Abstract
Regulation of stomata movements is crucial for plants ability to cope with their changing environment. Guard cell’s (GC) water potential directs water flux inside/outside this cell, which eventually is causing the stoma to open or close, respectively. Some of the osmolytes which accumulates in the GC cytoplasm and are known to play a role in stomata opening are sugars, arising from chloroplast starch degradation. During stomata closure, the accumulated osmolytes are removed from the GC cytoplasm. Surprisingly little is known about prevention of starch degradation and forming additional sugars which may interfere with osmotic changes that are necessary for correct closure of stomata.
One of the early events leading to stomata closure is production of reactive oxygen species (ROS) in various sub-cellular sites and organelles of the stoma. Here we report that ROS production during abscisic acid (ABA) and methyl jasmonate (MJ) stimuli in Arabidopsis GC chloroplasts were more than tripled. Moreover, ROS were detected on the sub-organelle level in compartments that are typically occupied by starch grains. This observation leads us to suspect that ROS function in that particular location is necessary for stomata closure. We therefore hypothesize that these ROS are involved in redox control that lead to the inactivation of starch degradation that takes place in these compartments, thus contributing to the stoma closure in an additional way.
Keywords: abscisic acid, guard cells, methyl jasmonate, osmolytes, reactive oxygen species, starch, stomata
Introduction
Starch breakdown and closure of stomata
Stomatal movements have been the subject of extensive investigation for their role in plant-environment interactions, especially during periods of drought.1,2 The opening and closing of the stomata pore is controlled by water fluxes that enter or exit the guard cells, resulting in cell swelling or shrinking, respectively. The water potential of guard cells, which determines the direction of the water flow is governed by osmoregulation,3 mainly using K+ and C1- ions or malate and sucrose (SUC) molecules.4 The opening of stomata in response to blue light was shown to be coupled to Starch degradation in guard cells’ chloroplasts, and the sugars that originate from that process were determined as an additional source of osmoticum.5 Moreover, starch degradation occurs also under natural illumination conditions, resulting in induction of stomatal opening.6
The closure of the stomata, however, must involve exclusion of osmolyte molecules from the cytoplasm of the opened guard-cells to the apoplast or to its vacuole. In addition, the formation of new additional osmoticum in the same interior has to be prevented. Therefore, starch degradation that sustains stomatal opening should be inactivated during that time. Surprisingly, to the best of our knowledge very little is known about the latter event and how is it regulated.
The guard cell chloroplasts (GCC) are different from the mesophyll cell chloroplasts (MCC) by several features. In many species the typical ultra-structure of GCC include small grana stacks and large starch grains, which occupy most of the plastid interior and the size of GCC is much larger than the size of MCC.7-9 Moreover, there are much fewer GCC per stomatal cell as compared with the high number of MCC in the mesophyll cells.
Recent studies showed that the mechanism of starch degradation in Arabidopsis leaves is very different from the well characterized starch breakdown in cereal endosperm, as well as in legume seeds.10,11 The role of the key enzyme, α-amylase, has been questioned,12 and the enzyme that breaks down the starch granule into glucans has not been identified.10 Analysis of starch degradation in Arabidopsis leaves was shown to be regulated by the cellular redox state.10,13,14 It is initiated by α-amylase, which catalyzes the cleavage of α-l,4-glucosidic bond of amylose and amylopectin. The enzyme is found in nearly all plants, animals and microorganisms. Accumulation of hydrogen peroxide in plant cells causes inactivation of the α-amylase.15,16 Osmotic stress was shown to impair the rate of diurnal starch accumulation in leaves of wild-type plants, but had no effect on starch accumulation in mutants of thioredoxin that cannot reduce β−amylase. Moreover, the mutants were impaired in stomatal opening, suggesting involvement of β−amylase activity in sustaining stomatal opening during the day.17 The thioredoxin was shown to activate starch degradation pathway in illuminated mesophyll cells upon osmotic stress, similar to the diurnal pathway of starch degradation in guard cells, which is also dependent on thioredoxin-regulated β−amylase. Furthermore, oxidized thioredoxin inactivated starch degradation by β−amylase in illuminated mesophyll cells.17
In this paper we studied the production of ROS in GCC during stomata closure in response to treatment with ABA or MJ. Our results show that ROS production was more than tripled during these stimuli. Moreover, ROS accumulated in compartments that are typically occupied by starch grains. That intriguing finding leads us to suspect that ROS has a role in that location necessary for stomata closure. We suggest that ROS control starch degradation by regulating organellar redox, thus preventing formation of additional sugars which may interfere with osmotic changes during stomata closure.
