Abstract
U373MG cells constitutively express glutathione S-transferase mu 2 (GSTM2) and exhibit 3H-dopamine uptake, which is inhibited by 2 µM of nomifensine and 15 µM of estradiol. We generated a stable cell line (U373MGsiGST6) expressing an siRNA against GSTM2 that resulted in low GSTM2 expression (26% of wild-type U373MG cells). A significant increase in cell death was observed when U373MGsiGST6 cells were incubated with 50 µM purified aminochrome (18-fold increase) compared with wild-type cells. The incubation of U373MGsiGST6 cells with 75 µM aminochrome resulted in the formation of autophagic vacuoles containing undigested cellular components, as determined using transmission electron microscopy. A significant increase in autophagosomes was determined by measuring endogenous LC3-II, a significant decrease in cell death was observed in the presence of bafilomycin A1, and a significant increase in cell death was observed in the presence of trehalose. A significant increase in LAMP2 immunostaining was observed, a significant decrease in bright red fluorescence of lysosomes with acridine orange was observed, and bafilomycin A1 pretreatment reduced the loss of lysosome acidity. A significant increase in cell death was observed in the presence of lysosomal protease inhibitors. Aggregation of TUBA/α-tubulin (tubulin, α) and SQSTM1 protein accumulation were also observed. Moreover, a significant increase in the number of lipids droplets was observed compared with U373MG cells with normal expression of GSTM2. These results support the notion that GSTM2 is a protective enzyme against aminochrome toxicity in astrocytes and that aminochrome cell death in U373MGsiGST6 cells involves autophagic-lysosomal dysfunction.
Keywords: autophagy, lysosome dysfunction, Parkinson disease, dopamine, aminochrome, glutathione transferase, siRNA, astrocytes
Introduction
Astrocytes take up dopamine through the norepinephrine transporter, and selective inhibitors of the norepinephrine transporter, nisoxetine, and desipramine, inhibit the specific uptake of both 3H-dopamine and 3H-norepinephrine in astrocytes.1 However, the physiological relevance of dopamine in astrocytes remains unknown, as dopamine is oxidized to aminochrome in the presence of oxygen at physiological pH.2 In dopaminergic neurons, aminochrome participates in several reactions, such as i) aminochrome polymerization to form neuromelanin, which accumulates with age in the human substantia nigra;3,4 ii) adduct formation with proteins such as SNCA/α-synuclein to enhance and promote neurotoxic protofibril formation,5,6 PARK2/parkin (parkin RBR E3 ubiquitin protein ligase) of the proteasome system,7 complex I and III of the mitochondrial electron transport chain,8 tubulin (a structural protein required for cytoskeleton architecture and autophagosome fusion with lysosomes)9 and actin (which is required for cytoskeleton architecture and axonal transport),9 iii) one-electron aminochrome reduction to form the leukoaminochrome-o-semiquinone radical, which is extremely reactive with oxygen and neurotoxic;10-20 iv) 2-electron aminochrome reduction to form leukoaminochrome, a process catalyzed through NQO1 [NAD(P)H dehydrogenase, quinone 1], which prevents aminochrome participation in neurotoxic reactions such as adduct formation with proteins and subsequent one-electron reduction;10-19,21-23 and v) aminochrome conjugation through GSTM2. Aminochrome conjugation has been proposed as a protective reaction, as this reaction prevents the one-electron reduction of aminochrome, and the formed conjugate (leukoaminochrome-GSH) is stable in the presence of biological oxidizing agents, such as dioxygen, superoxide radicals, and hydrogen peroxide.24,25 In addition, GSTM2 prevents the formation of aminochrome through the conjugation of dopamine o-quinone to 5-glutathionyl-dopamine,26 the precursor of 5-cysteinyl dopamine.27 Indeed, 5-S-cysteinyl-dopamine has been detected in neuromelanin and in the cerebrospinal fluid of PD patients as well as in dopamine-rich brain regions such as the caudate nucleus, putamen, globus pallidus, and substantia nigra.28-30
Therefore, the aim of this study was to test the hypothesis that GSTM2 protects astrocytes from aminochrome toxicity. We transduced the human glioblastoma astrocytoma (U373MG) cell line with a pSuper.retro.puro plasmid encoding a siRNA to suppress GSTM2 expression.
Results
U373MG as a model cell line
The human astrocytoma cell line U373MG was used as a model cell line to study the protective role of GSTM2 against aminochrome. U373MG cells constitutively express GSTM2, as determined by western blotting (Fig. 1A and B), showing that 3H-dopamine uptake increases with time (Fig. S1A). Dopamine uptake was 90 ± 3 nmol/min/mg protein at 15 min and significantly decreased to 47 ± 6 and 44 ± 6 nmol/min/mg protein in the presence of 2 µM nomifensine (P < 0.05) and 15 µM estradiol (P < 0.05), respectively (Fig. S1B). To determine the possible identity of the dopamine transporter in U373MG, we measured the mRNA expression of dopamine transporters through reverse transcriptase PCR. We observed that the mRNA expression of SLC6A3/dopamine transporter [solute carrier family 6 (neurotransmitter transporter), member 3] was higher than that of SLC22A1 [solute carrier family 22 (organic cation transporter), member 1], and SLC29A4/plasma membrane monoamine transporter [solute carrier family 29 (equilibrative nucleoside transporter), member 4] (Fig. S1C). The expression of SLC22A2, SLC22A3, SLC6A2/norepinephrine transporter [solute carrier family 6 (neurotransmitter transporter), member 2], and SLC6A4/serotonin transporter [solute carrier family 6 (neurotransmitter transporter), member 4] mRNA was not detectable using RT-PCR (not shown).
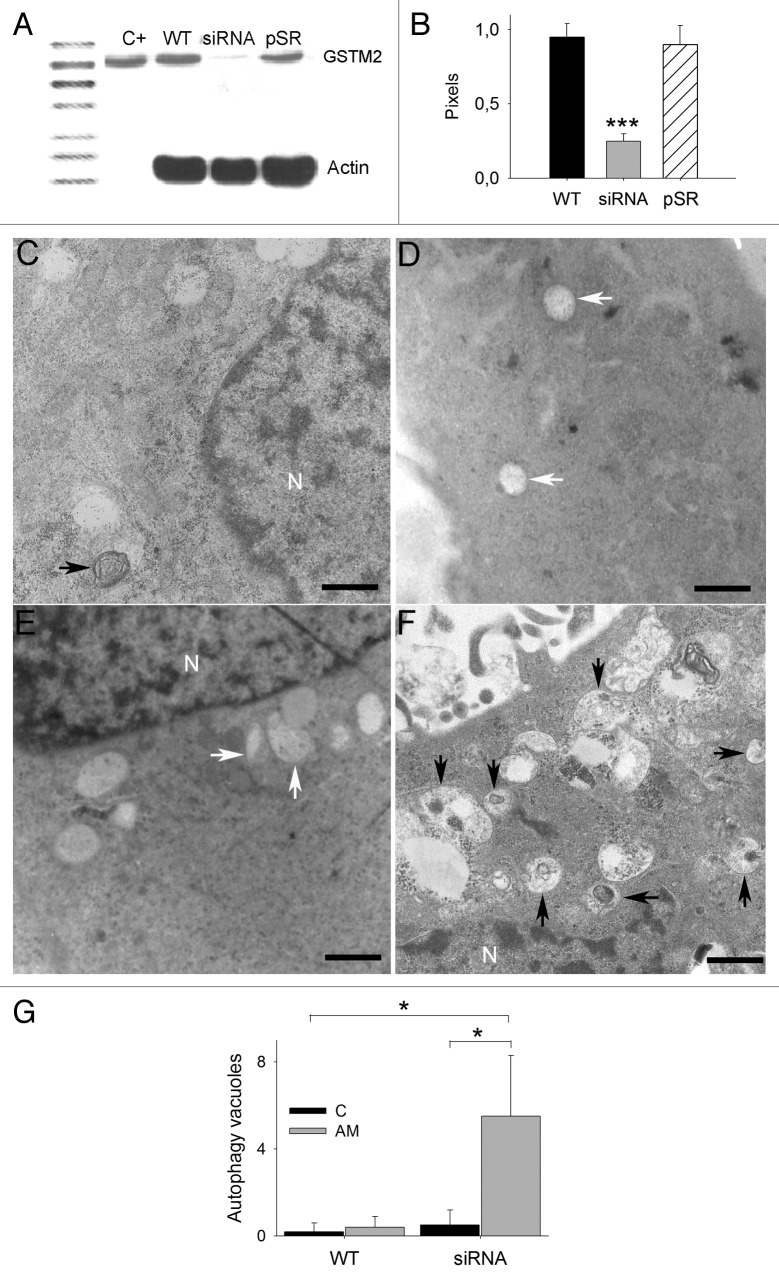
Figure 1. GSTM2 expression and ultrastructure of U373MG in the presence of aminochrome. (A) A significant decrease in GSTM2 in U373MGsiGST6 cells (siRNA) was determined using western blotting. U373MG wild-type cells (WT) and U373MGpSR empty vector cells (pSR) were used as a control. As a positive control for GSTM2 antibodies, we used pure GSTM2 recombinant enzyme (C+). (B) The western blot results were plotted as pixels of GSTM2/pixels actin; autophagic and nonautophagic vacuoles were observed in U373MG (C) and U373MGsiGST6 cells (E) incubated with cell culture medium during 24 h. In the presence of 75 µM of aminochrome for 24 h, we observed vacuoles with undigested cellular components in U373MGsiGST6 cells (F) in contrast with the vacuoles of U373MG cells incubated with 75 µM aminochrome (D). The autophagic vacuoles in (C–F) are indicated with black arrows, and nonautophagic vacuoles are indicated with white arrows. (G) The number of autophagic vacuoles observed was quantified and plotted. Scale bars: (C–F) 1.5 µm; nucleus (N).
