Abstract
Biotic and abiotic stress conditions produce reactive oxygen species (ROS) in plants causing oxidative stress damage. At the same time, ROS have additional signaling roles in plant adaptation to the stress. It is not known how the two seemingly contrasting functional roles of ROS between oxidative damage to the cell and signaling for stress protection are balanced. Research suggests that the plant growth regulator auxin may be the connecting link regulating the level of ROS and directing its role in oxidative damage or signaling in plants under stress. The objective of this review is to highlight some of the recent research on how auxin’s role is intertwined to that of ROS, more specifically H2O2, in plant adaptation to oxidative stress conditions.
Keywords: auxin, hydrogen peroxide, signaling, oxidative stress
Auxin and ROS
The plant growth regulator auxin has been well known for regulating many growth and developmental processes such as meristem development, cell division, cell elongation and maintenance of polarity.1,2 More recently, auxin’s function has also been connected to plant defense against stress. Oxidative stress is a component of many abiotic stress conditions such as drought,3 high temperature stress,4 salinity5 and heavy metal stress6 and biotic stress conditions such as herbivory7 and plant pathogen interactions.8 During these stress conditions, levels of reactive oxygen species (ROS) increase, potentially resulting in oxidations of DNA, proteins and lipids. During plant adaptation, however, cellular repair machineries reduce at least some of these oxidized macromolecules. At the same time, ROS have additional signaling roles in plant adaptation to the stress (Fig. 1).
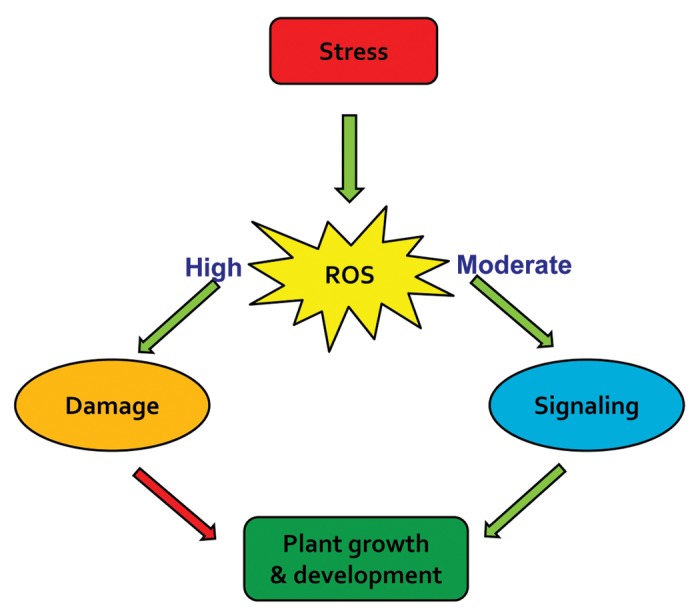
Figure 1. Dual role of reactive oxygen species (ROS) during stress. Green arrows indicate positive effects and red negative effects.
Auxin and ROS are rapidly altered by environmental stress factors. ROS can have effects on auxin biosynthesis, transport, metabolism and signaling.9 Major ROS molecules in cells include superoxide anion (O2−), hydroxyl radical (·OH), singlet oxygen (1O2) and hydrogen peroxide (H2O2). They are produced in various cell compartments mainly chloroplasts, mitochondria, peroxisomes, endoplasmic reticulum, plasma membrane, cell wall and apoplast and this aspect has been extensively reviewed.10,11 Hydrogen peroxide is one of the major ROS compounds produced in and outside the cells during abiotic and biotic stress conditions.12 Studies show that exogenous auxin application induces H2O2 in plants13,14 and that may be the mode of action for auxin type herbicides.15 On the contrary, exogenous auxin reduced the H2O2 accumulation in the roots of tomato through altered expression and activity of H2O2 scavenging enzymes catalases, Cu-Zn-superoxide dismutase (SOD) and peroxidases.16 Reactive oxygen species production was shown to be essential for auxin-regulated gravitropism in maize roots.17 Scavenging of ROS by the addition of antioxidants inhibited root gravitropism.17 It was shown that phosphatidylinositol 3-kinase activation was required for auxin-induced H2O2 production and root gravitropism.18 Pre-treatment with inhibitors of phosphatidylinositol 3-kinase stopped ROS production in root tissue and root protoplasts of maize, while the addition of exogenous auxin induced phosphatidylinositol 3-kinase activity.18 A study on barley root tip indicated that the application of auxin signaling inhibitor reduced cadmium-induced H2O2 production and growth response.19 Auxin-induced plant cell elongation is mediated by the production of ·OH, H2O2 and O2.-.20 The production of ·OH from O2− and H2O2 from peroxidase reactions act as cell wall loosening agents20 and help in extensibility by breaking the backbones of cell wall polysaccharides.21
