Abstract
Ontak®, a conjugate between IL-2 and a diphtheria toxin fragment, was recently investigated in cancer clinical trials aiming to kill CD25+ regulatory T cells (Tregs). We found that the activity of Ontak® was more complex on Tregs and conventional T cells (Tconvs) than anticipated, including a novel strong influence on dendritic cells (DCs).
Keywords: Ontak, dendritic cells, T cells, regulatory T cells, interleukin-2 receptor
Pharmacological intervention strategies aiming to modulate immune responses include toxins conjugated to ligands that recognize cognate receptors on target cells. Ligand-receptor binding purportedly initiates internalization of the toxin-ligand-receptor complex, thereby affecting target cell killing. Such simplistic approaches, however, postulate rudimentary biological functions that may, in actuality, be far more complex processes in vivo.
Immunization with vaccines against tumor antigens or infusion of cancer-specific T cells is expected to ablate residual malignant cells and reduce metastatic burden. However, activation of the adaptive immune system naturally elicits the counter-activity of endogenous tolerance mechanisms. These include the immunosuppressive activity mediated by Tregs functioning to maintain self-tolerance. Tregs also limit and ultimately terminate immune responses against foreign antigens. Since tumors are composed of autologous cells typically exhibiting moderate immunological phenotypic alterations relative to the originating tissue cell type, Treg infiltration and immunosuppressive activity is frequently observed to occur at tumor sites. Therefore, either the direct pharmacological depletion of Tregs, or alternatively, the application of agents designed to influence their differentiation state or immune-inhibitory properties, could enhance native anticancer immune responses. One strategy to deplete Tregs is to target them with Ontak®, a fusion molecule of the truncated coding fragment A and the membrane-associated domain B of diphtheria toxin genetically linked to human interleukin-2 (IL-2).2
Cellular toxicity of Ontak® requires the IL-2 receptor (IL-2R)-mediated uptake of the chimeric toxin into acidic intracellular vesicles and subsequent transfer into the cytoplasm where it binds to the eukaryotic translation elongation factor 2 (EEF2) inhibiting protein synthesis precipitating apoptotic cell death.3 Ontak®-susceptible cell types express the α chain for the IL-2R, more commonly known as CD25+. CD25 is present on lymphoid cancer cells, mature dendritic cells (DCs), activated and antigen-specific conventional T cells (Tconv), and Tregs, all of which also constitutively express both the IL-2R β (CD122) and common γ chains (CD132) to form the high affinity IL-2Rαβγ complex.
The hypothesis that CD25+ leukemia and lymphoma cells could be killed by cytocidal therapy with Ontak® was tested in the first clinical trials using this drug. Since then successful application of Ontak® against undesirable CD25+ Tconv responses has been reported in mouse models in vivo4 and in studies assaying human cells in vitro.5 These studies implied that Ontak® could potentially dampen overshooting immunologic responses or treat autoimmune-related pathologies (such as psoriasis) leading to clinical trials with good therapeutic outcomes.6
More recently, cytolytic effects of Ontak® on immunosuppressive CD25+ Tregs have been reported. Injection of Ontak® into mice has been observed to deplete Tregs and enhance T-cell immunity.7 However, in the non-obese diabetic (NOD) mouse model of human type-1 diabetes, we reported that Ontak® either promoted disease or had no effect, depending on the time point of application.8 Several clinical studies (cited in ref. 1) reported the successful depletion of Tregs by Ontak® in treated cancer patients, although others have failed to detect Ontak®-mediated Treg depletion in melanoma patients.9 The reasons underlying these incongruous outcomes are obscure.
Our own clinical data obtained from Ontak®-treated melanoma patients shortly before undergoing tumor-specific DC vaccinations indicated that Ontak® prevented the development of CD4+ and CD8+ T-cell responses against the vaccine.1 This prompted us to investigate the effects of Ontak® on immature and mature DCs, as well as resting and activated Tregs and Tconvs, in more detail in vitro.
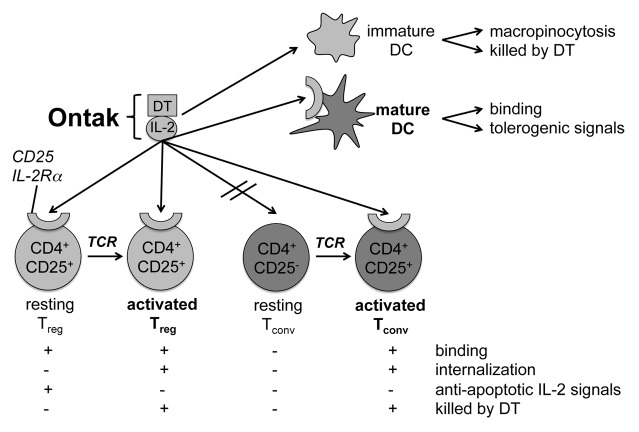
One outstanding question was whether Ontak® could selectively target CD25+ Tregs or CD25+ Tconvs in different diseases, and if so, how, considering that both cell types are simultaneously present in lymphoid and effector tissues during autoimmunity and tumor-immunity. In our patients we failed to detect reliable depletion of Tregs after Ontak® application. Thus, we investigated the susceptibility of resting and activated Tregs and Tconvs to Ontak® in vitro.1 Our results indicate that the cytotoxicity of Ontak® correlates with the activation state of both T-cell types (Fig. 1). At a resting state, high doses of Ontak® are required to induce apoptosis in both cell types whereas low doses are anti-apoptotic. In contrast, freshly activated Tregs and Tconvs are both readily killed by Ontak®.