GSTM2-silencing with siRNA
We used siRNA to silence the expression of GSTM2 in U373MG cells. The siRNA duplex oligonucleotide was inserted into a pSuper.retro.puro plasmid (pSR) and transfected into HEK-293T cells to produce retroviral particles to infect U373MG cells. The transfection efficiency of retroviral particles in U373MG cells was tested using siRNA for GFP in U373MG cells transfected with a plasmid encoding GFP (not shown). We transduced U373MG cells with a supernatant fraction containing retroviral particles with a pSR plasmid encoding siRNA for GSTM2 collected at 72 h. The selection of U373MGsiGST6 cells expressing siRNA for GSTM2 was performed after adding 6 µg of puromycin to the cell culture medium at 24 h after transduction, as the pSR plasmid carries a resistance gene against this antibiotic. As a control, we transduced U373MG cells with the pSR plasmid without siRNA (U373MGpSR cells). A 74% decrease in GSTM2 protein expression was determined through western blotting in U373MGsiGST6 cells compared with U373MG wild-type cells. As expected, no significant decrease in GSTM2 protein expression was observed in U373MGpSR cells compared with U373MG cells (Fig. 1A and B). The quantification of GSTM2 mRNA expression was determined using quantitative real-time PCR. An 87% decrease in GSTM2 mRNA expression in U373MGsiGST6 cells was observed compared with that in the wild-type U373MG cell line. No decrease in the expression of GSTM2 was observed in U373MGpSR cells (Fig. S1D).
GSTM2 protects against aminochrome toxicity
The protective effect of GSTM2 against aminochrome-dependent cell toxicity was tested after incubating U373MG cells for 24 h with increasing concentrations of aminochrome (0 to 100 µM), and no cell death was observed until 50 µM of aminochrome was reached. However, the incubation of U373MGsiGST6 cells with 50 µM of aminochrome for 24 h induced a significant 18-fold increase in cell death compared with the incubation of wild-type U373MG cells. At 100 µM of aminochrome, we observed significant cell death in both U373MG (16 ± 2% cell death; P < 0.001), and U373MGsiGST6 (57 ± 11 cell death; P < 0.01) cells. At this concentration, no significant differences between U373MG and U373MGpSR cells were observed (Fig. S2).
Aminochrome effect on autophagy
The ultrastructure of U373MGsiGST6 cells incubated with 75 µM of aminochrome for 24 h showed autophagy vacuoles with double membranes containing undigested cell components (Fig. 1F). In the absence of aminochrome, U373MGsiGST6 cells showed non-autophagic vacuoles (Fig. 1E). Wild-type U373MG cells also showed autophagic and non-autophagic vacuoles, but the cells treated with 75 µM of aminochrome did not contain autophagic vacuoles with undigested material (Fig. 1C and D, respectively). The U373MGsiGST6 cells treated with 75 µM of aminochrome showed a significant increase in the number of autophagic vacuoles (5.5 ± 3; P < 0.05) compared with both untreated and treated U373MG and untreated U373MGsiGST6 cells (0.2 ± 0.4, 0.4 ± 0.5, and 0.5 ± 0.7 respectively; Fig. 1G).
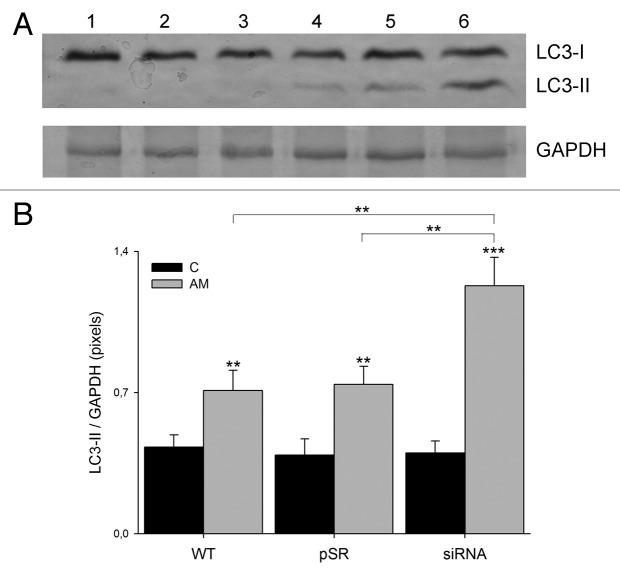
We determined the levels of LC3-I and -II expression using western blot analysis. A significant 2.9-fold (P < 0.001) increase in LC3-II expression was observed in U373MGsiGST6 cells treated with 75 µM of aminochrome compared with U373MG cells without aminochrome (Fig. 2B).
Figure 2. The effect of aminochrome on LC3-II expression. (A) Determination of LC3-II expression through western blot analysis. A total of 30 µg of U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) untreated cell homogenate (control) was applied in lanes 1, 2, and 3, respectively. The same amount of protein from U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) cell homogenate treated with 75 µM aminochrome (AM) was applied to lanes 4, 5, and 6, respectively. (B) The values represent the means ± SD (n = 3). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test (*P < 0.01; ***P < 0.001).
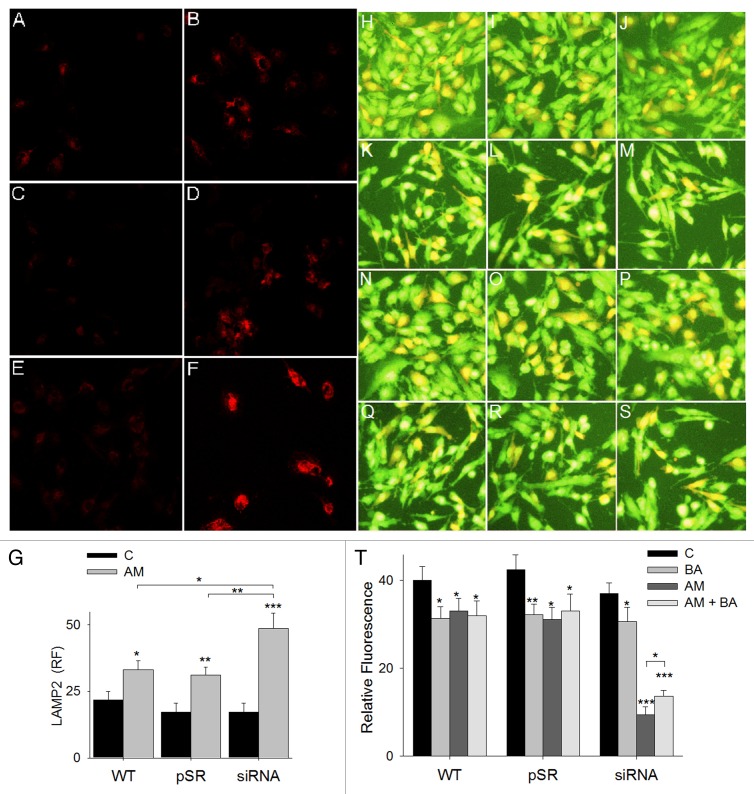
To determine the role of autophagy in aminochrome-induced cell death, we used bafilomycin A1, an inhibitor of vacuolar-type H+-ATPase31,32 required for the lysosomal degradation of protein and damaged organelles. The preincubation of U373MG cells with bafilomycin A1 for 2 h prior to the addition of 75 µM of aminochrome induced a significant decrease in aminochrome-induced cell death in U373MGsiGST6 cells from 26 ± 2% to 21 ± 2% cell death with 10 nM bafilomycin A1 (P < 0.05; Fig. 3A). A similar but more pronounced effect was observed when U373MGsiGST6 cells treated with 75 µM of aminochrome were preincubated with 100 nM bafilomycin A1, showing a decrease in cell death from 32.5 ± 2.4% to 18.9 ± 2.2% (P < 0.001; not shown).