Arsenite (AsIII) is a toxic metalloid known to induce oxidative damage in cells. We used this as a tool to identify the role of auxin in oxidative stress tolerance in Arabidopsis.22 The auxin transporter mutant aux1 was more sensitive to AsIII than the wild-type. During AsIII stress, compared with aux1, wild-type Arabidopsis plants produced increased H2O2 which helped them tolerate the stress better than the mutant. This indicated that AUX1 had a positive role in induction of H2O2 production during stress.22 Our results are corroborated by a study conducted on auxin signaling mutant.23 The auxin signaling mutant, tir1afb2 (double mutant for auxin receptors TIR1-Transport Inhibitor Response1 and AFB2-auxin signaling F-box 2)24 showed reduced accumulation of H2O2 and superoxide anion, and had enhanced activities of antioxidant enzymes catalase and ascorbate peroxidase.23 These results indicate that auxin homeostasis in specific tissues is important to regulate the production of H2O2 through altered expression of antioxidant enzymes.
Auxin and ROS Signaling
Production of superoxide by NADPH oxidase is the first step in the formation of H2O2.25,26 Auxin-induced NADPH oxidase activity has been recorded in isolated vesicles and elongating hypocotyls of soybean.27 This activity was inhibited by the addition of thiol reagents like dithiothreitol, and reduced glutathione.27 In Arabidopsis root, it is shown that transient increase in extracellular ATP(eATP) is perceived by the plasma membrane leading to the production of reactive oxygen species mainly through the action of NADPH oxidase (AtRBOHC) followed by the activation of Ca2+ channels.28 AtrbohC mutants were impaired for eATP buildup, ROS production, increase in Ca2+ and transcription of mitogen-activated protein kinase 3 (MAPKinase3).28 Mitogen-activated protein kinase was found to be induced by H2O2 treatment which in turn was able to activate antioxidant enzymes.29 During salt stress tolerance, the H2O2 and Ca2+ signaling was triggered by H+ coupled ion transporters like H+-ATPase in Populus euphratica.30 Exogenous supply of auxin induced H+-ATPase activity in petunia pollen by hyper polarization of plasma membrane and transient increase in cytosolic Ca2+.31 Inhibitors of NADPH oxidase of plasma membrane blocked this process. Hydrogen peroxide application mimicked the exogenous IAA application in the male gametophyte indicating the process is mediated by production of ROS.31
Mitochondrial electron transport chain is a site of ROS production. A study on Arabidopsis mutant abo6 coding a mitochondrial DEXH box RNA helicase indicated that these mutants accumulated more ROS than the wild-type and were impaired for auxin signaling, suggesting ABA’s role in its enhancement of auxin signaling.32
There are lines of evidence in Arabidopsis for localized accumulation of auxin increasing H2O2 production.14 Exogenous auxin application was found to produce H2O2 and induced an accumulation of irreversible inactive form of auxin, 2-oxindole-3-acetic acid (oxIAA).14 This form of auxin was not transported from cell to cell and was found at high levels in auxin transporter (ABCB) mutants. The oxIAA was not able to activate auxin signaling suggesting the importance of auxin metabolism in manipulating auxin signaling.14
Thiol Reduction Systems in Auxin Regulation
Thiol reduction systems, NADPH-dependent thioredoxin reductases and glutathione (GSH) affect the developmental processes in Arabidopsis by interfering with auxin signaling.33 In this study, Trx reductase (ntra ntrb-mutant with inactivated cytosolic and mitochondrial thioredoxin reductases) and glutathione biosynthesis mutations (cad2-Cd hypersensitive) negatively altered auxin transport and metabolism and the triple mutant ntra ntrb cad2 had defects in the auxin-regulated phenotypes.33 Low glutathione availability correlated with the reduction in expression of PIN auxin transporters PIN1, PIN2, PIN3, PIN4 and AUX1 and auxin response marker gene IAA1.33 Triple mutant ntra ntrb cad2 had flowerless phenotype similar to the pin mutants which was rescued by the addition of GSH. Also, the mutant calli lacked the ability to regenerate shoots in the absence of exogenous auxin. In the same way, mutants of ROXY1 and ROXY2, CC-type glutaredoxins show abnormalities in petal and anther development in flowers of Arabidopsis,34-36 suggesting auxin-related phenotypes.
