Abstract
Tumor immunosurveillance can be readily observed in mice and humans. Here, we examine how T-cell responses are primed during tumorigenesis, a condition in which immunostimulatory antigens are extraordinarily scarce. We recently demonstrated that the HSP-CD91 pathway is indispensable for antigen cross-presentation, and thus immunosurveillance, in cancer.
Keywords: CD91, HSP, T cells, cross-presentation, tumor
Immunosurveillance of cancer requires innate and adaptive immune cells and their corresponding effector molecules to eliminate aberrant neoplastic cells.1 Since the 1990s, experiments have shown that there is a higher incidence of tumors in immunodeficient mice, such as those lacking V(D)J recombination activating gene (rag), perforin, interferon-γ receptor (IFNγR), or IL-12, as compared with wild type mice.1,2 This phenomenon has been shown to manifest in chemically-induced murine cancers1 as well, as in mice genetically predisposed to developing spontaneous tumors.2 Increased susceptibility to cancer naturally occurs in immunodeficient people also.3 A well-known consequence of immunosurveillance is the presence of tumor-specific T cells in cancer patients. Cancer immunoediting in the absence of a normally functioning immune system is reduced relative to that generated in immunocompetent animals.1 T cells, the major contributors to immunosurveillance, are primed by antigen presenting cells (APCs) following recognition of tumor antigens in the context of co-stimulation. Conventional mechanisms of antigen cross-presentation and co-stimulation that have been primarily derived from our understanding of immune responses to pathogens require re-examination for 2 reasons. First, most tumors, being of self-origin, lack classical pathogen-associated molecular patterns that are recognized by corresponding pattern recognition receptors (PRRs). PRRs initiate intracellular signaling pathways leading to upregulation of co-stimulatory molecules and release of cytokines. What are the origins of these signals during tumorigenesis? Second, the amount of (mutated) tumor antigen present in the first few aberrant cells during tumorigenesis cannot be more than a picogram, even for abundant proteins.4 Clearly, antigen transfer and cross-presentation as studied in numerous systems in vitro and during immunologic responses to viral pathogenesis do not apply because such systems introduce micro- to milligram quantities of antigen. Tumors generally lack sufficient native antigen for cross-priming.5,6 However, it is clear that the amount of tumor cell-associated antigen in vivo is sufficient for cross-priming.5 What is the nature of the tumor cell-associated antigen, and how is this cross-presented by APCs sufficient for efficacious T-cell priming? In our recent manuscript, we demonstrated that heat shock proteins (HSPs), together with their chaperoned tumor antigens, are the singular entity that satisfies these 2 conundrums.7 We further showed that tumor-derived HSP-peptide complexes and their receptor CD91 presented on the surface of APCs are essential for tumor immunosurveillance. These observations are supported by additional experimental evidence accumulated over the past 3 decades.
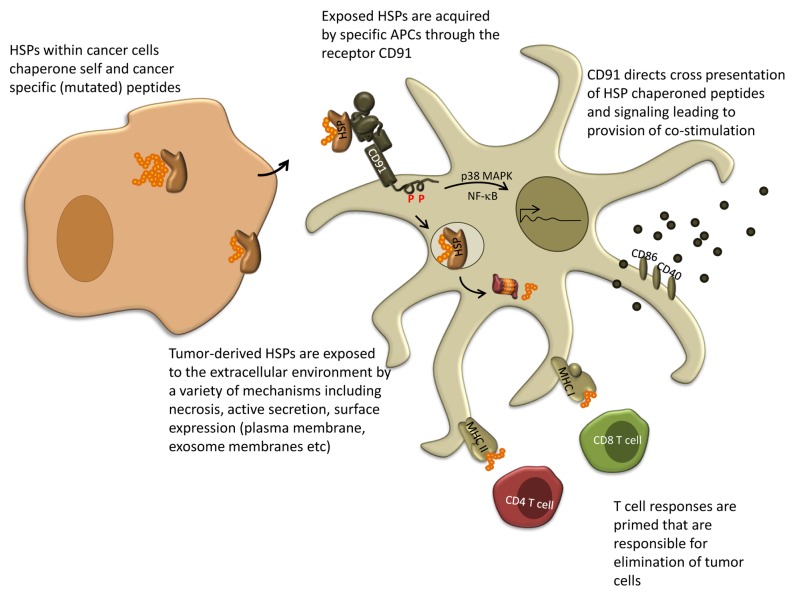
The HSPs, gp96, hsp70, hsp90, calreticulin, hsp110, and grp170 are immunogenic.8 These HSPs share 2 essential properties that allow them to elicit specific immune responses (Fig. 1). First, HSPs are chaperones of peptides which comprise the entire complement of the cellular proteome. HSP-chaperoned peptides include antigenic peptides produced by a particular cell. Thus, HSPs purified from tumor cells harbor the entire antigenicity of that tumor encompassed by the chaperoned peptides. Structural crystallography has identified peptide binding sites on at least 4 HSPs. Cross-presentation of HSP-chaperoned peptides has been examined in great detail in vivo and in vitro. HSP-chaperoned peptides can be presented via MHC class I or MHC class II molecules. Most relevant to this review is that HSP-mediated cross-presentation of chaperoned peptides satisfies the quantitative issue. A microgram of total immunogenic HSPs (the amount that will be present in 10 000 cells), typically chaperones about a nanogram of a specific antigenic/mutated peptide, but this HSP-peptide complex is sufficient for cross priming. Second, HSPs are capable of binding to receptors expressed on APCs. We first identified CD91 as a receptor for gp96, hsp70, hsp90, and calreticulin.8 CD91 has been shown to be the endocytic receptor necessary for cross-presentation of HSP-chaperoned peptides. CD91-dependent cross-presentation greatly augments and apparently fosters the efficiency of the process. Recently CD91 was also shown to be a signaling receptor for HSPs, capable of directing multiple downstream pathways in APCs. The engagement of HSP dictates the pattern of phosphorylation of CD91 cytosolic tyrosines, thus eliciting distinct cytokine profiles and the display of co-stimulatory molecules by the stimulated APC. These 2 properties underlie the ability of tumor-derived HSPs to stimulate specific anticancer immune responses.