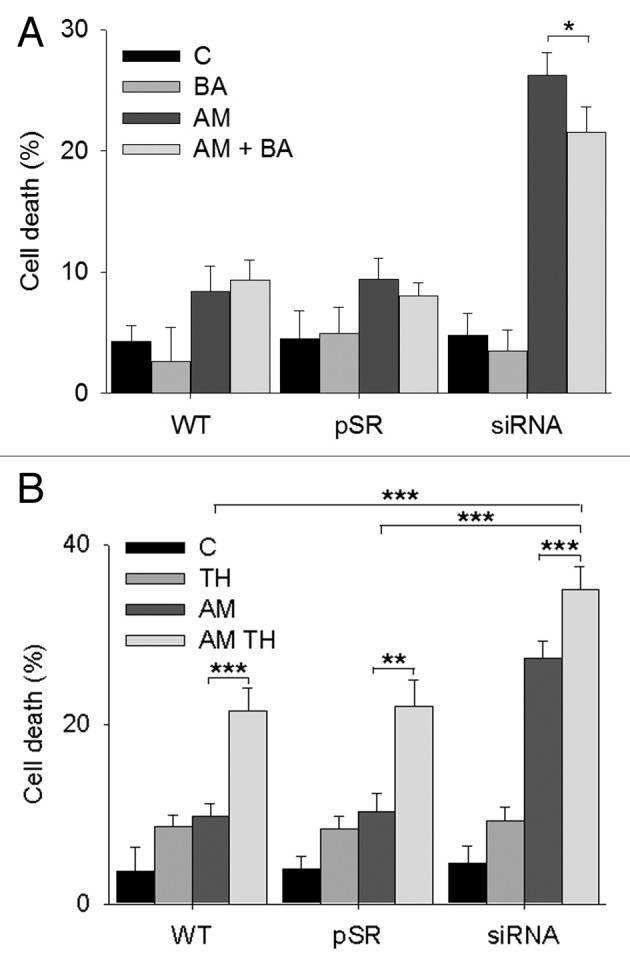
Figure 3. The role of autophagy in aminochrome-induced cell death. (A) The role of autophagy in aminochrome-induced cell death was determined by preincubating the cells for 2 h with 10 nM bafilomycin A1 (BA) prior to the addition of 75 µM aminochrome (AM) for 24 h to prevent the degradation of autophagic vacuoles. U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) cells were incubated with cell culture medium (C); 10 nM bafilomycin A1 (BA); 75 µM aminochrome (AM), or 75 µM aminochrome with 10 nM bafilomycin A1 pretreatment (AM + BA), which induces a significant decrease in cell death. (B) The role of increased autophagy in aminochrome-induced cell death was determined by incubating the cells with the autophagy-inducer trehalose. The cells were pretreated with 100 mM trehalose (TH) for 24 h before the addition of 75 µM aminochrome (AM). A significant increase in cell death was observed in cells treated with 75 µM of aminochrome and trehalose (AM + TH). The values are the means ± SD (n = 3). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test. (*P < 0.05; **P < 0.01; ***P < 0.001).
We used trehalose as an inducer of autophagy33 to determine whether an increase in autophagy protects the cells against aminochrome-induced cell death, as U373MG cells are rapamycin-resistant.34 A significant increase in cell death was observed in U373MG, U373MGpSR, and U373MGsiGST6 cells that were pretreated with 100 mM of trehalose for 24 h before the addition of 75 µM of aminochrome (21.5 ± 2.5%; 22 ± 2.9% and 35 ± 2.6% cell death; P < 0.001; P < 0.01, and P < 0.01, respectively; Fig. 3B).
Aminochrome effect on lysosomes
The protection against aminochrome-induced cell death, provided through pretreatment with bafilomycin A1 in U373MGsiGST-6 cells, suggests aminochrome-mediated lysosomal dysfunction, as bafilomycin A1 inhibits cell death caused by lysosomotropic agents.35-37 To characterize the potential lysosomal alterations induced through aminochrome treatment in U373MG cells, we stained lysosomes for lysosomal-associated membrane protein 2 (LAMP2) and performed an immunofluorescence analysis. The incubation of U373MG, U373MGpSR, and U373MGsiGST6 cells with 75 µM aminochrome for 24 h induced a significant increase in LAMP2 immunofluorescence intensity (33 ± 3, P < 0.05; 31 ± 3, P < 0.01; 49 ± 6, P < 0.001) compared with control conditions incubated with cell culture medium alone (22 ± 3; 17 ± 3; 17 ± 4, respectively) (Fig. 4G); this effect was more intense in U373MGsiGST6 cells with low GSTM2 expression. The increase in LAMP2-positive immunostaining was accompanied with a significant increase in lysosome size (not shown). To determine whether aminochrome induced lysosome dysfunction, we treated U373MG, U373MGpSR, and U373MGsiGST6 cells using 75 µM of aminochrome for 24 h, followed by staining with 2 µg/ml of acridine orange. The acidic compartments show red fluorescence with acridine orange.38 A significant decrease in percentage of cells with red acridine orange fluorescence was observed in U373MG, U373MGpSR, and U373MGsiGST6 cells treated with aminochrome, with a greater effect on these latter cells (33 ± 3%, P < 0.05; 31 ± 4%, P < 0.05; 9.4 ± 2%, P < 0.001; Fig. 4T) in comparison with cells incubated with cell culture medium (40 ± 3%; 43 ± 3%; 37 ± 3% red fluorescence, respectively; Fig. 4T). Interestingly, the pretreatment of cells with 10 nM bafilomycin A1 for 2 h significantly inhibited the decrease in red fluorescence induced by aminochrome, but only in U373MGsiGST6 cells (14 ± 1% red fluorescence vs. 9 ± 2% in U373MGsiGST6 treated with aminochrome without bafilomycin A1 pretreatment; P < 0.05; Fig. 4M, S, and T). Higher effect was observed when U373MGsiGST6 cells were incubated with 100 nM bafilomycin A1 for 2 h (16 ± 2% red fluorescence vs. 6.3 ± 3% red fluorescence in cells incubated with aminochrome alone) (P < 0.01; not shown).
Figure 4. The effect of aminochrome on LAMP2 and acridine orange staining. The effect of aminochrome on LAMP2 expression was determined using immunofluorescence. U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) cells were incubated with cell culture medium (A, C, and E, respectively) or 75 µM aminochrome (B, D, and F, respectively) for 24 h. The quantification of fluorescence was plotted in (G). The effect of aminochrome on lysosomal function determined with acridine orange fluorescence staining after preincubating U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) cells for 2 h with 10 nM bafilomycin A1 prior to the addition of 75 µM aminochrome to the culture medium for 24 h (AM + BA; Q–S). As a control, we incubated the cells with cell culture medium (C; H–J); 75 µM aminochrome (AM; K–M) or we only preincubated them with bafilomycin A1 (BA; N–P). Interestingly, bafilomycin A1 inhibited the aminochrome-induced decrease in red acridine orange fluorescence in U373MGsiGST6 cells (S). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test (*P < 0.05; **P < 0.01; ***P < 0.001).
These results suggested a protective effect of bafilomycin A1 against aminochrome-induced lysosomal dysfunction. Therefore, to obtain more information regarding the role of lysosomal function in aminochrome toxicity, we also studied aminochrome-induced cell death in the presence of lysosomal protease inhibitors such as pepstatin A (CTSD [cathepsin D]) inhibitor and leupeptin (inhibitor of other proteases, such as CTSB [cathepsin B]).39,40 Treatment with 50 µM leupeptin for 24 h significantly increased aminochrome-induced cell death in U373MG WT (15 ± 2%; P < 0.05) and U373MGpSR (16 ± 1.9%; P < 0.01) cells, but not in U373MGsiGST6 (24.6 ± 2%) cells, compared with cells treated with aminochrome alone (8.8 ± 1.7%, 9.6 ± 1.6%, and 26.3 ± 1.6%, respectively; Fig. 5A). However, the pretreatment of cells with 100 µM of pepstatin A for 24 h significantly increased aminochrome-induced cell death in U373MG WT, U373MGpSR, and U373MGsiGST6 cells (25.5 ± 2.0%, P < 0.001; 27.6 ± 2.6, P < 0.001; 34.2 ± 2.4%, P < 0.01, respectively) compared with U373MG WT, U373MGpSR, and U373MGsiGST6 cells treated with aminochrome alone (8.4 ± 2%, 8.1 ± 3%, and 25.2 ± 2%, respectively; Fig. 5B).
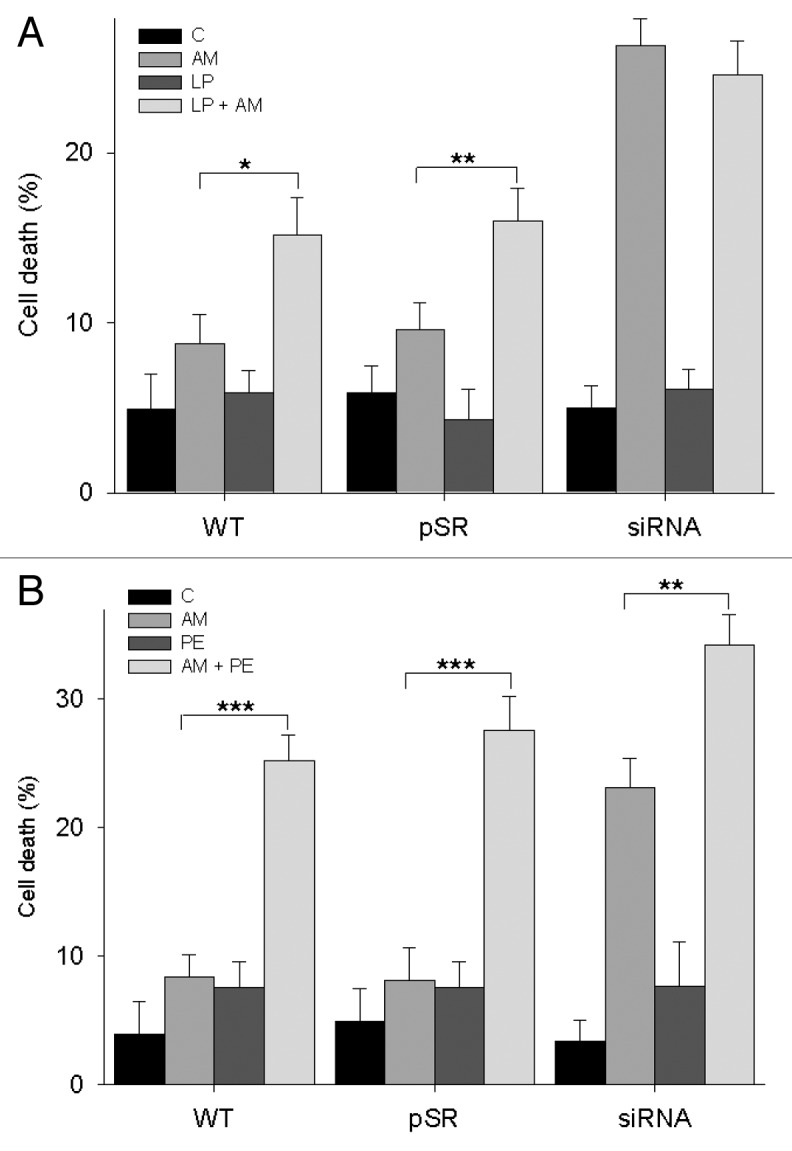
Figure 5. Aminochrome cytotoxicity in the presence of protease inhibitors. (A) 1.-U373MG (WT), U373MGpSR (pSR), and U373MGsiGST6 (siRNA) cells were incubated with cell culture medium (C), 50 µM leupeptin (LP), 75 µM aminochrome (AM), 50 µM leupeptin, and 75 µM aminochrome (AM + LP) for 24 h. Leupeptin treatment increased aminochrome-induced cell death in U373MG and U373MGpSR cells, but not in U373MGsiGST6 cells. (B) The cells were incubated with cell culture medium (C), 100 µM pepstatin A (PE), 75 µM aminochrome (AM), 100 µM pepstatin A, and 75 µM aminochrome (AM + PE) for 24 h. Pepstatin A treatment increased aminochrome-induced cell death in U373MG WT, U373MGpSR, and U373MGsiGST6 cells. The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test (*P < 0.05; **P < 0.01; ***P < 0.001).