Parallel to these observations, atgrxs17 Arabidopsis mutant for a GSH-dependent thiol transferase (glutaredoxin) were sensitive to high temperature stress and accumulated higher amounts of ROS and displayed altered auxin response phenotype.37 Arabidopsis mutants for AtGrxS17 displayed post embryonic growth phenotypes similar to that of auxin perception mutants.37 These mutants had altered auxin sensitivity and polar auxin transport37 compared with wild-type plants. Exogenous GSH application rescued hyponastic leaf curling caused by altered auxin levels in catalase2 (cat2) mutant which accumulates high levels of H2O2.38 These results together indicate that thiol reduction pathways are involved in the regulation of auxin homeostasis and resulting phenotypes.
Role of H2O2 and Auxin in Abiotic Stress Tolerance
Reactive oxygen species in plants are known to be produced during biotic and abiotic stress conditions having dual roles (Fig. 1) of causing damage and signaling to induce defense responses.39,40 Several studies suggest a link between auxin homeostasis and H2O2 in plant stress tolerance but the mechanistic details are not well understood. Auxin transport mutant aux1 was more sensitive to arsenite stress than wild-type seedlings in Arabidopsis.22 Wild-type plants recorded increased H2O2 on arsenite stress treatment but not in aux1 mutant indicating a positive role of auxin transporter in production of ROS. Auxin transport mutant aux1 was also found to be hypersensitive to high temperature and salt stress indicating a common functional role of auxin transport in stress tolerance to heat, salt and arsenite.22 Increase in H2O2 was also correlated with reduced transcription of the catalase-3 in the wildtype.22 This can be attributed to the reduced auxin transport and accumulation in the roots of aux1 mutant and its inability to induce H2O2 signaling.
Several other phenotypes of the ROS signaling mutants were reversible by the addition of exogenous auxin signifying the cross-link between auxin and ROS. Defects in shoot regeneration from the calli and secondary root production in Trx reductase mutant ntra ntrb cad2 were rescued by exogenous supply of auxin suggesting that the mutants were less sensitive to auxin or auxin was limiting in these mutants.33 High temperature causes tissue specific reduction in auxin which leads to pollen sterility. Application of auxin completely reversed male sterility in barley and Arabidopsis.41 This is consistent with the report in Arabidopsis that auxin transporter PIN8, expressed in the male gametophyte is critical for auxin homeostasis and normal development of the male gametophyte.42In the presence of cytosolic Ca, calmadulin (CaM) a calcium binding protein was able to bind to and activate catalase extracted from tobacco leaves.43 Activation of catalase by Ca2+CAM was found to be unique to plants and no such activation was found in bovine, human or fungal catalases, thus suggesting unique regulation of H2O2 concentration and signaling to environmental signals.43 Because auxin could increase cytosolic calcium, the control on H2O2 levels by auxin could be due to CaM’s effects on catalase activity. A recent study demonstrated that a catalase-deficient mutant (cat2) had reduced catalase activity, increased H2O2 accumulation and reduced auxin levels in the Arabidopsis leaves, leading to an “up curling phenotype” under photorespiratory conditions.38 This implies that auxin levels in the leaves were modulated by H2O2 levels. Exogenous application of auxin was also able to rescue the mutant phenotype.38 The same results of rescued phenotype could be achieved by the application of GSH which was found to induce the transcription of auxin biosynthetic genes.38
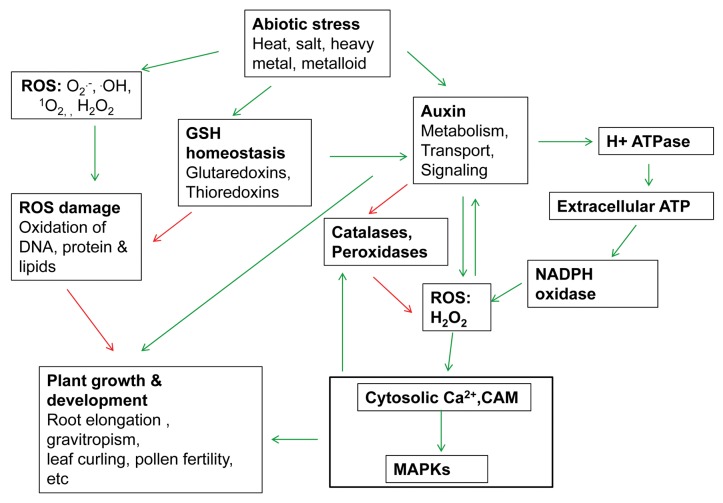
Accumulating evidence from studies in different plant species indicate that auxin’s function in plants growth, development and more recently in stress response could be mediated via ROS signaling. Key events relating to auxin and ROS regulating plant growth, development and stress tolerance are summarized in Figure 2. The regulated processes include root gravitropism, extension growth, pollen tube growth, arsenite tolerance and high temperature stress tolerance. Further studies are required to examine the link between auxin and ROS homeostasis and the signaling cascades. These studies also open new possibilities to alter crop tolerance to stress by engineering auxin synthesis and metabolism, auxin signaling, ROS signaling and thiol-mediated regulatory pathways.44 New uses for exogenous auxin to optimize crop performance under stress could be explored41.
