Abstract
Upon entry into mitosis, many microtubules are nucleated that coordinately integrate into a stable, yet dynamic, mitotic spindle apparatus. In a recent publication, we examined microtubule-generating pathways within a single model system, the Drosophila syncytial embryo. We found that, following depolymerisation of metaphase spindle microtubules by cold treatment, spindles regenerate predominantly from microtubules nucleated within the vicinity of chromatin. We also showed this chromatin-mediated microtubule nucleation is mediated by the Drosophila homolog of a vertebrate spindle assembly factor (SAF), HURP and is dependent on the conserved microtubule amplifying protein complex, Augmin. Here, we expand our investigation into Drosophila SAFs, providing evidence that, in vitro, both D-HURP and D-TPX2 are able to bind to and stabilize microtubules. We show that GFP-D-HURP purified from embryos interacts with Importin-β and Augmin and, consistent with this, demonstrate that the underlying basis of chromatin-mediated microtubule nucleation in Drosophila syncytial embryos is dependent on Ran-GTP.
Keywords: microtubule, mitosis, Drosophila, Ran, HURP, Augmin
In vertebrate systems, mitotic chromatin-mediated generation of microtubules is facilitated by activating a set of proteins termed SAFs.1 Two SAFs, TPX2 and HURP, are the most thoroughly studied in a variety of experimental systems. We recently demonstrated that the putative Drosophila HURP homolog (D-HURP), but not the putative Drosophila TPX2 (D-TPX2), is required for chromatin-mediated microtubule generation in syncytial embryos.2 This is in contrast to vertebrates, in which there is a clear requirement for both proteins in this process. To test whether the requirement of D-HURP and apparent non-requirement of D-TPX2 in chromatin-mediated microtubule generation correlate with the biochemical properties of the two proteins, we generated recombinant D-TPX2 and D-HURP and investigated their ability to bind and stabilize/nucleate microtubules in vitro. We found that both His-D-TPX2 and MBP-D-HURP bind microtubules directly in a standard microtubule co-sedimentation assay (Fig. 1A). An additional assay, designed to test the microtubule stabilizing ability of the proteins, demonstrated that both D-HURP and D-TPX2 possess microtubule stabilizing (and/or nucleating) capacity (Fig. 1B). Therefore, biochemically, both Drosophila proteins behave similarly to their putative human homologs.
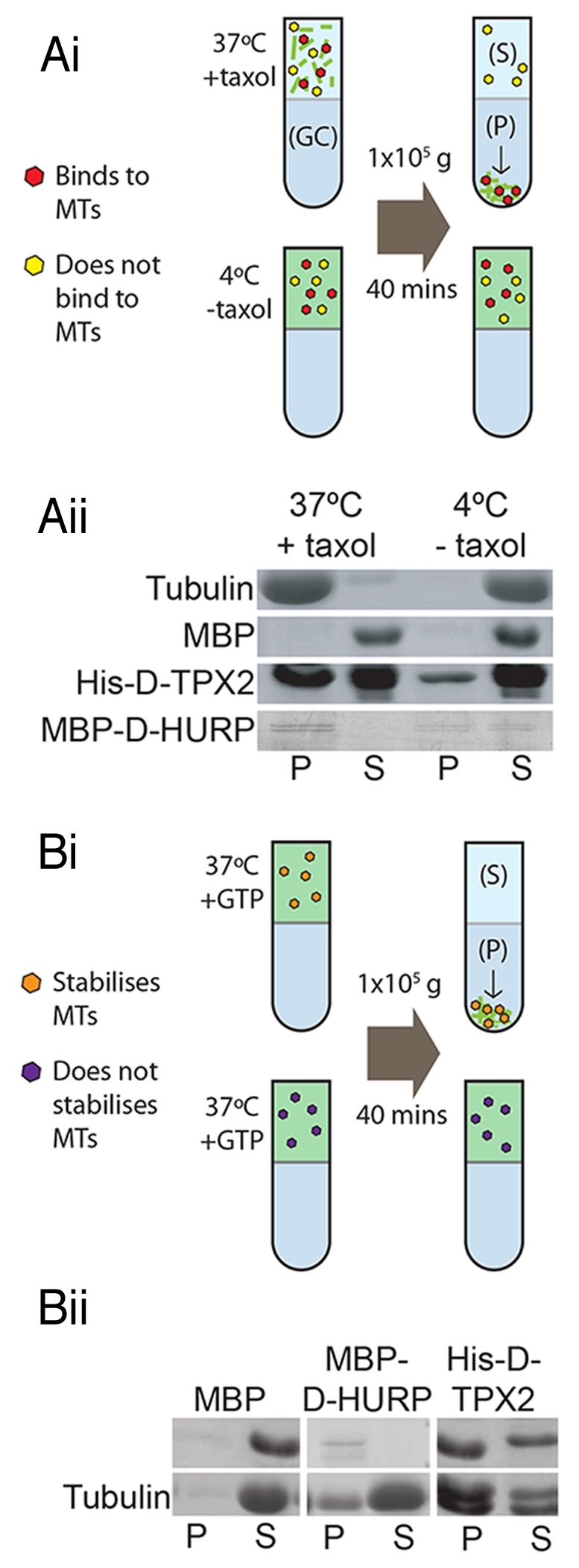
Figure 1. In vitro characteristics of Drosophila SAFs. (A) Microtubule co-sedimentation assay. Recombinant proteins are incubated with either preassembled microtubules (at 37 °C with Taxol) or with unpolymerised Tubulin (at 4 °C) then centrifuged at 100,000 g through a glycerol cushion (GC). Microtubules and microtubule binding proteins will pellet (P) while Tubulin and proteins that do not bind microtubules will remain in the supernatant (S) (i). SDS-PAGE/Western blots demonstrating that His-D-TPX2 and MBP-D-HURP are both found at higher amounts in the pellets of + Taxol samples, indicating they are both