Aminochrome effect on TUBA
U373MGsiGST6 cells treated with 75 µM of aminochrome for 24 h showed an alteration in shape from elongated to spherical (not shown); therefore, we measured the effect of aminochrome on the cell cytoskeleton by determining TUBA immunofluorescence. We observed that aminochrome induced morphological changes in U373MG, U373MGpSR, and U373MGsiGST6 cells treated with aminochrome (75 µM) for 24 h (Fig. S3B, S3D, and S3F, respectively). Aminochrome treatment induced a significant increase in the number of cells with TUBA aggregation and cytoskeleton architecture disruption in U373MG (11 ± 3%), U373MGpSR (13 ± 3%; P < 0.01), and U373MGsiGST6 (22.9 ± 3.4%; P < 0.01) cells compared with untreated cells (5.3 ± 2%, 4.4 ± 2%, and 5.5 ± 3%, respectively, Fig. S3G).
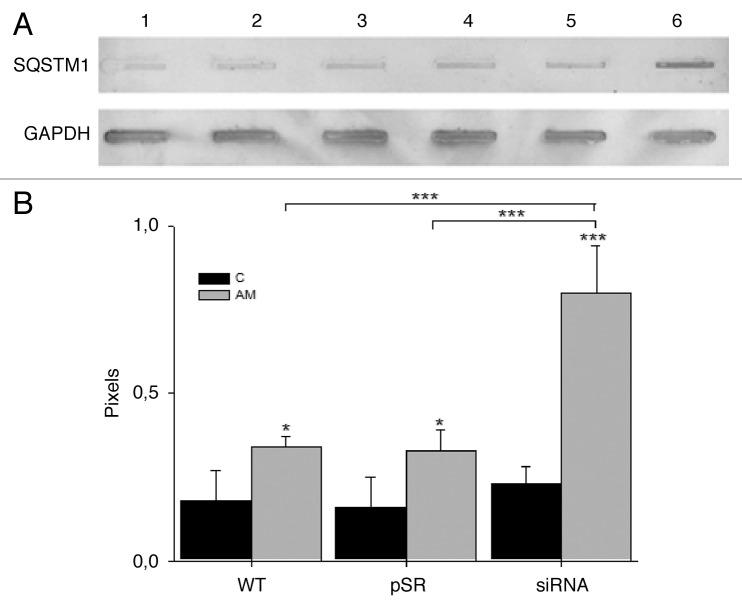
The aggregation of TUBA suggests that microtubule formation was impaired, and the fusion of autophagic vacuoles and lysosomes, mediated through microtubules, might have also been affected, reflecting aminochrome-induced autophagic dysfunction. Therefore, we determined whether aminochrome inhibits autophagy through the degradation of SQSMT1, which accumulates when autophagy is inhibited.41 Treatment with 75 µM aminochrome induced a significant 4.4-fold accumulation of SQSMT1 in U373MGsiGST6 cells (P < 0.001) compared with untreated U373MG cells (Fig. 6B).
Figure 6. The effect of aminochrome on SQSMT1 degradation. (A) The SQSMT1 accumulated in U373MGsiGST6 cells (AM, lane 6) treated with 75 µM aminochrome (AM) for 24 h, in contrast with U373MG WT (C, lane 1), U373MGpSR (C, lane 2), and U373MGsiGST6 C, (lane 3) cells incubated with cell culture medium (C) and also U373MG WT (AM, lane 4) and U373MGpSR (AM, lane 5) cells treated with 75 µM aminochrome for 24 h. The results from analysis of 4 immunoblots are plotted in (B). The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test (*P < 0.05; ***P < 0.001).
Aminochrome effect on macrolipophagy
Moreover, the ultrastructure analysis of U373MGsiGST6 cells using electron microscopy revealed a dramatic increase in the number of lipid droplets in the cytosol when cells were incubated with 75 µM aminochrome for 24 h (50 ± 5 lipid droplets per cell; P < 0.001), in contrast with U373MGsiGST6 and U373MG cells incubated with cell culture medium and U373MG cells incubated with 75 µM aminochrome (7 ± 1, 6 ± 1, and 6 ± 1, respectively; Fig. 7).
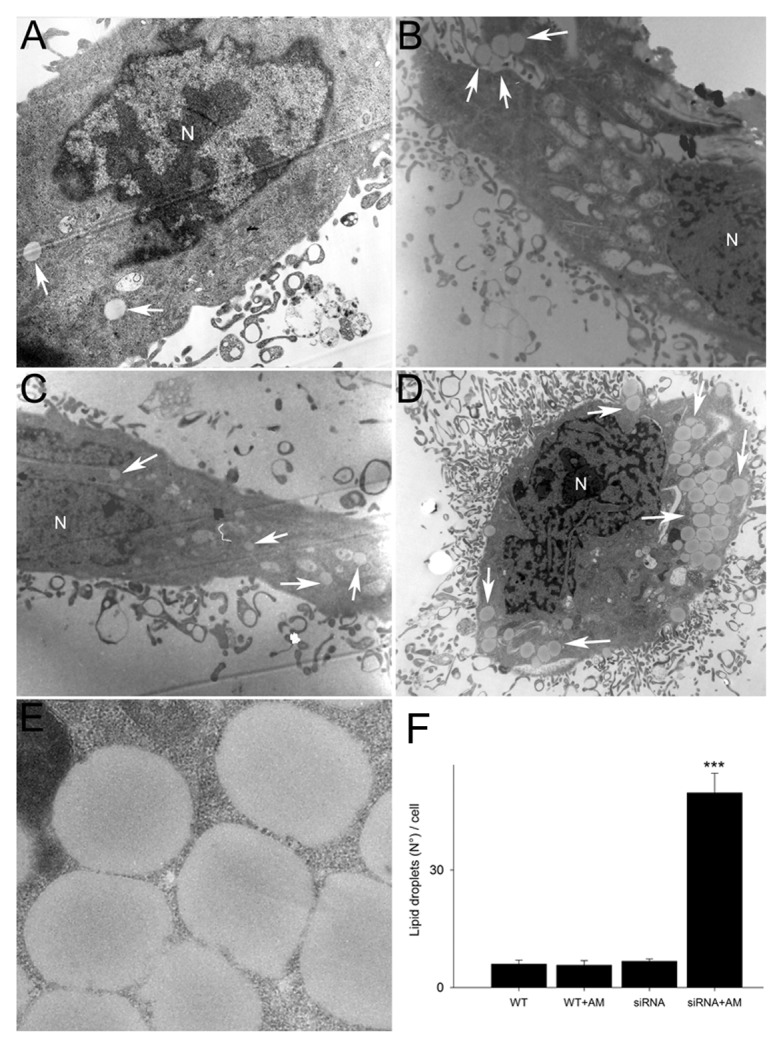
Figure 7. The effect of aminochrome on lipid droplets in U373MG cells. Normal amount of lipid droplets was observed in U373MG incubated with cell culture medium (A) or 75 µM aminochrome (B) for 24 h. U373MGsiGST6 cells incubated with cell culture medium (C) also presented normal amount of lipid droplets contrasting with a significant increase of lipid droplets observed in U373MGsiGST6 cells incubated with 75 µM aminochrome for 24 h (D and E). The lipid droplets are indicated with white arrows. Scale bars: (A–D) 2 µm; (E) 0.25 µm; nucleus (N).