Figure 2. The metabolic interplay between auxin and hydrogen peroxide to control plant growth, development and stress tolerance. Green arrows indicate positive effects and red negative effects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
Funding support by the Consortium for Plant Biotechnology Research Inc. (GO12026-315) , BASF Plant Science LLC and the College of Agriculture and Life Sciences, University of Florida, is gratefully acknowledged.
References
- 1.Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot. 2005;95:707–35. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol. 2008;24:55–80. doi: 10.1146/annurev.cellbio.23.090506.123214. [DOI] [PubMed] [Google Scholar]
- 3.Moran J, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas R, Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta. 1994;194:346–52. doi: 10.1007/BF00197534. [DOI] [Google Scholar]
- 4.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–95. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez JA, Corpas FJ, Gomez M, del Rio LA, Sevilla F. Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant. 1993;89:103–10. doi: 10.1111/j.1399-3054.1993.tb01792.x. [DOI] [Google Scholar]
- 6.Sytar O, Kumar A, Latowski D, Kuczynska P, Strzalka K, Prasad MNV. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant. 2013;35:985–99. doi: 10.1007/s11738-012-1169-6. [DOI] [Google Scholar]
- 7.Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–7. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant JJ, Yun BW, Loake GJ. Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J. 2000;24:569–82. doi: 10.1046/j.1365-313x.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 9.Tognetti VB, Mühlenbock P, Van Breusegem F. Stress homeostasis - the redox and auxin perspective. Plant Cell Environ. 2012;35:321–33. doi: 10.1111/j.1365-3040.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 10.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P. A. BJ, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012:217037. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 12.Voothuluru P, Sharp RE. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. J Exp Bot. 2013;64:1223–33. doi: 10.1093/jxb/ers277. [DOI] [PubMed] [Google Scholar]
- 13.Peer WA, Murphy AS. Flavonoids as signal molecules: Targets of flavonoid action. In E Grotewold, ed. The Science of Flavonoids. New York, DA. Springer publishing, 2006:239-68. [Google Scholar]
- 14.Peer WA, Cheng Y, Murphy AS. Evidence of oxidative attenuation of auxin signalling. J Exp Bot. 2013;64:2629–39. doi: 10.1093/jxb/ert152. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann K, Kwiatkowski J, Tresch S. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galium aparine L.) J Exp Bot. 2001;52:1811–6. doi: 10.1093/jexbot/52.362.1811. [DOI] [PubMed] [Google Scholar]
- 16.Ja T, Dunajska K, Mazurek P. Piotrowska Ba, Tretyn A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol Plant. 2009;31:249–60. doi: 10.1007/s11738-008-0225-8. [DOI] [Google Scholar]
- 17.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–60. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett. 2005;579:1243–8. doi: 10.1016/j.febslet.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Tamás L, Bočová B, Huttová J, Liptáková L, Mistrík I, Valentovičová K, et al. Impact of the auxin signaling inhibitor p-chlorophenoxyisobutyric acid on short-term Cd-induced hydrogen peroxide production and growth response in barley root tip. J Plant Physiol. 2012;169:1375–81. doi: 10.1016/j.jplph.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–8. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- 21.Schweikert C, Liszkay A, Schopfer P. Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry. 2000;53:565–70. doi: 10.1016/S0031-9422(99)00586-5. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamurthy A, Rathinasabapathi B. Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ. 2013 doi: 10.1111/pce.12093. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias MJ, Terrile MC, Bartoli CG, D’Ippólito S, Casalongué CA. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol. 2010;74:215–22. doi: 10.1007/s11103-010-9667-7. [DOI] [PubMed] [Google Scholar]
