Abstract
NADPH oxidase is a cellular enzyme devoted to the production of reactive oxygen species (ROS). NOX4 and NOX2 are the main isoforms of NADPH oxidase in the cardiovascular system. In our recent study, we demonstrated that NOX4, but not NOX2, is a critical mediator of the cardiomyocyte adaptive response to energy stress. NOX4 activity and protein levels are increased in the endoplasmic reticulum (ER) but not in mitochondria of cardiomyocytes during the early phase of energy deprivation. NOX4-derived production of ROS in the ER is a critical event that activates autophagy through stimulation of the EIF2AK3/PERK-EIF2S1/eIF-2α-ATF4 pathway. NOX4-dependent autophagy is an important mechanism to preserve cellular energy and limit cell death in energy-deprived cardiomyocytes. Aside from elucidating a crucial physiological function of NOX4 during cellular energy stress, our study dissects a novel signaling mechanism that regulates autophagy under this condition.
Keywords: autophagy, oxidative stress, endoplasmic reticulum, glucose deprivation, Nox4, PERK
Oxidative stress significantly modulates autophagy. How ROS are produced during the induction of autophagy and what targets are modulated by ROS to activate autophagy are, however, less well understood. In our study we clarified the exact source of ROS modulating a signaling cascade for the induction of autophagy in response to energy deprivation. Although activation by ROS during a lack of oxygen sounds somewhat counterintuitive, NOX4 is activated through transcriptional mechanisms in response to hypoxia and energy stress. We have now extended this finding by demonstrating that energy stress upregulates NOX4 in the ER, which in turn induces autophagy. NOX4 levels and activity are increased in the ER, but not in mitochondria or the nucleus in the early phase of glucose deprivation. This may be dependent upon the presence of multiple ER-localization signals in the NOX4 primary structure, or upon alternative splicing of NOX4 transcripts, particularly in the untranslated regions, or posttranslational modifications of the NOX4 nascent protein, such as N-myristoylation. These mechanisms for differential protein targeting were previously demonstrated for other proteins residing in multiple subcellular compartments, such as CYB5R (NADH-cytochrome b5 reductase).
Oxidative modifications of amino acids, including disulfide bond formation, nitrosylation, and glutathionylation, affect both the structure and function of proteins. ROS directly activate autophagy during amino acid deprivation in ovarian cells by oxidizing and inhibiting ATG4, thereby increasing LC3 lipidation and autophagosome formation. Although it is hard to exclude the possibility that direct oxidative modification of the ATG proteins by the ER-derived ROS affects autophagy, thus far we have found that NOX4-dependent ROS in the ER activate autophagy through stimulation of the EIF2AK3/PERK-EIF2S1/eIF-2α-ATF4 pathway which is dependent upon inhibition of the ER-specific prolyl hydroxylase enzyme P4HTM. Prolyl hydroxylase enzymes inhibit PERK, likely through hydroxylation, whereas prolyl hydroxylase enzymes are inhibited by ROS through oxidation of the enzyme-bound iron.
The EIF2AK3/PERK signaling pathway promotes autophagy through ATF4- and DDIT3/CHOP-dependent upregulation of genes involved in autophagosome formation and maturation. The unfolded protein response is activated in cancer cells, and the EIF2AK3/PERK-EIF2S1/eIF-2α-ATF4 pathway is an important inducer of autophagy in cancer. EIF2AK3/PERK activation during energy deprivation in cardiomyocytes is more likely a part of the integrated stress response, since activation of EIF2AK3/PERK signaling occurs independently of the unfolded protein response. Therefore, our study reveals an unexpected role of EIF2AK3/PERK-ATF4 signaling activated by NOX4 as a transduction cascade devoted to the regulation of autophagy during energy deprivation.
The eIF-2α kinase EIF2AK4/GCN2 has been recently found to promote autophagy in response to amino acid deprivation in mouse embryonic fibroblasts. Thus, it will be interesting to evaluate whether NOX4-derived ROS are also able to activate EIF2AK4/GCN2 or whether they may regulate additional parallel mechanisms in the ER that can contribute to autophagy activation. NOX4-derived ROS promote the activation of the HIF1A signaling pathway in response to hypoxia in cancer cells. HIF1A was previously shown to induce the upregulation of autophagy-related genes. NOX4 can regulate autophagy in response to misfolded protein accumulation through KRAS-MAPK1/ERK2 activation. Finally, the ER is a major site for autophagosome formation in starved cells. This evidence suggests that NOX4 activation in the ER may also take part in other mechanisms regulating the autophagic machinery.