Results and Discussion
ROS formation in guard cell chloroplasts during stomatal closure
Recent studies showed that plant treatment by several phytohormones increased the production of Reactive Oxygen Species (ROS) in guard cells, resulting in stomatal closure.18,19 However, the exact localization of ROS in the cell remained obscure. We showed that the production of ROS in the Arabidopsis stomata is localized in many different sub-cellular organelles, such as nucleus, cytoplasm, endosomes and chloroplasts.18 Accumulation of H2O2 in GCC during ABA treatment was also studied in guard cells of Vicia-faba.20,21 However, those studies lacked high resolution of the sub-organellar ROS localization during induction in the chloroplastids.
Sub-organellar localization of ROS in GCC during treatment with ABA or MJ
Here we show highly localized ROS production in GCC (Fig. 1A). Measurements of H2O2 levels showed that they were more than tripled in Arabidopsis GCC during ABA and MJ treatments (Fig. 1B). Moreover, the induction of ROS in GCC was not spread evenly but appeared restricted to some inner compartments of the chloroplast (Fig. 1A). High resolution study that included endomembrane staining with MitoFluor™ Red 589 in addition to H2DCFDA showed that the accumulation of ROS in GCC occurred in large spaces, typically occupied by starch grains (Fig. 2, full complete Z stacks are presented in Figs. S1, S2 and S3). It is notable, that the signal was not detected on the inner membranes of chloroplasts that represent the small grana stacks, where the PSI and PSII are located.
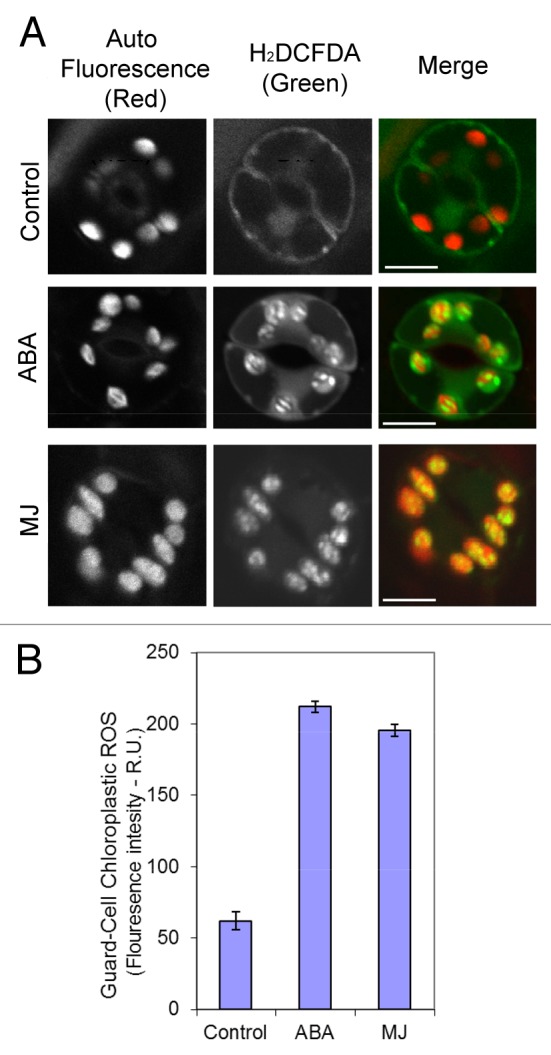
Figure 1.Induction of ROS production in Arabidopsis guard cells’ chloroplasts by ABA and MJ. (A) Leaf disks were prepared from wild-type plants and treated with ABA and MJ. After incubation of leaf discs in solution with the phytohormones, cells were loaded with H2DCFDA as described in methods. Shown are representative images of single section confocal microscopy (1 μm thick) of guard cells from the different treatments. Scale bar = 10 µm. (B) Quantification of the H2DCFDA-dependent fluorescence of individual chloroplasts shown in (A). Quantification was done using ImagePRO-Plus program from projected images of 2–3 confocal Z stack sections of individual chloroplasts, as described in methods. Shown is a representative one of three replicate experiments. n = 30 chloroplasts from 10 guard cells sampled from three different young rosette leaves (± SE). R.U. relative unites.