DIscussion
Astrocyte protection
Astrocytes provide neighboring neurons with different metabolites, including energy substrates;42-45 precursors of neurotransmitters, such as glutamine46 and glutamate;47 and precursors for the neuronal synthesis of the antioxidant GSH.48,49 Neuronal GSH synthesis is dependent on astrocytes that supply all 3 constituent amino acids of GSH.50 Therefore, the disruption of the metabolic pathways required for the generation of such cellular components in astrocytes will also affect neuronal antioxidant defenses. It has been proposed that glial cells might represent a primary target of degenerative disease processes;51 however, it is unknown whether the degeneration of melanin-containing dopaminergic neurons in Parkinson disease is an initial event of neurodegeneration or a secondary process as a consequence of astrocyte degeneration. The protective effect of astrocytes on dopaminergic neurons has been postulated and supported through several lines of evidence: i) astrocyte-mediated neuroprotection, afforded through increased levels of glutathione peroxidase, is associated with the increased expression of F2R/PAR-1 in astrocytes in substantia nigra pars compacta;52 ii) the transplantation of GDNF-transduced astrocytes to the substantia nigra at one week prior to the intrastriatal 6-hydroxydopamine lesion showed a significant protection of nigral tyrosine hydroxylase-positive cells,53 and astrocytes protect neurons against 6-OHDA toxicity;54,55 iii) arundic acid mediates the protection of dopaminergic neurons against MPTP neurotoxicity in mice and ameliorates neurological deficits through the modulation of astrocytic activation, which includes the inhibition of S-100 protein synthesis;56 and iv) the astrocyte-specific overexpression of NFE2L2/Nrf2 provides neuroprotection against SNCA (A53T)-mediated toxicity by promoting the degradation of SNCA (A53T) through the autophagy-lysosome pathway in vivo.57 Notably, NFE2L2 is an antioxidant transcription factor that induces the transcriptional activation of genes encoding phase II-detoxifying enzymes and antioxidants, such as NQO1 and glutathione S-transferases.58
U373MG as model cell line
As a model cell line, we used the well-characterized permanent human astrocytoma U373MG cell line derived from a patient with malignant anaplastic astrocytoma grade III glioblastoma.59 This cell line was used to study the effect of MPP+, suggesting that these cells express the dopamine transporter.60 Our results showed that U373MG cells express dopamine transporter mRNA because nomifensine, a dopamine transporter inhibitor, inhibited 3H-dopamine uptake in U373MG cells. Our results suggest additional neuronal transporters of dopamine, such as SLC22A1, SLC22A2, and SLC22A3, because β-estradiol, an inhibitor of SLC22A1, SLC22A2, and SLC22A3,61 inhibited 3H-dopamine uptake in U373MG cells. However, only SLC22A1 mRNA was expressed in U373MG cells. The mRNA expression of SLC29A4, the non-neuronal dopamine transporter, was also observed in U373MG cells.
GSTM2 protection against aminochrome toxicity
The ability of astrocytes to take up dopamine and the possibility that dopamine oxidizes to aminochrome at physiological pH in the presence of oxygen likely indicate a plausible mechanism for the degeneration of astrocytes surrounding and protecting dopaminergic neurons. Aminochrome is neurotoxic in cell lines with catecholaminergic features9,12-20 and in dopaminergic neurons in the substantia nigra of rats.62 The incubation of U373MG cells with aminochrome revealed that this complex was not toxic, contrasting with a significant 18-fold increase in cell death when U373MGsiGST6 cells expressing the siRNA for GSTM2 were incubated with aminochrome. Therefore, our results support the proposed protective role of aminochrome conjugation with GSH catalyzed through GSTM2 against aminochrome toxicity.24,25,27 GSTM2 catalyzes the conjugation of aminochrome with GSH, preventing the one-electron reduction of aminochrome and generation of the leukoaminochrome o-semiquinone radical, which is extremely reactive with oxygen.11 The formed conjugate (leukoaminochrome-GSH) is stable in the presence of biological oxidizing agents, such as dioxygen, superoxide radicals, and hydrogen peroxide.24 Dopamine oxidizes to dopamine-o-quinone, which spontaneously cyclizes to aminochrome at physiological pH,21 and GSTM2 prevents the formation of aminochrome through the conjugation of dopamine-o-quinone, the precursor of aminochrome, to 5-glutathionyl dopamine, which is the precursor of 5-cysteinyldopamine.63 Indeed, 5-S-cysteinyldopamine has been detected in neuromelanin and in the cerebrospinal fluid of Parkinson disease patients as well as in dopamine-rich brain regions, such as the caudate nucleus, putamen, globus pallidus, and substantia nigra.28-30
Aminochrome inhibits autophagy and causes lysosome dysfunction
The ultrastructural analysis of U373MG and U373MGsiGST6 cells in the presence of aminochrome revealed the formation of autophagic vacuoles containing undigested cellular components in U373MGsiGST6 cells, as previously demonstrated in a neuronal cell line.18 Determination of endogenous LC3-II expression through western blotting for LC3 revealed an increase of LC3-II expression in U373MGsiGST6 cells incubated with aminochrome. The increase in LC3-II expression reflects an increase in autophagy or the accumulation of LC3-II as a consequence of the inhibition of autophagosome degradation in lysosomes. Our results suggest that the increased LC3-II expression in U373MGsiGST6 cells treated with aminochrome might have resulted from the accumulation of autophagosomes that cannot be degraded in lysosomes as a consequence of an inhibition of the fusion between autophagosomes and lysosomes mediated through aminochrome. Aminochrome also induces the aggregation of α- and β-tubulin (TUBA and TUBB) in catecholaminergic neuron-like cells9 and U373MG cells (Fig. S3), preventing the microtubule formation required for the fusion of autophagosomes with lysosomes.26,64 The significant accumulation of SQSMT1 in U373MGsiGST6 cells treated with aminochrome supports the idea that aminochrome inhibits autophagy by preventing autophagosome degradation and that GSTM2 protects against aminochrome-mediated autophagy dysfunction. The protein SQSTM1 binds LC3-II and targets ubiquitinated protein aggregates for lysosomal degradation; SQSTM1 is selectively degraded via autophagy.41 The role of autophagy in aminochrome-induced cell death was studied using bafilomycin A1, a reversible inhibitor of vacuolar-type H+-ATPase required for the lysosomal degradation of protein and damaged organelles that promotes the accumulation of autophagic vacuoles,31,32 and trehalose, an activator of autophagy in an MTOR-independent manner through the inhibition of inositol-monophosphatase.33 Bafilomycin A1 caused a significant decrease in aminochrome-induced cell death in U373MGsiGST-6 cells, suggesting that aminochrome induces lysosomal dysfunction. The protective effect of bafilomycin A1 at both low (≤ 1 nM) and high (100 nM) concentrations against cell death induced through lysosomotropic agents, such as chloroquine,36,37 quinolone antibiotics, and hydroxychloroquine,35,65 has been reported. The increase in LAMP2 immunofluorescence (a lysosome marker) and the decrease in acridine orange red fluorescence (loss of lysosome acidity) in U373MGsiGST6 cells support the idea that aminochrome causes lysosomal dysfunction in these cells and that GSTM2 protects against this aminochrome-induced cellular alteration. Interestingly, the loss or deficiency of ATP13A2 function leads to lysosome dysfunction, accompanied by lysosome accumulation, increased LAMP1 and LAMP2 immunostaining, and impaired lysosome acidification in fibroblasts from Parkinson disease patients with ATP13A2 mutations.66,67 Thus, the increase in LAMP2 immunofluorescence reported in our work suggests a compensatory response to aminochrome-induced lysosomal dysfunction to generate new functional lysosomes. However, trehalose induced a significant increase in aminochrome-induced cell death resulting from autophagy stress. The aminochrome-induced lysosome dysfunction and inhibition of the fusion of autophagic vacuoles with lysosomes resulted in autophagic stress as a consequence of autophagy imbalance between the increase in autophagy induced through trehalose and the cell capacity to degrade damaged organelles and proteins.68 The fact that trehalose effect on aminochrome-induced cell death is greater in U373MG and U373MGpSR cells than U373MGsiGST6 cells, can probably be explained by the existence of a greater autophagic stress in these cell lines as a consequence of a lower effect of aminochrome on TUBA in U373MG and U373MGpSR cells. The microtubules are not only important for the fusion of autophagic vacuoles and lysosomes but also play a role in the formation and transport of autophagosomes.69-71 To obtain more information regarding the importance of lysosome function in aminochrome-induced cell death, we measured cell death in the presence of lysosomal protease inhibitors such as leupeptin and pepstatin A. A significant increase in aminochrome-induced cell death was observed in the presence of pepstatin A, a CTSD inhibitor, in U373MG, U373MGpSR, and U373MGsiGST6 cells, suggesting that CTSD plays a protective role against aminochrome toxicity. Unlike most of the lysosomal enzymes, which are active only in an acidic environment, CTSD is active at both acid and the neutral pH.39,72,73 In contrast, CTSB that is inhibited by leupeptin, is only active between pH 5.0–6.040,74 and does not increase aminochrome-induced cell death in U373MGsiGST6 cells, which show an aminochrome-dependent loss of acidity. These results suggested that functional lysosomes play a protective role in aminochrome toxicity.