microtubule binding proteins, whereas MBP remains in the supernatant (ii). (B) Microtubule stability assay. Proteins are added to Tubulin and GTP and incubated at 37 °C, then centrifuged through a glycerol cushion. The level of Tubulin found in the pellet is indicative of the microtubule stabilizing/nucleating capabilities of the protein (i). Higher levels of Tubulin are found in the pellet of MBP-D-HURP and His-D-TPX2 samples compared with MBP as a negative control, indicating that both stabilize/nucleate microtubules (ii).
Next, we utilized GFP-TRAP-based affinity purification and mass spectrometry (AP-MS) of extracts from syncytial embryos expressing GFP-fusions to either D-HURP or D-TPX2 to identify proteins that interact with the two proteins. We failed to identify D-TPX2 by MS or western blotting from GFP-D-TPX2 extracts (not shown), implying that the GFP epitope in this expressed transgene is unavailable to the GFP-TRAP nanobody. However, D-HURP was isolated as the protein with highest MS score from extracts of embryos expressing GFP-D-HURP (Table 1). We identified nine additional specific interacting proteins, of which the two Drosophila Importin-β proteins, Fs(2)Ketel and Karyopherin-β3 were the most abundant, with scores similar to D-HURP itself. We also identified the microtubule-associated protein Ensconsin, a PP1A isoform, ArfGAP3, an uncharacterised protein (CG2017), and three subunits of the Augmin complex, all at substantially lower abundance than Importin-β, probably reflecting the mixed cell cycle population of these embryos (~80% interphase: 20% mitosis). The remaining Augmin subunits were also present in our immuno-precipitates, but with scores below our stringent cut-off such that we cannot conclude whether they are specific or non-specific interactors (see Materials and Methods).
Table 1. List of specific proteins identified by GFP-D-HURP AP-MS.
| Protein Name | % Coverage | No. Peptides | MW [kDa] | Score |
| D-HURP (Mars) | 72.20 | 77 | 101.9 | 1378.32 |
| Fs(2)Ketel (Importin β)* | 63.12 | 52 | 98.6 | 1372.07 |
| Karybeta3 (Importin β)* | 61.36 | 61 | 123.5 | 1192.46 |
| PP1A-96A | 33.64 | 10 | 37.3 | 75.78 |
| Ensconsin | 18.73 | 7 | 92.0 | 51.34 |
| Dgt6 | 21.25 | 10 | 72.8 | 48.34 |
| CG2017 | 16.19 | 7 | 75.1 | 43.86 |
| Dgt5 | 15.33 | 9 | 77.9 | 37.81 |
| Dgt3 | 20.71 | 8 | 65.8 | 31.68 |
| ArfGAP3 * | 17.93 | 5 | 54.4 | 31.13 |
GFP-D-HURP embryo extracts were incubated with GFP-TRAP-A beads, washed and subjected to Trypsin digestion, LC-MSMS and database searches. The proteins in Table 1 constitute those that were: (i) either identified in negative controls with scores of at least 4-fold less than in GFP-D-HURP (asterisks) or not identified in negative controls and (ii) had an MS score of > 30 and (iii) had > 10% coverage (% Coverage represents the % of the protein sequence covered by the peptides identified by LC-MSMS). The Importin-β proteins, Fs(2)Ketel and Karybeta3, are found at ~7 and 5-fold greater amounts respectively in GFP-D-HURP AP-MS, in relation to control experiments. Augmin subunits Dgt6, Dgt5 and Dgt3 were not present in the negative controls.