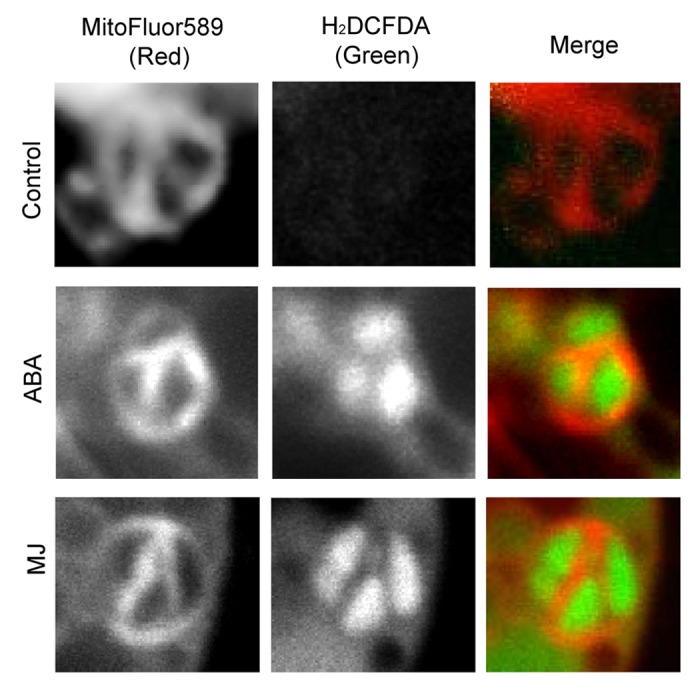
Figure 2. Sub-organellar localization of ROS in guard cell chloroplasts during ABA and MJ stimuli. Simultaneous staining of ROS by H2DCFDA and the intracellular membrane dye MitoFluor589 combined with red auto fluorescence, in guard cell chloroplasts of wild type epidermal peals. ABA and MJ were applied as in Figure 1. Loading of dyes and fluorescence detection were as described by Leshem et al. (2010). Shown are representative single section (1 um thick) confocal images of individual chloroplasts from the different treatments. For the complete Z stack image series see Supplemental Figures 1,2 and 3 for control, ABA and MJ treatments, respectively. Scale bar = 2 µm.
Concluding Remarks
In summary, we found ROS accumulation in GCC in compartments typically occupied by starch grains, following treatments with ABA and MJ. We suspect that the function of ROS in this location is to block the source of osmoticum formation by inactivating starch degradation. This suggestion is in line with previous in vitro studies in Arabidopsis chloroplasts that showed regulation of starch degradation by redox potential, which controlled the activity of amylases in chloroplasts.16 Additional effects of ROS production in GCC might influence other activities, such as gene expression22 and transport of molecules between cytoplasm and chloroplasts.
Experimental Procedures
Plant material
WT Arabidopsis plants (COL) were grown in soil pots located in growth chamber (light 120 μE in short day conditions, 8 h light). Leaf epidermal strips or leaf discs were harvested and treated as described in Leshem et al. (2010). MJ and ABA were applied at 20 μM for 2 h.
ROS detection and Confocal microscopy
Treated leaf epidermal strips or discs were floated in 20 mM KCl, 1 mM CaCl2, 5 mM MES-KOH, pH 6.15, at 20 °C and loaded with 10 μM H2DCFDA (Molecular Probes-Invitrogen, Eugene OR) and 6 μM MitoFluorTM Red 589 (Molecular Probes- Invitrogen) and the fluorescent signal was detected using confocal microscope as described by Leshem et al. (2010). All confocal images were processed by ImagePro Plus software package (Media Cybernetics, Bethesda, MD). Quantification of the H2DCFDA-dependent signal in GCC was performed by projecting Z-stack images of a single chloroplast (2–3 sections, 1 μm thick) of the separate H2DCFDA filter and analyzing the mean signal intensity of the integrated image by ImagePro Plus analysis.