In addition, our results suggested that the protective effect of bafilomycin A1 against aminochrome-induced cell death depends on the ability of this reagent to reduce the aminochrome-induced loss of lysosomal acidity, as bafilomycin A1 inhibited the aminochrome-induced decrease in red acridine orange fluorescence in U373MGsiGST6 cells. This result is consistent with competition between bafilomycin A1 and aminochrome for the same substrate. Bafilomycin A1 is a specific and reversible inhibitor of the proton pump vacuolar type H+-ATPase.31 Aminochrome also inhibits the same proton pump in chromaffin granule membrane ghosts, but this effect is irreversible likely because of the formation of an adduct with the H+-ATPase.75,76 The inhibitory effect of aminochrome on this vacuolar type H+-ATPase was found to be mediated by the formation of leukoaminochrome o-semiquinone during aminochrome one-electron reduction75,76 and the conjugation of aminochrome with glutathione catalyzed with GSTM2 prevents the formation of this o-semiquinone.24 Therefore, the incubation of U373MGsiGST6 cells with bafilomycin A1 during 2 h before the addition of new medium containing aminochrome resulted probably in i) prevention of aminochrome adducts with the vacuolar type H+-ATPase; ii) formation of irreversible adducts of aminochrome with other proteins such as parkin, DJ-1, UCHL-1, actin, TUBA, and TUBB, complex I, III, and V of mitochondria electron transport and oxidative phosphorylation, etc7-9; iii) dissociation of bafilomycin A1 binding with the vacuolar type H+-ATPase after 1 h of incubation with a medium free of bafilomycin A1; and iv) significant recovery of lysosome acidity after 24 h incubation in comparison with aminochrome without bafilomycin A1. The reversibility of the effects of bafilomycin A1 has been reported many times in different cell types.31,32 In summary, these results suggest that aminochrome, at least in part, induces cell death through the induction of autophagy-lysosome dysfunction and that GSTM2 protects against these cellular alterations.
Macrolipophagy
Autophagy is not only necessary for the degradation of protein and damaged organelles but also for lipid droplet degradation in a process called macrolipophagy.77 The pharmacological inhibition of autophagy or knockdown of the autophagy gene ATG5 increases triglyceride levels with a concomitant increase in the number of lipid droplets.77 Therefore, the dramatic increase in lipid droplets in U373MGsiGST6 cells incubated with aminochrome supports the idea that aminochrome inhibits both macroautophagy and macrolipophagy. Interestingly, the aminochrome-dependent inhibition of protein degradation is not limited to autophagy. Aminochrome also i) inhibits the proteasomal system,78 as aminochrome inactivates parkin, a proteasomal system ligase 3, through the formation of parkin adducts,7 and ii) aminochrome induces and stabilizes the formation of α synuclein protofibers,5 which inhibit chaperone-mediated autophagy.79
Conclusions
In conclusion, our results suggest that aminochrome toxicity in U373MG cells is mediated through the induction of autophagy-lysosome dysfunction as a consequence of lysosomal acidity loss and TUBA aggregation, induced through aminochrome, which prevent the formation of the microtubules required for fusion between autophagic vacuoles and lysosomes. However, GSTM2 prevents aminochrome toxicity, supporting the proposed protective role of GSTM2 against aminochrome toxicity (Fig. S4).24,25,27 GSTM2 plays an important protective role in astrocytes surrounding the synaptic terminals of dopaminergic neurons during neurotransmission, where the released dopamine into the synaptic cleft is removed through dopamine transporters present in both dopaminergic neurons and astrocytes.
Materials and Methods
Chemicals
Bafilomycin A1 and trehalose were purchased from Sigma-Aldrich (B1793-10UG and T0167-10G, respectively). LIVE/DEAD Viability/Cytotoxicity kit was purchased from Invitrogen (L3224). Acridine orange was from Molecular Probes (A3568). Aminochrome was prepared by oxidizing dopamine with tyrosinase (Sigma Aldrich, H8502-10G and T3824-50KU, respectively) and purified according as we described before.9 We used the following antibodies from Santa Cruz Biotechnology: Anti-TUBA (sc-23948); anti-MAP1LC3B (N-20; sc-16755) anti-GAPDH (FL-335; sc-25778); anti-SQSTM1 (H-290; sc-25575); anti-CTSD (C-20; sc-6486); anti-goat IgG-AP (sc-2351); anti-rabbit IgG-AP (sc-2034). The anti-LAMP2 antibodies were from Abcam (ab25631); Cy3 goat anti-mouse was from Invitrogen (A10521); biotinylated goat anti-mouse was from Abcam (ab64255); DTAF-conjugated streptavidin was from Jackson ImmunoResearch (016-010-084); and anti-actin rabbit was from SigmaAldrich (A2668). Polyclonal anti-GSTM2 was obtained from rabbits injected with recombinant GSTM2 obtained by heterologous expression in Escherichia coli and purified by affinity chromatography.10
Cell lines
As the model cell line to test the effect of siRNA against GSTM2, we used U373MG cells, a well-characterized permanent human astrocytoma cell line derived from patients with malignant anaplastic astrocytoma grade III-glioblastoma.59 We used 3 cell lines in this study: U373MG as the wild-type cell line; U373MGsiGST6 cells expressing an siRNA directed against GSTM2; and U373pSR cells transduced with the pSuper.retro.puro plasmid vector (pSR) alone. All cells were incubated in RPMI-1640 medium (ATCC, 30-2001) containing 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/l glucose, and 1500 mg/l sodium bicarbonate, supplemented with 10% fetal bovine serum Fetalclone III, Hyclone (Thermo Scientific, SH30109.03), 10 U / ml sodium penicillin, 10 U / ml streptomycin sulfate, and amphotericin, under an atmosphere of 5% CO2 at 37 °C. For the production of retroviral particles, HEK-293T cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, 11550-043) supplemented with 4.5 g/l glucose, 110 g/l sodium pyruvate, 10 U/ml sodium penicillin, 10 U/ml streptomycin sulfate, and 10 U/ml amphotericin under an atmosphere of 5% CO2 at 37 °C.
Dopamine uptake
The 3H-dopamine uptake into U373MG cells was measured as previously described.19 The uptake was assessed after an incubation period of 5, 15, 30, and 45 min at room temperature. To inhibit the SLC6A3 and the SLC22A1, 2 μM nomifensine (SigmaAldrich, N1530) and 15 µM of β-estradiol (SigmaAldrich, E8875), respectively, were used.
Reverse transcriptase-PCR
Reverse transcriptase-PCR was performed to determine the expression of the dopamine transporters in U373MG using the following primers for SLC29A4: 5′- CGGGCGTGAT GATCTCTCTG AGCCGCATC-3′ and 5′-GGTTGAACAG ACCCATGATG AGGATGGGCA-3′; SLC22A1: 5′-GGCTGGCTAC ACCCTAATCA CAG-3′ and 5′-AGTCCGTGAA CCACAGGTAC ATC-3′; SLC22A2: 5′-GTACAACTGG TTCACGAGCT CTG-3′ and 5′-CGCCAAGATT CCTAATGAAT GTGGG-3′; SLC6A3: 5′-AGCAGAACGG AGTGCAGCT-3′ and 5′-GTATGCTCTG ATGCCGTCT-3′; norepinephrine transporter: 5′-ATCACGCCAG AGAACGAGCA C-3′ and 5′-TTTCTCTCTT CGGTGGCTTT CG-3′; serotonin transporter: 5′-TCCCAGCCTC CTCTTCATCA CG-3′ and 5′-TTTCTGATGA GCCCGCCACA ACTACGA-3′. The following primers were used to determine the expression of GSTM2: 5′-TACATTGCCC GCAAGCAC-3′ and 5′-TTCAAGGCCC TACTTGTTGC-3′.
Construction of siRNA duplexes and plasmid
The siRNA duplexes used in this study were designed according to previously described guidelines.80-82 A specific sequence was chosen to target the human GSTM2 gene. The alignment procedure was performed as previously described.83 The siRNA duplexes were synthesized with 2-nt deoxythymidine 3′-overhangs (TAG Copenhagen) as previously described.80-82 The cDNA-targeted region and the sequence of the siRNA duplexes was 339-GACATTTTGG AGAACCAGT-365 (Gene bank NM_000848.2 GI:23065549). We synthesized the following oligonucleotides: sense siRNA 5′-GATCCCCGAC ATTTTGGAGA ACCAGTTTCA AGAGAACTGG TTCTCCAAAA TGTCTTTTTG GAAA-3′ and antisense siRNA 5′-AGCTTTTCCA AAAAGACATT TTGGAGAACC AGTTCTCTTG AACTGGTTCT CCAAAATGTC GGG-3′. The plasmid Super.retro.puro (pSR) and the oligonucleotides encoding the siRNA were incubated with the restriction enzymes HindI and BgIII and ligated under standard conditions. The plasmid pSR and the oligonucleotides encoding the siRNA were digested using the restriction enzymes HindI and BgIII and ligated using a 1:10 plasmid:oligonucleotide ratio.
Formation of retroviral particles
HEK-293T cells were transfected with a combination of 6 µg DNA of pSR encoding siGST, 6 µg of the DNA packing plasmid pMDG and FuGENE HD (Promega, E2311) in an 8:2 (DNA:FuGENE HD) ratio in 300 µl of cell culture medium without serum. After 1 h, the serum was added to the cells, followed by incubation for 24, 48, and 72 h. The supernatant fraction was collected and filtered using a 0.45-µm filter. As controls, pSR without a DNA insert and pMDG were transfected using FuGENE HD as described above.
Transduction of U373MG cells
U373MG cells were incubated in T-75 flasks to 40 to 50% confluence, after which 2 ml of the supernatant fraction containing the retroviral particles was added to the cells. The cells transduced with retroviral particles were selected after incubation with puromycin at different concentrations (2, 4, 6, 8, and 10 µg/ml). After 48 h, the antibiotic was removed by changing the cell culture medium. The cells that survived the highest puromycin concentration were trypsinized and subcultured. U373pSR cells were obtained after transducing cells with viral particles containing pSuper.retro.puro plasmid alone.