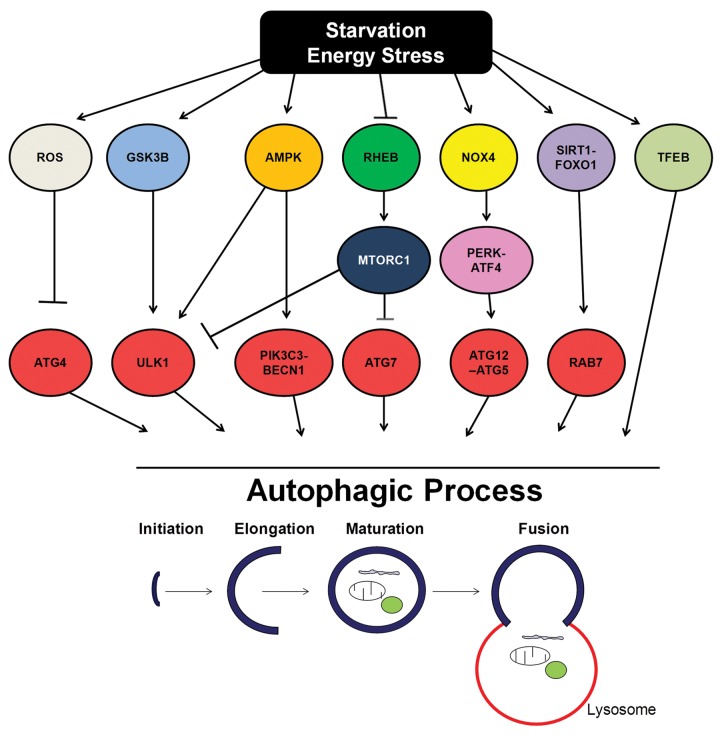
Multiple independent signaling pathways are essential for regulating distinct steps of autophagy from autophagosome formation to lysosomal degradation during energy deprivation. Activation of AMPK and GSK3B and inhibition of RHEB-MTORC1 signaling regulate autophagosome formation during energy deprivation, through either posttranslational activation of ULK1 and PIK3C3/VPS34 or transcriptional regulation of important autophagy-related genes, such as Atg7. SIRT1/FOXO1 signaling and TFEB stimulate autophagy and autophagic flux during energy deprivation through the upregulation of autophagic proteins, such as RAB7. ROS can also promote autophagy in starved ovary cells through the direct inactivation of ATG4. The EIF2AK3/PERK-ATF4 signaling pathway promotes ATG12–ATG5 conjugation and the upregulation of other autophagic proteins. It is possible that these pathways may crosstalk at multiple levels to regulate autophagy during energy deprivation (Fig. 1).
Figure 1. Schematic representation of the multiple signaling pathways regulating the autophagic genes and machinery during starvation and energy stress.
NOX4-dependent autophagy activation in energy-deprived cardiomyocytes is an important mechanism to limit cell death and function. NADPH oxidase localized in the ER is activated by mitochondrial dysfunction and aging in yeast. It will be interesting to investigate whether NOX4 is associated with activation of cargo-specific forms of autophagy, such as mitophagy or ribophagy, and determining their role in mediating quality control of organelles and proteins during aging and stress. In addition, mitophagy is increased during glucose deprivation. It is possible that crosstalk between mitochondria and the ER that promotes NOX4 upregulation and mitophagy exists during energy stress.
Whether NOX4-dependent autophagy is protective in other types of stress and cells remains to be demonstrated. NOX4 is activated in aggressive cancers where it favors the formation of metastases through the activation of TGFB1/TGF-β signaling. Autophagy is also activated in invasive cancers where it promotes the survival of cancer cells. It will be interesting to evaluate whether NOX4 activation stimulates autophagy, which in turn promotes survival in cancer cells. In support of this hypothesis, PERK-induced autophagy is an important survival mechanism in cancer cells, particularly in response to therapy. Therefore, even in the case of NOX4-EIF2AK3/PERK-ATF4-dependent autophagy, the oncologist should probably inhibit what the cardiologist desires to activate.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Daniela Zablocki, Christopher D Brady, and Dominic P Del Re for the critical reading of the manuscript and suggestions. This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039, and AG27211. This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence. SS has been supported by a postdoctoral fellowship from the Founders Affiliate, American Heart Association, and partially by a grant from the Italian Society of Cardiology and Italian Society of Hypertension.