Figure 1. A mechanism for antigen transfer, cross-presentation, and priming of T-cell responses to cancer antigens. Extracellular HSP-peptide complexes engage CD91 on the surface of APCs. CD91 allows for cross-presentation of the chaperoned antigens and promotes downstream signaling cascades culminating in co-stimulation of T cells. APC, antigen-presenting cell; HSP, heat-shock protein
HSP-peptide complexes are prime candidates accounting for the generation of tumor-specific immune responses during immunosurveillance. In our recent work, we created mice lacking CD91 expression in CD11c+APCs and tested whether HSPs were required to elicit anticancer immune responses using transplantable tumors. CD91−/− mice harboring tumors failed to mount immune responses, even though the mice were still capable of mounting efficient responses to CD91-independent immunogens.7 This was due to a deficiency in cross-presentation of HSP-chaperoned peptides, an observation recapitulated using endogenous CD91 inhibitors in wild-type mice. The transplantable tumor model allowed for a careful titration of antigen abundance in the system. Only under conditions in which antigen quantity was limiting was the HSP-CD91 pathway found to be essential. The opposite appeared to be true—CD91 was dispensable when tumor antigen was abundant, presumably due to alternative modes of antigen transfer.9
In our system the elicitation of tumor-associated immunity was unperturbed by in vitro experimental manipulation. However, several outstanding questions regarding the HSP-CD91 pathway remain. The role of CD91 in the induction of tumors de novo needs to be empirically tested. We anticipate that tumors elicited in CD91-deficient animals will be less immunoedited than those from wild-type mice. Consistent with this premise, CD91 is dispensable at higher antigen quantities. Although the role of HSPs under these conditions has not been tested, alternative mechanisms may be utilized by HSPs. The APCs essential for HSP-mediated immunogenicity have recently been characterized10 and these findings will elucidate receptor utilization. Lastly, the contribution of alternative CD91 ligands such as α2-macroglobulin to the overall co-stimulatory environment remains to be examined, given that HSPs are capable of priming helper T cells, including Th1, Th2, Th17, and regulatory T cells.
Disclosure of Potential Conflicts of Interest
R.J.B. is a named inventor of intellectual property that is being evaluated under his NIH grants, and the technology has been licensed to a company in which R.J.B. has no ownership interest or consulting contract and from which he has no sponsored research agreements. His significant financial interest consists only of a share of license fees and possible future royalties from the commercialization of his invention.
Citation: Zhou YJ, Binder RJ. The heat shock protein-CD91 pathway mediates tumor immunosurveillance. OncoImmunology 2014; 3:e28222; 10.4161/onci.28222
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–6. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 3.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohmé I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frödin L, et al. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer. 1995;60:183–9. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 4.Kropp LE, Garg M, Binder RJ. Ovalbumin-derived precursor peptides are transferred sequentially from gp96 and calreticulin to MHC class I in the endoplasmic reticulum. J Immunol. 2010;184:5619–27. doi: 10.4049/jimmunol.0902368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–9. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YJ, Messmer MN, Binder RJ. Establishment of tumor-associated immunity requires interaction of Heat Shock Proteins with CD91. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-13-0132. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder RJ. Heat-shock protein-based vaccines for cancer and infectious disease. Expert Rev Vaccines. 2008;7:383–93. doi: 10.1586/14760584.7.3.383. [DOI] [PubMed] [Google Scholar]
- 9.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messmer MN, Pasmowitz J, Kropp LE, Watkins SC, Binder RJ. Identification of the cellular sentinels for native immunogenic heat shock proteins in vivo. J Immunol. 2013;191:4456–65. doi: 10.4049/jimmunol.1300827. [DOI] [PMC free article] [PubMed] [Google Scholar]