Abstract
Cytokine-induced killer (CIK) cells are T lymphocytes expanded ex vivo that are endowed with MHC-independent tumoricidal activity. We have recently demonstrated, in a preclinical setting, that CIK cells are active against autologous bone and soft tissue sarcomas. In particular, CIK cells killed a putative sarcoma stem cell population that may underlie disease relapse and chemoresistance.
Keywords: adoptive immunotherapy, CIK cells, cancer stem cells, sarcomas, solid tumors
Metastatic recurrent bone sarcomas (BSs) and soft tissue sarcomas (STSs) are unmet clinical challenges. Conventional chemotherapies have indeed reached a plateau, and metastatic or unresectable diseases are currently considered as incurable.1,2 In this setting, clinical relapses and chemoresistance are generally attributed to a small subset of cancer cells endowed with stem-like features, which as generically defined as “cancer stem cells” (CSCs). The identification, characterization, and targeting of CSCs represent major challenges for anticancer therapy.3 Although a unique biomarker for CSCs has not yet been identified, several molecules exposed on the cell surface such as prominin 1 (PROM1, also known as CD133) and STRO-1, as well as the re-expression of specific genes including POU class 5 homeobox 1 (POU5F1, also known as OCT3 or OCT4), Nanog homeobox (NANOG), and various members of the SOX family, have been proposed to identify putative CSCs and allow for their targeting.4,5 Immunotherapy, which for a long time has been viewed only as a promising strategy for the treatment of solid tumors, has nowadays found its way to successful clinical applications.6,7 Among multiple immunotherapeutic strategies currently under investigation, our group has focused on the use of cytokine-induced killer (CIK) cells. CIK cells are T lymphocytes expanded ex vivo that exhibit a mixed T-natural killer (NK)-cells phenotype and are endowed with MHC-unrestricted tumoricidal activity, targeting both solid and hematologic malignancies.8
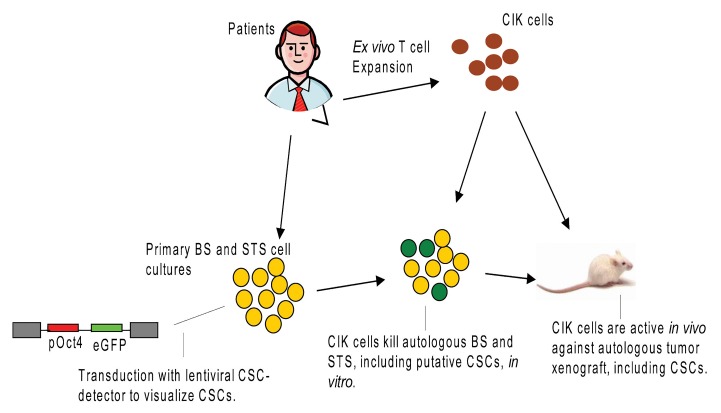
We have recently characterized the ability of CIK cells to kill autologous BS and STS cells in a preclinical setting.9 Our model was autologous with the intent of accounting as much as possible for intrinsic biological variables. Thus, primary sarcoma cultures were generated from fresh surgical biopsies. We could confirm that both BS and STS cells express ligands recognized by CIK cells, which efficiently killed autologous osteosarcoma, liposarcoma, leiomiosarcoma, gastrointestinal sarcoma, and pleomorphic sarcoma cells in vitro. The antitumor activity of CIK cells was confirmed in vivo in a murine tumor xenograft model (Fig. 1). In this experimental setup, the adoptive infusion of autologous CIK cells delayed indeed tumor growth and resulted in significant reduction of tumor proliferative index. Consistently with other immunotherapeutic strategies, objective responses, notably the reduction in tumor mass, induced by CIK cells were not dramatic, and could better be appreciated by the significant drop in the tumor proliferative index. This is an issue consistently faced when immunotherapeutic regimens are tested in clinical trials. Indeed, dimensional parameters such as those employed for Response Evaluation Criteria In Solid Tumors (RECIST) may not be the most appropriate approach to appreciate clinical responses to immunotherapy. Thus, new criteria to monitor the response of neoplastic lesions to immunotherapy, including metabolic variables assessed by positron emission tomography-computer tomography (CT-PET), histological data like the degree of necrosis of the tumor proliferation index, and immunological parameters, should be evaluated and possibly incorporated into preclinical and clinical evaluations. From our findings we could not conclude on what the optimal schedule of administration for CIK cells would be. However, based on available information and considering the extremely safe profile of CIK cells, it seems reasonable to hypothesize that multiple infusions should be performed to exploit the total number of CIK cells available. The optimal clinical setting to employ CIK cells may be represented by patients with minimal residual disease or at high risk of relapse upon the surgical resection of metastases.