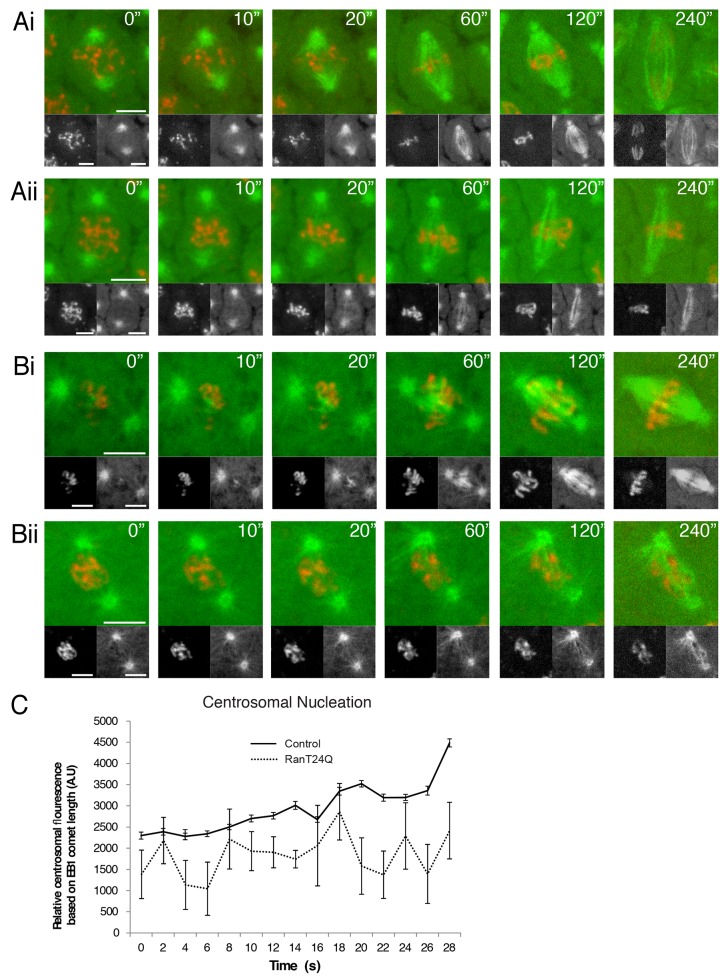
The specific interaction between D-HURP and Importin-β suggested to us that chromatin-mediated microtubule nucleation in the early embryo is most likely Ran-GTP dependent. This process is, in general, reliant on a high concentration of the GTP-bound form of the small GTPase Ran around chromatin and is achieved by converting the mitotically inactive Ran-GDP into Ran-GTP through localizing the guanine-nucleotide-exchange factor RCC1 to condensing chromatin. Ran-GTP subsequently activates SAFs and other proteins contributing to microtubule nucleation and stabilization in the vicinity of chromosomes, through their release from Importins.3 GST-RanT24N, a recombinant Ran allele locked in the GDP bound form, has previously been shown to exhibit a dominant-negative effect on Ran-GTP in Drosophila syncytial embryos.4 However, the precise effects of Ran inhibition upon microtubule generation during spindle formation in this system are not clear. By microinjecting GST-RanT24N into the cytoplasm of Drosophila embryos expressing GFP-α-Tubulin and Histone-RFP, we were able to inhibit Ran-GTP function and analyze the consequences on spindle formation and chromosome dynamics concomitantly. In cycling embryos injected with GST-RanT24N, the centrosomes initiated spindle formation in a similar timeframe to controls. However these spindles, although able to initially align chromosomes, were substantially less dense than control embryos, exhibiting a thin appearance and arresting prior to anaphase (Fig. 2A; Supplementary Videos 1 and 2). Given this phenotype is more severe than the d-hurp mutant in which chromatin-mediated microtubule nucleation is completely absent,2 we asked whether nucleation of microtubules from centrosomes is reduced upon Ran-GTP perturbation. Quantitative analysis of the intensity of EB1-GFP comets emanating from the centrosomal region in control and GST-RanT24N injected cycling embryos confirmed a significant decrease in intensity (Fig. 2C). To assess the requirement of Ran-GTP activity for chromatin-mediated microtubule nucleation, we followed microtubule organization in control and GST-RanT24N injected cold-treated embryos. As previously described, during recovery from cold treatment in control embryos, microtubules are generated around chromatin and spindles formed in an “inwards-out” manner (Fig. 2Bi; Supplementary Video 3). However, in cold-treated embryos injected with GST-RanT24N, microtubules were nucleated solely from centrosomes (Fig. 1Bii; Supplementary Video 4). These spindles appeared to be highly disorganized, and although a bipolar structure could be formed in an “outwards-in” manner, chromosomes could not be successfully aligned. Thus Ran-GTP activity functions during mitosis in Drosophila embryos through regulating microtubule generation at both centrosomes and chromosomes.
