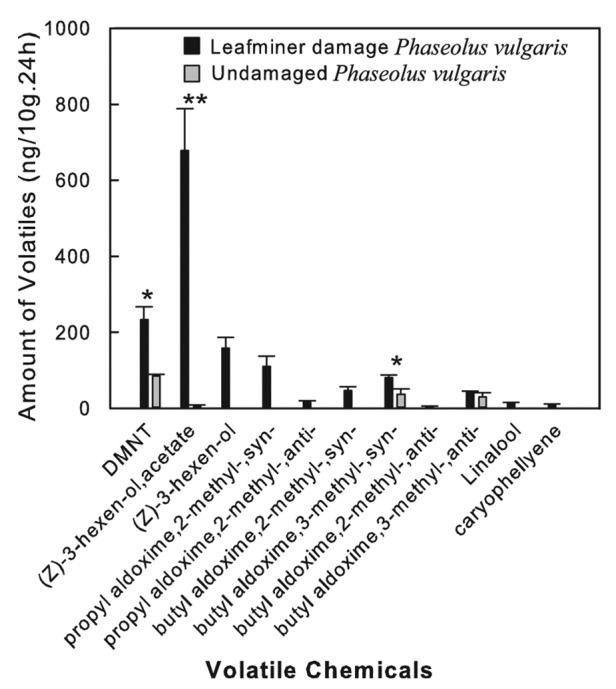
Figure 1. Types and amounts of volatile compounds released from healthy and leafminer-infested kidney bean plants during a 24 h period (mean ± SE).
Release rhythm of total VOCs from healthy and leafminer-damaged kidney bean plants
Only small amounts of VOCs were released from healthy kidney bean plants, and no obvious rhythm was observed. However, there were 2 main peaks of VOCs release from leafminer-damaged kidney bean plants. The total VOCs emission started to increase at the beginning of the photophase and peaked between 17:00 and 20:00, then gradually decreased and remained at low levels between 23:00 and 02:00 during the dark phase. Subsequently, the amount of VOCs released gradually increased over the following several hours (Fig. 2).
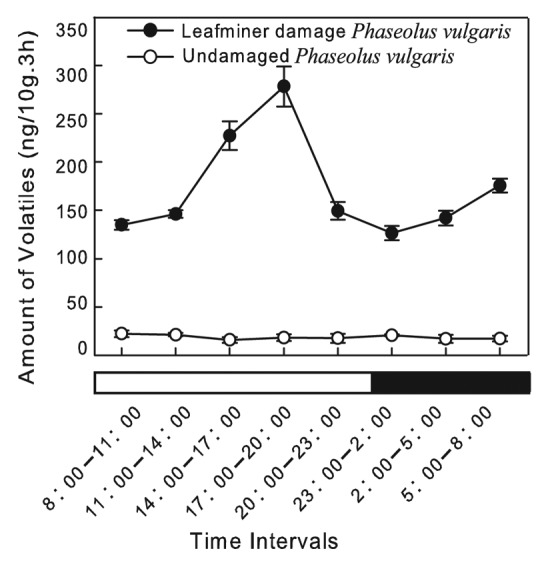
Figure 2. Diurnal rhythm of total volatiles released from healthy and leafminer-infested kidney bean plants (mean ± SE). Bars under X-axis show photoperiodic cycle: white, light phase; black, dark phase.