Figure 1. Preclinical model unveiling the activity of cytokine-induced cells against autologous bone sarcoma and soft tissue sarcoma. Primary sarcoma cultures were generated from fresh surgical biopsies. Putative cancer stem cells (CSCs) were visualized upon the transduction of malignant cells with a lentiviral CSC-detecting vector encoding the enhanced variant of green fluorescent protein (eGFP) under the control of the OCT4 promoter (pRRL.sin.PPT.hOct4.eGFP.Wpre). Patient-derived cytokine-induced killer (CIK) cells were active in vitro and in vivo against autologous bone sarcoma (BS) and soft tissue sarcoma (STS) cells, including putative CSCs.
As a further investigational step, we designed a gene-transfer strategy to visualize putative CSCs within BSs and STSs and evaluate their susceptibility to the cytotoxic activity of CIKs. To this aim, we employed a lentiviral CSC-detecting vector encoding the enhanced variant of green fluorescent protein (eGFP) under the control of the OCT4 promoter.10 The underlying rationale was that the Oct4 promoter would be active only in putative CSCs, which would therefore become green and detectable. By means of this approach, we could estimate that putative CSCs are present in variable proportions in BS and STS cell cultures (mean 14.3%) and express ligands recognized by CIK cells similar to their differentiated counterparts.
It is important to clarify that putative stemness-associated genes are an intriguing tool to visualize cells endowed with stem-like features, but they do not necessarily identify bona fide CSCs, which must be characterized by means of adequate functional assays. Indeed, our eGFP+ putative CSCs displayed a significantly reduced proliferative ability in vitro as compared with their differentiated cells. Serial transplantations into animals are currently considered as the most accredited method to uncover the true staminal nature of cancer cells as opposed to the presence of “transiently amplifying” cell subsets. From a clinical-therapeutic perspective, further experiments are required to explore the degree of chemoresistance and the actual role of putative CSCs in tumor relapse. This area of investigation is being actively pursued in our laboratory.
Patient-derived CIK cells killed autologous CSCs in vitro as efficiently as their differentiated counterparts. While these data demonstrate the susceptibility of CSCs to CIK cells in vitro, the situation is more complex in vivo. We indirectly assessed the activity of CIK cells against sarcoma CSCs in vivo analyzing the residual fraction of putative CSCs in tumor samples explanted from mice adoptively treated with CIK cells. In this setting, tumors that clinically and histologically responded to immunotherapy failed to manifest a relative enrichment in eGFP+ putative CSCs, as if they were resistant to CIK cells. This indicates that CIK cells were equally capable of killing CSCs and more differentiated malignant cells in vivo. Future studies will have to confirm these findings, perhaps exploring the self-renewal and tumorigenic capacity of residual tumor cells explanted from animals that underwent immunotherapy. An important issue to be considered when targeting CSCs is the possible involvement of non-malignant stem cells, bearing a significant risk of side effects. CIK may be advantageous from this angle, as they recognize CSCs based on malignant, rather than stem-like, features, an ability that should (at least theoretically) spare normal stem cells from immunotherapy.
If confirmed in additional studies, our findings will endow CIK cells with a new therapeutic value, supporting their evaluation in clinical trials enrolling BS and STS patients, either as a single immunotherapeutic intervention or combined with conventional treatments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Sangiolo D, Mesiano G, Gammaitoni L, Aglietta M, Grignani G. Activity of cytokine-induced killer cells against bone and soft tissue sarcoma. OncoImmunology 2014; 3:e28269; 10.4161/onci.28269
References
- 1.Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G, Kabisch H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Casali PG, Blay JY, ESMO/CONTICANET/EUROBONET Consensus Panel of experts Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v198–203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 3.Borovski T, De Sousa E Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 4.Wirths S, Malenke E, Kluba T, Rieger S, Müller MR, Schleicher S, Hann von Weyhern C, Nagl F, Fend F, Vogel W, et al. Shared cell surface marker expression in mesenchymal stem cells and adult sarcomas. Stem Cells Transl Med. 2013;2:53–60. doi: 10.5966/sctm.2012-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ, Gimotty PA, Guerra M, Guo W, Xu X. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene. 2012;31:4898–911. doi: 10.1038/onc.2011.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo Diego L, Carnevale-Schianca F, Fagioli F, Piacibello W, Aglietta M, Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–84. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 9.Sangiolo D, Mesiano G, Gammaitoni L, Leuci V, Todorovic M, Giraudo L, Cammarata C, Dell’Aglio C, D’Ambrosio L, Pisacane A, et al. Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas. Cancer Res. 2014;74:119–29. doi: 10.1158/0008-5472.CAN-13-1559. [DOI] [PubMed] [Google Scholar]
- 10.Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, Steindler DA, Gibbs CP. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–55. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]