Supplementary Material
Acknowledgments
We thank Dr Naomi Melamed-Book for assistance with confocal microscopy and Dr Miriam Hassidim for helpful discussions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Luan S. Signalling drought in guard cells. Plant Cell Environ. 2002;25:229–37. doi: 10.1046/j.1365-3040.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Xiao Y-G, Li X, Ni M. Light-regulated stomatal aperture in Arabidopsis. Mol Plant. 2012;5:566–72. doi: 10.1093/mp/sss039. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane TT, Cochrane TA. Differences in the way potassium chloride and sucrose solutions effect osmotic potential of significance to stomata aperture modulation. Plant Physiol Biochem. 2009;47:205–9. doi: 10.1016/j.plaphy.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Talbott LD, Zeiger E. Central Roles for Potassium and Sucrose in Guard-Cell Osmoregulation. Plant Physiol. 1996;111:1051–7. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallman G, Zeiger E. Light quality and osmoregulation in vicia guard cells : evidence for involvement of three metabolic pathways. Plant Physiol. 1988;88:887–95. doi: 10.1104/pp.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritte G, Raschke K. Metabolite export of isolated guard cell chloroplasts of Vicia faba. New Phytol. 2003;159:195–202. doi: 10.1046/j.1469-8137.2003.00789.x. [DOI] [PubMed] [Google Scholar]
- 7.Vaughn KC. Two immunological approaches to the detection of ribulose-1,5-bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1987;84:188–96. doi: 10.1104/pp.84.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whatley JM. The Ultrastructure of Guard Cells of Phaseolus vulgaris. New Phytol. 1972;71:175–9. doi: 10.1111/j.1469-8137.1972.tb04825.x. [DOI] [Google Scholar]
- 9.Henry Y, Steer MW. A reexamination of the induction of phloem transfer cell-development in pea leaves (Pisum-sativum) Plant Cell Environ. 1980;3:377–80. doi: 10.1111/1365-3040.ep11581888. [DOI] [Google Scholar]
- 10.Smith AM, Zeeman SC, Smith SM. Starch degradation. Annu Rev Plant Biol. 2005;56:73–98. doi: 10.1146/annurev.arplant.56.032604.144257. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd JR, Kossmann J, Ritte G. Leaf starch degradation comes out of the shadows. Trends Plant Sci. 2005;10:30–137. doi: 10.1016/j.tplants.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Yu T-S, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue W-L, et al. alpha-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem. 2005;280:9773–9. doi: 10.1074/jbc.M413638200. [DOI] [PubMed] [Google Scholar]
- 13.Silver DM, Silva LP, Issakidis-Bourguet E, Glaring MA, Schriemer DC, Moorhead GBG. Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J. 2013;280:538–48. doi: 10.1111/j.1742-4658.2012.08546.x. [DOI] [PubMed] [Google Scholar]
- 14.Glaring MA, Skryhan K, Kötting O, Zeeman SC, Blennow A. Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana. Plant Physiol Biochem. 2012;58:89–97. doi: 10.1016/j.plaphy.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Lin LL, Lo HF, Chiang WY, Hu HY, Hsu WH, Chang CT. Replacement of methionine 208 in a truncated Bacillus sp. TS-23 alpha-amylase with oxidation-resistant leucine enhances its resistance to hydrogen peroxide. Curr Microbiol. 2003;46:211–6. doi: 10.1007/s00284-002-3846-y. [DOI] [PubMed] [Google Scholar]
- 16.Sparla F, Costa A, Lo Schiavo F, Pupillo P, Trost P. Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis. Plant Physiol. 2006;141:840–50. doi: 10.1104/pp.106.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valerio C, Costa A, Marri L, Issakidis-Bourguet E, Pupillo P, Trost P, et al. Thioredoxin-regulated β-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J Exp Bot. 2011;62:545–55. doi: 10.1093/jxb/erq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leshem Y, Golani Y, Kaye Y, Levine A. Reduced expression of the v-SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid-dependent stomatal closure. J Exp Bot. 2010;61:2615–22. doi: 10.1093/jxb/erq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jannat R, Uraji M, Hossain MA, Islam MM, Nakamura Y, Mori IC, et al. Catalases negatively regulate methyl jasmonate signaling in guard cells. J Plant Physiol. 2012;169:1012–6. doi: 10.1016/j.jplph.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang L, Dong FC, Gao JF, Galbraith DW, Song CP. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–48. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Wang H, Takemiya A, Song CP, Kinoshita T, Shimazaki K-i. Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 2004;136:4150–8. doi: 10.1104/pp.104.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, et al. Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol. 2011;156:185–201. doi: 10.1104/pp.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