Release diurnal rhythms of different VOCs from healthy and leafminer-damaged kidney bean plants
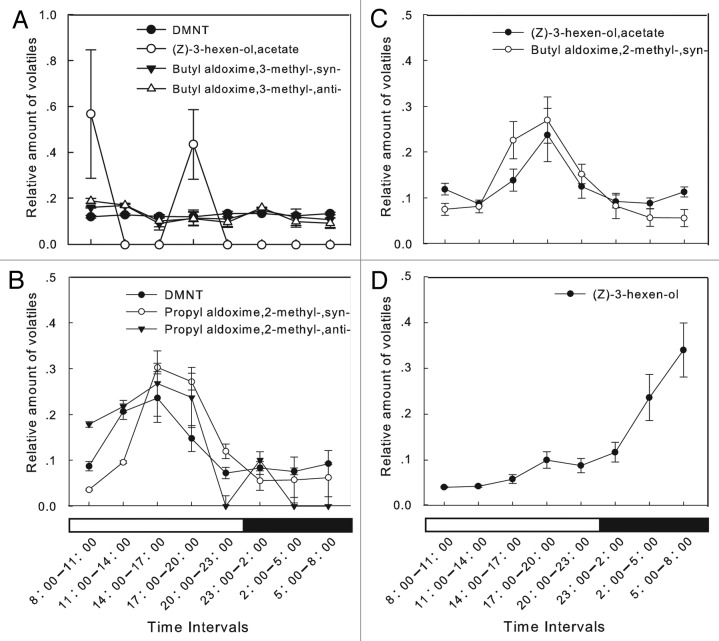
Although healthy kidney bean plants released only small amounts of volatiles with no obvious release rhythms (Fig. 3A), there were variations in the amounts of (Z)-3-hexenyl acetate released (Fig. 3A); this compound was detected only at 2 time intervals (8:00–11:00 and 17:00–20:00) and in very small quantities. In contrast, the leafminer-damaged plants released VOCs with obvious rhythms. The dominant VOCs could be divided into 3 main categories based on their release rhythms. The first category consisted of DMNT, propyl aldoxime, 2-methyl, syn-, and propyl aldoxime, 2-methyl, anti-; the amounts of these compounds emitted increased rapidly from 8:00, peaked between 14:00 and 17:00, remained at high levels from 17:00 to 20:00, and then decreased from 20:00 until the next day (Fig. 3B). The second category consisted of (Z)-3-hexenyl acetate and butyl aldoxime, 2-methyl, syn-. The emissions of these compounds were low in the morning, rapidly increased from 14:00, peaked between 17:00 and 20:00, then gradually decreased and remained at low levels from 23:00 to 05:00 during the dark phase (Fig. 3C). The third category consisted of one VOC, (Z)-3-hexen-ol, which was released at low levels during the day, with emission markedly increasing later, and peaking between 05:00 and 08:00 in the dark phase (Fig. 3D). The other five kinds of volatiles did not show clear rhythms of release.
Figure 3. Different diurnal release rhythms of VOCs from healthy and leafminer-infested kidney bean plants (mean ± SE). Bars under X-axis show photoperiodic cycle: white, light phase; black, dark phase. (A) Diurnal release rhythms of different volatiles from healthy kidney bean plants. (B–D) Diurnal release rhythms of different volatiles from leafminer-infested kidney bean plants.
Discussion
We detected the VOCs release rhythms of kidney bean, an important economic crop. Only 4 kinds of chemicals were released from healthy bean plants, but those damaged by leafminer released greater quantities of the four previously detected chemicals, and seven new volatile compounds (11 compounds in total). The chemicals released in response to insect attack were DMNT, (Z)-3-hexenyl acetate, (Z)-3-hexen-ol, propyl aldoxime,2-methyl-,syn-, propyl aldoxime,2-methyl-,anti-, butyl aldoxime,2-methyl-,syn-, butyl aldoxime,3-methyl-,syn-, butyl aldoxime,2-methyl-,anti-, butyl aldoxime,3-methyl-,anti-, linalool, and caryophyllene. Among the 11 volatiles, the most abundant were DMNT, (Z)-3-hexenyl acetate, and (Z)-3-hexen-ol. Six compounds showed clear release rhythms. These 6 compounds could be divided into 3 categories according to their release patterns; those compounds whose releases peaked at 14:00–17:0, 17:00–20:00, and 5:00–8:00, respectively. Our results indicated that the volatiles from kidney bean show a complex pattern of release. This highlights the importance of analyzing both the constituents of volatiles mixtures and their release rhythms, to characterize the VOCs responses of plants.
Our results showed that DMNT, oximes, and (Z)-3-hexenyl acetate were released mainly in the daytime, while (Z)-3-hexen-ol accumulated rapidly during the night. These patterns of volatiles release were similar to those reported for lima bean.13 Several plant species are known to release terpenes during the daytime. For example, insect-damaged cotton seedlings released terpenes, with peak emissions in the afternoon;8 Batocera horsfieldi-damaged Viburnum awabuki released a large amount of VOCs, mainly terpenes, during the day.14 However, volatiles that accumulate during the dark are rare. In this work, (Z)-3-hexen-ol release was mainly detected at night, possibly because (Z)-3-hexen-ol was transformed into (Z)-3-hexenyl acetate in the light.