Quantitative real-time PCR
Total RNA from the U373MG WT and U373MGsiGST6 cells was extracted with TRIZOL reagent (Invitrogen, 15596-026) according to the manufacturer’s protocol and was quantified using Nanodrop 3300 (Thermo). The cDNA was synthesized using oligo-dT (IDT) and epicenter RT reagents according to the manufacturer’s instructions. Comparative quantitation real-time PCR for the GSTM2 gene (Fw: 5′-AAACCAAGTA TTTGAGCCCA GCTGCC-3′; Rv: 5′-TCAAGGCCCT ACTTGTTGCC CCA-3′) was performed in triplicate using Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix (Agilent Technologies, 600886) in a Stratagene Mx3000p Detection System. The mRNA level was normalized through the housekeeping genes RPLP0/Ribosomal protein, large, P0 (Fw: 5′-GCGCAGCCAA TAGACAGGAG CG-3′, RV: 5′-ACGATGTCAC TTCCACGAGG ACGC-3′) and ACTB/Actin, β (Fw: 5′-AGCCTCGCCT TTGCCGATCC G-3′, Rv: 5′-CATGCCGGAG CCGTTGTCGA C-3′). The following experimental run protocol was used: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s, and 10 min at 95 °C, ending with a melting program ranging from 70 °C to 95 °C with continuous fluorescence measurement. The real-time data were analyzed using Mx3000P Software.
Western blot
The level of GSTM2 expression was determined after separating 100 µg of U373MG cell homogenate through SDS-PAGE (10% w/v) and subsequent electrophoretic transfer onto a 0.2 μm nitrocellulose membrane. The membrane was incubated with polyclonal primary antibodies against GSTM2 and anti-goat alkaline phosphatase-linked antibodies. GSTM2 was detected using BCIP/NBT (Invitrogen, N6547). Recombinant GSTM2 was used as a positive control.
Cell death
Cell death was determined by LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes, L-3224) after incubating the cells in culture medium for 24 h in the presence of aminochrome, purified as previously described.9
Transmission electron microscopy
After the treatments, U373MG and U373MGsiGST6 cells were washed 3 times with PBS, pH 7.4, and fixed in 3% glutaraldehyde for 240 min. The cells were washed 3 times and post-fixed in 2% osmium tetroxide for 60 min at room temperature, as previously described.9
LC3-II determination
Western blot analysis using anti-MAP1LC3B N(20) goat sc-16755 antibody diluted 1:500 was performed according to the western blot methodology describe above. The plotted result represents the pixels of LC3-II/GAPDH.
LAMP2 determination
LAMP2 was determined through immunofluorescence using the anti-LAMP2 monoclonal mouse antibody, diluted 1:250 according to the immunofluorescence methodology described before.18 ImageJ software was used to obtain the average fluorescence intensity.
SQSTM1 determination
SQSTM1 was measured using dot blot with anti-SQSTM1 rabbit polyclonal antibody diluted 1:500 according to the methodology described before.18 The plotted result represents the pixels of SQSTM1-GAPDH.
TUBA immunofluorescence
TUBA immunofluorescence analysis with confocal microscopy was performed as previously described.9 Confocal microscopy (Nikon D-Eclipse) at 100× was used to study the cells. The sample illumination was performed using a He–Ne laser with a 488 nm excitation filter and a 544 nm emission filter.
Acridine orange staining
The acidic lysosomes were visualized using acridine orange staining. The cells after treatment with 75 µM aminochrome for 24 h were stained with 2 µg/ml acridine orange (Molecular Probes, A3568) for 30 min at 37 °C and analyzed as previously described, with modifications.84 The number of cells showing red acridine orange fluorescence was plotted.
Protease inhibitors
The cytotoxicity of aminochrome was performed using a Live/Dead assay with 100 µM pepstatin A (CTSD inhibitor; Sigma Aldrich, P5318) pretreatment for 24 h, followed by cotreatment with aminochrome for an additional 24 h and 50 µM leupeptin (Sigma Aldrich, L2884) pretreatment for 24 h to inhibit other proteases, such as CTSB.74
Lipid droplets quantification
The number of lipid droplets per cell for each condition was determined using transmission electron microscopy, and 20 cells per condition were examined.
Statistical analysis
All data were expressed as the means ± SD values. The statistical significance was assessed using analysis of variance (ANOVA) for multiple comparisons and Student t test.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported through funding from FONDECYT (1100165, 1061083, 1120337, and 7040028), CONICYT doctoral thesis support scholarship Nº 24121454 and the Swedish Cancer Society.
Glossary
Abbreviations:
- CTSD
cathepsin D
- GSTM2
glutathione S-transferase mu 2 (muscle)
- U373MGsiGST6
U373MG cell expressing siRNA against GSTM2
- U373MGpSR
U373MG cells expressing plasmid SuperRetro
- LAMP2
lysosomal-associated membrane protein 2
- PARK2
parkin RBR E3 ubiquitin protein ligase
- SLC22A1
solute carrier family 22 (organic cation transporter), member 1
- SLC6A3/DAT
solute carrier family 6 (neurotransmitter transporter), member 3
- SLC29A4/PMAT
solute carrier family 29 (equilibrative nucleoside transporter), member 4
- SNCA
synuclein, alpha (non A4 component of amyloid precursor)
- SQSTM1/p62
sequestosome 1
- TUBA/α-tubulin
tubulin, alpha
References
- 1.Takeda H, Inazu M, Matsumiya T. Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:620–3. doi: 10.1007/s00210-002-0640-0. [DOI] [PubMed] [Google Scholar]
- 2.Linert W, Herlinger E, Jameson RF, Kienzl E, Jellinger K, Youdim MB. Dopamine, 6-hydroxydopamine, iron, and dioxygen--their mutual interactions and possible implication in the development of Parkinson’s disease. Biochim Biophys Acta. 1996;1316:160–8. doi: 10.1016/0925-4439(96)00020-8. [DOI] [PubMed] [Google Scholar]
- 3.Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002;510:216–20. doi: 10.1016/S0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- 4.Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem. 2008;106:1866–75. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- 5.Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–9. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 6.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr. Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science. 2001;294:1346–9. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 7.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–21. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 8.Van Laar VS, Mishizen AJ, Cascio M, Hastings TG. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis. 2009;34:487–500. doi: 10.1016/j.nbd.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- 10.Baez S, Linderson Y, Segura-Aguilar J. Superoxide dismutase and catalase enhance autoxidation during one-electron reduction of aminochrome by NADPH-cytochrome P-450 reductase. Biochem Mol Med. 1995;54:12–8. doi: 10.1006/bmme.1995.1002. [DOI] [PubMed] [Google Scholar]
- 11.Segura-Aguilar J, Metodiewa D, Welch CJ. Metabolic activation of dopamine o-quinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim Biophys Acta. 1998;1381:1–6. doi: 10.1016/S0304-4165(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 12.Paris I, Dagnino-Subiabre A, Marcelain K, Bennett LB, Caviedes P, Caviedes R, Azar CO, Segura-Aguilar J. Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J Neurochem. 2001;77:519–29. doi: 10.1046/j.1471-4159.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 13.Paris I, Perez-Pastene C, Martinez-Alvarado P, Graumann R, Fuentes P, Lozano J, et al. On the mechanism of dopamine-dependent copper toxicity. Neurotox Res. 2005;8:330. [Google Scholar]
- 14.Paris I, Perez-Pastene C, Martinez-Alvarado P, Graumann R, Fuentes P, Lozano J, et al. DT-Diaphorase, monoaminergic transporter inhibitors and norepinephine prevent dopamine-dependent iron toxicity in cells derived from the Substantia nigra. Neurotox Res. 2005;8:335. [Google Scholar]
- 15.Paris I, Martinez-Alvarado P, Cárdenas S, Perez-Pastene C, Graumann R, Fuentes P, Olea-Azar C, Caviedes P, Segura-Aguilar J. Dopamine-dependent iron toxicity in cells derived from rat hypothalamus. Chem Res Toxicol. 2005;18:415–9. doi: 10.1021/tx0497144. [DOI] [PubMed] [Google Scholar]
- 16.Paris I, Cardenas S, Lozano J, Perez-Pastene C, Graumann R, Riveros A, Caviedes P, Segura-Aguilar J. Aminochrome as a preclinical experimental model to study degeneration of dopaminergic neurons in Parkinson’s disease. Neurotox Res. 2007;12:125–34. doi: 10.1007/BF03033921. [DOI] [PubMed] [Google Scholar]
- 17.Paris I, Perez-Pastene C, Couve E, Caviedes P, Ledoux S, Segura-Aguilar J. Copper dopamine complex induces mitochondrial autophagy preceding caspase-independent apoptotic cell death. J Biol Chem. 2009;284:13306–15. doi: 10.1074/jbc.M900323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paris I, Muñoz P, Huenchuguala S, Couve E, Sanders LH, Greenamyre JT, Caviedes P, Segura-Aguilar J. Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol Sci. 2011;121:376–88. doi: 10.1093/toxsci/kfr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arriagada C, Paris I, Sanchez de las Matas MJ, Martinez-Alvarado P, Cardenas S, Castañeda P, Graumann R, Perez-Pastene C, Olea-Azar C, Couve E, et al. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol Dis. 2004;16:468–77. doi: 10.1016/j.nbd.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes P, Paris I, Nassif M, Caviedes P, Segura-Aguilar J. Inhibition of VMAT-2 and DT-diaphorase induce cell death in a substantia nigra-derived cell line--an experimental cell model for dopamine toxicity studies. Chem Res Toxicol. 2007;20:776–83. doi: 10.1021/tx600325u. [DOI] [PubMed] [Google Scholar]
- 21.Segura-Aguilar J, Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine:prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact. 1989;72:309–24. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 22.Segura-Aguilar J, Cardenas S, Riveros A, Fuentes-Bravo P, Lozano J, Graumann R, Paris I, Nassif M, Caviedes P. DT-diaphorase prevents the formation of alpha-synuclein adducts with aminochrome. Soc Neurosci Abstr. 2006;824:17. [Google Scholar]