Figure 2. Ran-GTP perturbation in Drosophila syncytial embryos. (A-B) Stills from movies of spindle formation in embryos expressing α-Tubulin-GFP (green and bottom right panels) to visualize microtubules and Histone-RFP (red and bottom left panels) to visualize chromosomes. (A) Spindle formation in a cycling embryo (i) and spindle formation in an embryo injected with Ran-T24N (ii). (B) Spindle formation following cold treatment in a non-injected embryo (i) and an embryo injected mid-way through cold treatment (ii). (C) Quantification of centrosomal microtubule nucleation in control embryos and embryos injected with Ran-T24N. Line graphs show relative EB1-GFP fluorescence in the area adjacent to the centrosome during the first 30 s following cold treatment.
The present study progresses our understanding of chromatin-mediated microtubule generation in two important ways. First, the demonstration that D-HURP: (i) stabilizes microtubules, (ii) interacts with Importin-β and (iii) that it and Ran-GTP are essential for chromatin-mediated microtubule nucleation, strongly suggest that D-HURP is a true Ran-dependent SAF. Although the localization of D-HURP-GFP differs to its mammalian counterpart, in that it progressively localizes to spindle microtubules as they are nucleated,2 D-HURP shares many of the biochemical, physical and functional properties of HURP. As such, it will be important to undertake a further analysis of D-HURP function in mitotic function outside of the embryo. Second, it demonstrates the importance of the Ran-GTP gradient in embryonic spindle formation. The phenotype of GST-RanT24N injected embryos is similar to that of embryos in which the function of Augmin has been disrupted: reduced spindle density, initial spindle elongation and mitotic arrest.2,5 Although there is currently no evidence to suggest that Augmin is regulated by Ran, and an AP-MS analysis of Augmin purified from cycling Drosophila embryos does not identify Importin subunits as interacting partners (not shown), it is possible that Augmin activity could be a downstream target of the Ran-GTP pathway. Alternatively, Ran-GTP may lead to activation of other microtubule generating pathways from the centrosome. In support of this scenario, a fraction of human Ran has been shown to associate with the centrosome where it appears to regulate γ-tubulin independent microtubule nucleation.6
In summary, the work presented here complements and enhances our previous study, demonstrating that Ran-GTP, most likely through D-HURP, is responsible for chromatin-mediated microtubule nucleation in the syncytial embryo. Whether this molecular pathway is utilized for other Drosophila mitoses such as the asymmetric divisions of stem cells and/or for the male meiotic divisions to enhance the fidelity of centrosome-driven spindle formation will be an important avenue of future research.