It may be ecologically significant that the peak releases of terpenes and (Z)-3-hexen-ol were during the day and night, respectively. Wei et al.15 found that (Z)-3-hexen-ol was the most important host location director for naïve Opius dissitus, the parasitoid of leafminer. The eclosion peak of Opius dissitus overlapped with the release of (Z)-3-hexen-ol,12 ensuring that the newly emerged naïve parasitoids could find their host via the (Z)-3-hexen-ol signal. Similarly, the peak emission of terpenes, which are efficient attractants for parasitoids and stimulators of their ovipositioning behavior, overlapped with the peak activity of parasitoid ovipositioning.12 Thus, these compounds may play an important signaling role in short-range host-insect location.
Selecting leafminer as the insect to impose damage has certain advantages for detecting the rhythm of VOCs release. Green leaf volatiles, such as (Z)-3-hexen-ol and (Z)-3-hexenyl acetate, are released rapidly after plant damage, but their release quickly stops once insects stop feeding.8 Most previous studies on the rhythms of plant volatiles releases have used chewing insects, which were normally removed to avoid interference. In this case, the insect damage has ceased by the time the volatiles are analyzed, and so compounds that are released very rapidly and transiently in response to insect damage can be missed. In our research, we selected leafminer as the pest model. The leafminer adults lay eggs in the leaves of the kidney bean plants, and the larvae feed and grow inside the leaves. Therefore, when we detected volatiles, the insect damage was ongoing, and all the chemicals were released according to their own rhythms without interference caused by removing the insect pest. Of course, the larvae may introduce some impurities that affect VOCs emission.
Pea leafminer is an important crop pest that feeds on more than 100 species in 22 plant families. During the adult stage, the female fly uses her ovipositor to penetrate the epidermis of host plant leaves. The eggs are then laid inside the leaves and/or the adult feeds at the wound site, which can greatly reduce photosynthesis and eventually kill young plants. At the same time, the larvae live inside the plant leaves and remain well hidden. Thus, the pea leafminer is hard to detect and control using normal methods. Taking advantage of plant volatile compounds to attract its natural parasitoid is an efficient and environment-friendly strategy to control pea leafminer and other pests. Thus, there has been a large body of research on the VOCs from pea leafminer-damaged plants in recent years.3,12,13,15 In this study, we analyzed the volatiles release rhythms from an economic crop, kidney bean. The results showed clear different release patterns for different kinds of chemicals after pest damage, indicating that different volatiles may perform different functions in plant defenses.
Materials and Methods
Plants and insects
Kidney bean (P. vulgaris) plants were individually sown in plastic pots (12 cm in diameter) in a potting medium consisting of peat and vermiculite (3:1). Plants were grown in an environmental chamber. Bean plants with two fully developed true leaves (approximately 2 wks old) were used in all experiments. The environmental conditions inside the chambers were set at 25 ± 1 °C, 60% ± 10% RH, and a 15 h light/9 h dark photoperiod, with light intensity of 12.65 W/m2 during the photophase.
The pea leafminers (L. huidobrensis) used in these experiments have been cultured under laboratory conditions for 5 y, using kidney bean as the host plant.
Insect infection treatments
In total, approximately 100 mated L. huidobrensis adults were released onto kidney bean leaves for oviposition. Before the experiment, the adults were fed only 10% diluent honey for 10 h, and then the kidney bean plants were placed into the cage containing adult leafminers. We tried to keep the number of leafminers constant in each replicate of the experiment (approximately 50 sec instar larvae per leaf). The adults were removed after 4 h.
Volatile collection
There were three treatments: 1) clean bags – the atmosphere within a clean oven bag (Reynolds Oven Bags, Reynolds Kitchens, Richmond) was collected for analysis as the control; 2) healthy kidney bean plants; and 3) kidney bean plants damaged by second instar leafminers (96 h after ovipositioning). Three plants were used for each volatile collection experiments.
The procedure for headspace volatile collection was as described previously.3 To measure the diurnal cycle of plant volatile emissions, one diurnal cycle was divided into eight, 3 h time intervals corresponding to those in the larvae feeding experiments. Volatiles were collected once every 3 h starting from 08:00. There were approximately 200–300 larvae in the kidney beans prepared for volatile collection (three bean plants), and this number was kept constant each time the experiment was repeated. The absorbing glass collector connected to each bag was replaced with a new one for each collection, and the collector was changed under a red light in the dark phase. All extracts were stored at −20°C until analysis. The plants were weighed immediately after collection and the number of leafminer larvae in the leaves was recorded. Each treatment had three replicates.