Figure 1. Binding of Ontak® to DC, Tconvs, and Tregs results in diverse effects. The IL-2 conjugate with diphtheria toxin (DT; i.e., Ontak®) binds to the high affinity IL-2R dictated by the α chain (CD25) component that associates with the constitutively expressed β and γ chains. Immature DCs do not express CD25 but internalize Ontak®, most likely via macropinocytosis, leading to their toxin-mediated killing. Mature CD25+ dendritic cells (DCs) bind Ontak® resulting in the stimulation of tolerogenic-signaling pathways. Resting CD25+ regulatory T cells (Tregs) bind Ontak® but do not internalize it. Therefore, the IL-2 component of Ontak® rather transmits anti-apoptotic signals through the IL-2R. Resting CD25− conventional T cells (Tconvs) remain unaffected due to a lack of Ontak® binding. Both T cell receptor (TCR)-activated Tconvs and Tregs bind and internalize Ontak® and are, therefore, subsequently killed by the toxin.
Additionally, we found a totally novel effect of Ontak® on DCs. Surprisingly, both immature and mature DCs were affected, thereby influencing both the tolerogenic and immunogenic stages of this antigen-presenting cell concurrently. Immature, CD25- DCs bound and ingested Ontak®, most likely by macropinocytosis, and then underwent massive apoptosis in a dose-dependent manner. In contrast, mature CD25+ DC were killed only at high doses of Ontak®, whereas low dosages downregulated essential costimulatory molecules such as CD83, CD70, and CD25 (Fig. 1).1 The latter finding is fatal, since Ontak® converts mature DCs that would normally initiate anticancer T-cell immune responses into an immature DC-phenotype capable of fostering tolerance against the presented tumor antigens.
Our combined in vitro and in vivo data demonstrate that Ontak® cannot selectively deplete or inactivate tolerogenic cells such as Tregs and immature DCs while successfully eliminating immunogenic cells such as activated Tconv and mature DC. The discrepancies between human reports in regards to the selective depletion of Tregs may be due to differences in the activation states of Tregs present in various diseases, disease stages, or under the influence of parallel pharmacological treatments. In any case, more rigorous analyses of each patient's unique immune system should be performed before Ontak® treatment. For further clinical applications of Ontak® beyond its approved use in T cell lymphoma, these multifaceted effects have to be considered encompassing T cell and DC subsets and their corresponding activation stages. The outcomes of Ontak® immunotherapy in the treatment of different human diseases cannot be easily predicted due to its complex biological activity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the German Research Foundation (DFG) via the Collaborative Research Center grant SFB 643.
Glossary
Abbreviations:
- DC
dendritic cell
- Tconv
conventional T cell
- Treg
regulatory T cell
- IL-2
interleukin-2, IL-2R, IL-2 receptor
- DT
diphtheria toxin
Citation: Lutz MB, Baur AS, Schuler-Thurner B, Schuler G. Immunogenic and tolerogenic effects of the chimeric IL-2-diphtheria toxin cytocidal agent Ontak® on CD25+ cells. OncoImmunology 2014; 3:e28223; 10.4161/onci.28223
References
- 1.Baur AS, Lutz MB, Schierer S, Beltrame L, Theiner G, Zinser E, Ostalecki C, Heidkamp G, Haendle I, Erdmann M, et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122:2185–94. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 2.Kiyokawa T, Williams DP, Snider CE, Waters CA, Nichols JC, Strom TB, Murphy JR. Protein engineering of DAB-IL-2 fusion toxins to increase biologic potency. Ann N Y Acad Sci. 1991;636:331–9. doi: 10.1111/j.1749-6632.1991.tb33463.x. [DOI] [PubMed] [Google Scholar]
- 3.Bacha P, Williams DP, Waters C, Williams JM, Murphy JR, Strom TB. Interleukin 2 receptor-targeted cytotoxicity. Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J Exp Med. 1988;167:612–22. doi: 10.1084/jem.167.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley VE, Bacha P, Pankewycz O, Nichols JC, Murphy JR, Strom TB. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988;85:3980–4. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousvaros A, Stevens AC, Strom TB, Murphy J, Lamont JT. Interleukin-2 fusion protein (DAB389IL-2) selectively targets activated human peripheral blood and lamina propria lymphocytes. Dig Dis Sci. 1997;42:1542–8. doi: 10.1023/A:1018891432581. [DOI] [PubMed] [Google Scholar]
- 6.Martin A, Gutierrez E, Muglia J, McDonald CJ, Guzzo C, Gottlieb A, Pappert A, Garland WT, Bagel J, Bacha P. A multicenter dose-escalation trial with denileukin diftitox (ONTAK, DAB(389)IL-2) in patients with severe psoriasis. J Am Acad Dermatol. 2001;45:871–81. doi: 10.1067/mjd.2001.117852. [DOI] [PubMed] [Google Scholar]
- 7.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinser E, Rössner S, Littmann L, Pangratz N, Schuler G, Steinkasserer A. The IL-2 diphtheria toxin fusion protein denileukin diftitox modulates the onset of diabetes in female nonobese diabetic animals in a time-dependent manner and breaks tolerance in male nonobese diabetic animals. J Immunol. 2012;189:1173–81. doi: 10.4049/jimmunol.1102691. [DOI] [PubMed] [Google Scholar]
- 9.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–92. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]