Methods
Information on fly lines, imaging techniques, image analysis and microinjection can be found at Hayward et al., 2014. The GST-RanT24N construct was a gift from A. Wilde and was produced as described previously.4,7 Full-length D-TPX2 (Mei-38) and D-HURP (Mars) cDNAs were amplified by PCR and cloned into pDEST17a or pMAL-c2x/DEST (a gift from Jason Carlyon8) vectors respectively, using the pENTR/D/TOPO system. Constructs were transformed into BL21 competent cells with induction and purification of His-tagged and MBP-tagged proteins performed as described previously.9,10 The microtubule co-sedimentation assay was adapted for use with recombinant proteins from Hughes et al.11; using 31.25 μg Tubulin, 1 μM GTP, pure protein (MBP at 2 mg/ml, His-D-TPX2 at 2 mg/ml or MBP-D-HURP at 10 mg/ml) and 25 μM Taxol where appropriate. The microtubule stability assay was performed using 50 μl C buffer containing 62.5 μg Tubulin, pure protein (same amounts as co-sedimentation assay) and 1 mM GTP, incubated at 37 °C for 25 min. Samples for both the microtubule binding and microtubule stability assays were run on standard SDS-PAGE gels and stained with Coomassie. AP-MS to identify interacting proteins will be described in detail elsewhere. Briefly, clarified (high speed supernatant) embryo extracts, made from 0.4 g of 0–3 h old GFP-D-TPX2 or GFP-D-HURP embryos as previously described,11 were incubated with 30 μl of GFP-TRAP-A bead slurry (Chromotek, Germany) in C buffer for 2 h at 4 °C with rotation. Beads were washed 5 x in 1 ml C buffer, frozen in N2 (l) and processed by the Bristol Proteomics Facility using Orbitrap nano-LC MSMS. Identified proteins were cross-referenced against an in-house database of non-specific (false-positive) proteins identified from 3 control experiments from embryos expressing different GFP-fusion proteins in which the bait protein was not precipitated (i.e., negative controls). Table 1 constitutes proteins from the GFP-D-HURP AP-MS which: (i) either were not identified in negative controls or were identified in negative controls with scores of at least 4-fold less than in GFP-D-HURP and (ii) had an MS score of > 30 and (iii) had > 10% peptide:protein coverage. Nine such interactors were identified with these stringencies. The remaining Augmin subunits were present specifically in GFP-D-HURP AP-MS but with scores of < 30.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Andrew Wilde for the kind gift of the Ran-based reagents used in this study and Kate Heesom at the Bristol Proteomics Facility for Mass Spectrometry analysis.
References
- 1.Kalab P, Heald R. The RanGTP gradient - a GPS for the mitotic spindle. J Cell Sci 2008; 121; 1577-1586; 18469014; 10.4161/cib.28512 [DOI] [PMC free article] [PubMed]
- 2.Hayward D, Metz J, Pellacani C, Wakefield JG. Synergy between multiple microtubule-generating pathways confers robustness to centrosome-driven mitotic spindle formation. Dev Cell 2014; 28; 81–93; 24389063; 10.1016/j.devcel.2013.12.001 [DOI] [PMC free article] [PubMed]
- 3.Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 2001; 104; 83–93; 11163242. [DOI] [PubMed]
- 4.Silverman-Gavrila RV, Wilde A. Ran is required before metaphase for spindle assembly and chromosome alignment and after metaphase for chromosome segregation and spindle midbody. Mol Biol Cell 2006; 17; 2069 –2080; 16481399. [DOI] [PMC free article] [PubMed]
- 5.Wainman A, Buster DW, Duncan T, Metz J, Ma A, Sharp D, Wakefield JG. A new augmin subunit, Msd1, demonstrates the importance of mitotic spindle-templated microtubule nucleation in the absence of functioning centrosomes. Genes Dev 2009; 23; 1876–1881; 19684111; 10.1101/gad.532209 [DOI] [PMC free article] [PubMed]
- 6.Keryer G, Di Fiore B. Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol Biol 2003; 14; 4260–4271; 14517334. [DOI] [PMC free article] [PubMed]
- 7.Wilde A, Zheng Y. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 1999; 284; 1359–1362; 10334991. [DOI] [PubMed]
- 8.Huang B, Troese MJ, Ye S, Sims JT, Galloway NL, Borjesson DL, Carlyon JA. Anaplasma phagocytophilum APH_1387 is expressed throughout bacterial intracellular development and localizes to the pathogen-occupied vacuolar membrane. Infect Immun 2010; 78; 1864–1873; 20600793; 10.1016/j.micpath.2010.06.009 [DOI] [PMC free article] [PubMed]
- 9.Hengen P. Purification of His-Tag fusion proteins from Escherichia coli. Trends Biochem Sci. 1995;20:285–6. doi: 10.1016/S0968-0004(00)89045-3. [DOI] [PubMed] [Google Scholar]
- 10.Gao S, Giansanti MG, Buttrick GJ, Ramasubramanyan S, Auton A, Gatti M, Wakefield JG. Australin: a chromosomal passenger protein required specifically for Drosophila melanogaster male meiosis. J. Cell Biol 2008; 180; 521–535; 18268101; 10.1083/jcb.200708072 [DOI] [PMC free article] [PubMed]
- 11.Hughes JR, Meireles AM, Fisher KH, Garcia A, Antrobus PR, Wainman A, Zitzmann N, Deane C, Ohkura H, Wakefield JG A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol 2008; 6; e98; 18433294; 10.1371/journal.pbio.0060098 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.