Chemical identification and quantification
The collected volatile compounds were identified using an Agilent gas chromatograph (GC) (6890N) coupled with a mass spectrometer (MS) (5973 MSD, Agilent Technologies, Inc.). The system was equipped with a DB-WAX polyethylene glycol 20000 column (60 min, 60.25 mm ID, 0.15 mm film thickness). For analyses, the initial oven temperature was kept at 40 °C for 4 min and then increased to 180 °C at a programmed rate of 5 °C min−1, then to 230 °C at a rate of 10 °C min−1. Volatile compounds were identified by comparing their retention times and spectra with those of synthetic standards. Referenced mass spectra from the NIST02 library (Scientific Instrument Services, Inc.) were also used.
A GC (7890A; Agilent Technologies, Inc.) coupled with an auto-inlet installation was used to quantify the collected volatiles. The system was equipped with the same DB-WAX column and the same template program as that described above. Heptanoic acid, ethyl ester and dodecanoic acid, ethyl ester (1, 5, 20, 50, and 100 ng mL−1) were used to develop standard curves to quantify volatiles in the samples.
Acknowledgments
This research was supported by the National Basic Research Program of China (973 Program) (No. 2012CB114105), and the National Nature Science Foundation of China (30921063 and 31200492).
Disclosure of Potential Conflicts of Interest
The authors have no potential conflicts of interest to declare.
References
- 1.Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, et al. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- 2.Bate NJ, Rothstein SJ. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998;16:561–9. doi: 10.1046/j.1365-313x.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 3.Wei J-N, Zhu J, Kang L. Volatiles released from bean plants in response to agromyzid flies. Planta. 2006;224:279–87. doi: 10.1007/s00425-005-0212-x. [DOI] [PubMed] [Google Scholar]
- 4.de Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol. 2004;30:2215–30. doi: 10.1023/B:JOEC.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- 5.Guerrieri E, Poppy GM, Powell W, Tremblay E, Pennacchio F. Induction and systemic release of herbivore-induced plant volatiles mediating in-flight orientation of Aphidius ervi. J Chem Ecol. 1999;25:1247–61. doi: 10.1023/A:1020914506782. [DOI] [Google Scholar]
- 6.Lombardero MJ, Ayres MP, Lorio PL, Jr., Ruel JJ. Environmental effects on constitutive and inducible resin defences of Pinus taeda. Ecol Lett. 2000;3:329–39. doi: 10.1046/j.1461-0248.2000.00163.x. [DOI] [Google Scholar]
- 7.Karban R, Myers JH. Induced plant responses to herbivory. Annu Rev Ecol Syst. 1989;20:331–48. doi: 10.1146/annurev.es.20.110189.001555. [DOI] [Google Scholar]
- 8.Loughrin JH, Manukian A, Heath RR, Turlings TC, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci U S A. 1994;91:11836–40. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arimura G-i, Köpke S, Kunert M, Volpe V, David A, Brand P, et al. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 2008;146:965–73. doi: 10.1104/pp.107.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008;180:722–34. doi: 10.1111/j.1469-8137.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- 11.Godard K-A, White R, Bohlmann J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry. 2008;69:1838–49. doi: 10.1016/j.phytochem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Wei J, Guo X, Liu T-X, Kang L. Functional synchronization of biological rhythms in a tritrophic system. PLoS ONE. 2010;5:e11064. doi: 10.1371/journal.pone.0011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Wei J, Kang L. Transcriptional analysis of Arabidopsis thaliana response to lima bean volatiles. PLoS ONE. 2012;7:e35867. doi: 10.1371/journal.pone.0035867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Yang W, Yang MF, Yang CP, Yang LG, Xutang XK. [Diurnal rhythm of Viburnum awabuki and Betula luminifera volatiles and electroantennogram response of Batocera horsfieldi] Ying Yong Sheng Tai Xue Bao. 2011;22:357–63. [PubMed] [Google Scholar]
- 15.Wei J, Wang L, Zhu J, Zhang S, Nandi OI, Kang L. Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE. 2007;2:e852. doi: 10.1371/journal.pone.0000852. [DOI] [PMC free article] [PubMed] [Google Scholar]