- 24.Savaldi-Goldstein S, Baiga TJ, Pojer F, Dabi T, Butterfield C, Parry G, et al. New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc Natl Acad Sci USA. 2008;105:15190–5. doi: 10.1073/pnas.0806324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, Ohtaki S, Makino R, Tanaka T, Ishimura Y. Superoxide anion is the initial product in the hydrogen peroxide formation catalyzed by NADPH oxidase in porcine thyroid plasma membrane. J Biol Chem. 1989;264:4759–61. [PubMed] [Google Scholar]
- 26.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–32. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morre DJ, Brightman AO, Hidalgo A, Navas P. Selective Inhibition of auxin-stimulated NADH oxidase activity and elongation growth of soybean hypocotyls by thiol reagents. Plant Physiol. 1995;107:1285–91. doi: 10.1104/pp.107.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009;58:903–13. doi: 10.1111/j.1365-313X.2009.03830.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang A, Jiang M, Zhang J, Tan M, Hu X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006;141:475–87. doi: 10.1104/pp.105.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Zhang X, Deng S, Zhang C, Wang M, Ding M, et al. Extracellular ATP signaling is mediated by H₂O₂ and cytosolic Ca²⁺ in the salt response of Populus euphratica cells. PLoS ONE. 2012;7:e53136. doi: 10.1371/journal.pone.0053136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voronkov AS, Andreev IM, Timofeeva GV, Kovaleva LV. Electrogenic activity of plasma membrane H+-ATPase in germinating male gametophyte of petunia and its stimulation by exogenous auxin: mediatory role of calcium and reactive oxygen species. Russ J Plant Physiol. 2010;57:401–7. doi: 10.1134/S102144371003012X. [DOI] [Google Scholar]
- 32.He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, et al. DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell. 2012;24:1815–33. doi: 10.1105/tpc.112.098707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, et al. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell. 2010;22:376–91. doi: 10.1105/tpc.109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Lauri A, Ziemann M, Busch A, Bhave M, Zachgo S. Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. Plant Cell. 2009;21:429–41. doi: 10.1105/tpc.108.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Xing S, Birkenbihl RP, Zachgo S. Conserved functions of Arabidopsis and rice CC-type glutaredoxins in flower development and pathogen response. Mol Plant. 2008;2:323–35. doi: 10.1093/mp/ssn078. [DOI] [PubMed] [Google Scholar]
- 36.Xing S, Zachgo S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008;53:790–801. doi: 10.1111/j.1365-313X.2007.03375.x. [DOI] [PubMed] [Google Scholar]
- 37.Cheng N-H, Liu J-Z, Liu X, Wu Q, Thompson SM, Lin J, et al. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem. 2011;286:20398–406. doi: 10.1074/jbc.M110.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Yuan H-M, Hu Y-Q, Li J, Lu Y-T. Mutation of Arabidopsis CATALASE2 results in hyponastic leaves by changes of auxin levels. Plant Cell Environ. 2013;••• doi: 10.1111/pce.12144. [DOI] [PubMed] [Google Scholar]
- 39.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–9. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Potters G, Horemans N, Jansen MAK. The cellular redox state in plant stress biology--a charging concept. Plant Physiol Biochem. 2010;48:292–300. doi: 10.1016/j.plaphy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, et al. Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA. 2010;107:8569–74. doi: 10.1073/pnas.1000869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding ZJ, Wang BJ, Moreno I, Dupláková N, Simon S, Carraro N, et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun. 2012;3:941. doi: 10.1038/ncomms1941. [DOI] [PubMed] [Google Scholar]
- 43.Yang T, Poovaiah BW. Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA. 2002;99:4097–102. doi: 10.1073/pnas.052564899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JI, Baek D, Park HC, Chun HJ, Oh DH, Lee MK, et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol Plant. 2013;6:337–49. doi: 10.1093/mp/sss100. [DOI] [PubMed] [Google Scholar]