- 23.Cardenas SP, Perez-Pastene C, Couve E, Segura-Aguilar J. The DT-diaphorase prevents the aggregation of a-synuclein induced by aminochrome. Neurotox Res. 2008;13:136. [Google Scholar]
- 24.Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J Biol Chem. 1997;272:5727–31. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- 25.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–8. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol. 2010;11:89. doi: 10.1186/1471-2121-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun. 2000;274:32–6. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 28.Cheng FC, Kuo JS, Chia LG, Dryhurst G. Elevated 5-S-cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: possible markers for and potential insights into the pathoetiology of Parkinson’s disease. J Neural Transm. 1996;103:433–46. doi: 10.1007/BF01276419. [DOI] [PubMed] [Google Scholar]
- 29.Rosengren E, Linder-Eliasson E, Carlsson A. Detection of 5-S-cysteinyldopamine in human brain. J Neural Transm. 1985;63:247–53. doi: 10.1007/BF01252029. [DOI] [PubMed] [Google Scholar]
- 30.Carstam R, Brinck C, Hindemith-Augustsson A, Rorsman H, Rosengren E. The neuromelanin of the human substantia nigra. Biochim Biophys Acta. 1991;1097:152–60. doi: 10.1016/0925-4439(91)90100-N. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–12. [PubMed] [Google Scholar]
- 32.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 35.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini J-L, Metivier D, Ojcius DM, Jäättelä M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197:1323–34. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pivtoraiko VN, Harrington AJ, Mader BJ, Luker AM, Caldwell GA, Caldwell KA, Roth KA, Shacka JJ. Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction. J Neurochem. 2010;114:1193–204. doi: 10.1111/j.1471-4159.2010.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shacka JJ, Klocke BJ, Shibata M, Uchiyama Y, Datta G, Schmidt RE, Roth KA. Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol Pharmacol. 2006;69:1125–36. doi: 10.1124/mol.105.018408. [DOI] [PubMed] [Google Scholar]
- 38.Traganos F, Darzynkiewicz Z. Lysosomal proton pump activity: supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994;41:185–94. doi: 10.1016/S0091-679X(08)61717-3. [DOI] [PubMed] [Google Scholar]
- 39.Bednarski E, Lynch G. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J Neurochem. 1996;67:1846–55. doi: 10.1046/j.1471-4159.1996.67051846.x. [DOI] [PubMed] [Google Scholar]
- 40.Guha S, Padh H. Cathepsins: fundamental effectors of endolysosomal proteolysis. Indian J Biochem Biophys. 2008;45:75–90. [PubMed] [Google Scholar]
- 41.Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–8. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Schousboe A, Bak LK, Sickmann HM, Sonnewald U, Waagepetersen HS. Energy substrates to support glutamatergic and GABAergic synaptic function: role of glycogen, glucose and lactate. Neurotox Res. 2007;12:263–8. doi: 10.1007/BF03033909. [DOI] [PubMed] [Google Scholar]
- 43.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 44.Pellerin L. Brain energetics (thought needs food) Curr Opin Clin Nutr Metab Care. 2008;11:701–5. doi: 10.1097/MCO.0b013e328312c368. [DOI] [PubMed] [Google Scholar]
- 45.Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia. 2007;55:1238–50. doi: 10.1002/glia.20376. [DOI] [PubMed] [Google Scholar]
- 46.McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–58. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- 47.Yang CZ, Zhao R, Dong Y, Chen XQ, Yu AC. Astrocyte and neuron intone through glutamate. Neurochem Res. 2008;33:2480–6. doi: 10.1007/s11064-008-9758-x. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee R, Vitvitsky V, Garg SK. The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends Biochem Sci. 2008;33:413–9. doi: 10.1016/j.tibs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–16. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 50.Hirrlinger J, Dringen R. The cytosolic redox state of astrocytes: Maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev. 2010;63:177–88. doi: 10.1016/j.brainresrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. 2006;112:517–30. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- 52.Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J Neuropathol Exp Neurol. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- 53.Ericson C, Georgievska B, Lundberg C. Ex vivo gene delivery of GDNF using primary astrocytes transduced with a lentiviral vector provides neuroprotection in a rat model of Parkinson’s disease. Eur J Neurosci. 2005;22:2755–64. doi: 10.1111/j.1460-9568.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 54.Safi R, Gardaneh M, Panahi Y, Maghsoudi N, Zaefizadeh M, Gharib E. Optimized quantities of GDNF overexpressed by engineered astrocytes are critical for protection of neuroblastoma cells against 6-OHDA toxicity. J Mol Neurosci. 2012;46:654–65. doi: 10.1007/s12031-011-9654-8. [DOI] [PubMed] [Google Scholar]
- 55.Gardaneh M, Gholami M, Maghsoudi N. Synergy between glutathione peroxidase-1 and astrocytic growth factors suppresses free radical generation and protects dopaminergic neurons against 6-hydroxydopamine. Rejuvenation Res. 2011;14:195–204. doi: 10.1089/rej.2010.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato H, Kurosaki R, Oki C, Araki T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004;1030:66–73. doi: 10.1016/j.brainres.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 57.Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci. 2012;32:17775–87. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 59.Perego C, Vanoni C, Massari S, Raimondi A, Pola S, Cattaneo MG, Francolini M, Vicentini LM, Pietrini G. Invasive behaviour of glioblastoma cell lines is associated with altered organisation of the cadherin-catenin adhesion system. J Cell Sci. 2002;115:3331–40. doi: 10.1242/jcs.115.16.3331. [DOI] [PubMed] [Google Scholar]
- 60.Chuang JI, Chen TH. Effect of melatonin on temporal changes of reactive oxygen species and glutathione after MPP(+) treatment in human astrocytoma U373MG cells. J Pineal Res. 2004;36:117–25. doi: 10.1046/j.1600-079X.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 61.Hayer-Zillgen M, Brüss M, Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–36. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Díaz-Véliz G, Mora S, Gómez P, Dossi MT, Montiel J, Arriagada C, Aboitiz F, Segura-Aguilar J. Behavioral effects of manganese injected in the rat substantia nigra are potentiated by dicumarol, a DT-diaphorase inhibitor. Pharmacol Biochem Behav. 2004;77:245–51. doi: 10.1016/j.pbb.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 63.Shen XM, Xia B, Wrona MZ, Dryhurst G. Synthesis, redox properties, in vivo formation, and neurobehavioral effects of N-acetylcysteinyl conjugates of dopamine: possible metabolites of relevance to Parkinson’s disease. Chem Res Toxicol. 1996;9:1117–26. doi: 10.1021/tx960052v. [DOI] [PubMed] [Google Scholar]
- 64.Webb JL, Ravikumar B, Rubinsztein DC. Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. Int J Biochem Cell Biol. 2004;36:2541–50. doi: 10.1016/j.biocel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–36. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 66.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32:4240–6. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron M-H, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A. 2012;109:9611–6. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65:423–32. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–16. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 70.Köchl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic. 2006;7:129–45. doi: 10.1111/j.1600-0854.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 71.Mackeh R, Perdiz D, Lorin S, Codogno P, Poüs C. Autophagy and microtubules - new story, old players. J Cell Sci. 2013;126:1071–80. doi: 10.1242/jcs.115626. [DOI] [PubMed] [Google Scholar]
- 72.Fan X, Luo G, Yang D, Ming M, Liu H, Pu P, Le W. Critical role of lysosome and its associated protein cathepsin D in manganese-induced toxicity in cultured midbrain astrocyte. Neurochem Int. 2010;56:291–300. doi: 10.1016/j.neuint.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Banay-Schwartz M, Dahl D, Hui KS, Lajtha A. The breakdown of the individual neurofilament proteins by cathepsin D. Neurochem Res. 1987;12:361–7. doi: 10.1007/BF00993246. [DOI] [PubMed] [Google Scholar]
- 74.Sevlever D, Jiang P, Yen S-HC. Cathepsin D is the main lysosomal enzyme involved in the degradation of α-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–87. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terland O, Flatmark T, Tangerås A, Grønberg M. Dopamine oxidation generates an oxidative stress mediated by dopamine semiquinone and unrelated to reactive oxygen species. J Mol Cell Cardiol. 1997;29:1731–8. doi: 10.1006/jmcc.1997.0412. [DOI] [PubMed] [Google Scholar]
- 76.Terland O, Almås B, Flatmark T, Andersson KK, Sørlie M. One-electron oxidation of catecholamines generates free radicals with an in vitro toxicity correlating with their lifetime. Free Radic Biol Med. 2006;41:1266–71. doi: 10.1016/j.freeradbiomed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–5. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zafar KS, Siegel D, Ross D. A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol. 2006;70:1079–86. doi: 10.1124/mol.106.024703. [DOI] [PubMed] [Google Scholar]
- 79.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 81.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 82.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinonen JE, Smith CI, Nore BF. Silencing of Bruton’s tyrosine kinase (Btk) using short interfering RNA duplexes (siRNA) FEBS Lett. 2002;527:274–8. doi: 10.1016/S0014-5793(02)03206-4. [DOI] [PubMed] [Google Scholar]
- 84.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.