Abstract
Adenovirus (Ad) type 7 can cause severe infection, including pneumonia, in military recruits and children. The initial inflammation is a neutrophilic interstitial infiltration with neutrophilic alveolitis. Subsequently, monocytes become evident and, finally, there is a predominantly lymphocytic infiltrate. We have established that Ad7 infection of epithelial cells stimulates release of the neutrophil chemotaxin interleukin (IL)-8, and have extended these studies to a human lung tissue model. Here, we studied cytokine responses to Ad7 in human alveolar macrophages (HAM) and our human lung tissue model. Both ELISA and RNase-protection assay (RPA) data demonstrated that, upon Ad7 infection, IP-10 and MIP-1α/β are released from HAM. IP-10 and MIP-1α/β protein levels were induced 2- and 3-fold, respectively, in HAM 24 h after Ad7 infection. We then investigated induction of specific cytokines in human lung tissue by RPA and ELISA. The results showed that IL-8 and IL-6 were induced 8 h after infection and, by 24 h, levels of IL-8, IL-6, MIP-1α/β and MCP-1 were all increased. IP-10, a monocyte and lymphocyte chemokine, was also induced 30-fold, but only 24 h after infection. Immunohistochemistry staining confirmed that IL-8 was only released from the epithelial cells of lung slices and not from macrophages. IP-10 was secreted from both macrophages and epithelial cells. Moreover, full induction of IP-10 is likely to require participation and cooperation of both epithelial cells and macrophages in intact lung. Understanding the cytokine and chemokine induction during Ad7 infection may lead to novel ways to modulate the response to this pathogen.
Introduction
Fifty-one different serotypes of human adenovirus (Ad) are documented, and are categorized into seven species (A–G) based on common serological group antigens and DNA sequence similarity. Ad type 7 belongs to the B subgroup. Three different epidemic patterns of Ad7 infection can be distinguished: the first appears during the winter among infants of median age below 2 years, and has characteristic symptoms of high fever and pneumonia with severe, sometimes fatal, outcome. The second appears in the autumn among children of a median age of 7 years, and has characteristic symptoms of high fever, pneumonia, abdominal symptoms and meningismus with an outcome that is favourable. The third has been seen as outbreaks of acute respiratory disease among military recruits (Carballal et al., 2002; Klinger et al., 1998; Wadell et al., 1980).
Ad infection is characterized pathologically by a time-dependent progression in the type of inflammatory cells present. The initial inflammation is a neutrophilic interstitial infiltration with neutrophilic alveolitis (Brody et al., 1994; Otake et al., 1998). Subsequently, monocytes become more evident, and the inflammatory infiltrate has a mixture of monocytes and neutrophils. Finally, in later stages of the illness, there is a transition from the monocytic/neutrophilic inflammation characteristic of the innate immune response to a lymphocytic inflammation representing the transition to adaptive immunity (Ginsberg et al., 1991). It is still unknown why there is extensive tissue injury during Ad7 pneumonia. It is probable that cytokine induction, with subsequent recruitment and activation of inflammatory cells, contributes to this injury.
The alveolar epithelial cell surface has the largest surface area for contact with both infectious agents and environmental agents in the body. The epithelium is the initial contact point for airway pathogens, and releases chemokines immediately following contact with pathogens (reviewed by Diamond et al., 2000). The lung epithelium, therefore, plays an important role in activation of the innate immune system. Release of these mediators recruits and activates inflammatory cells, including macrophages and neutrophils, which aid in the initial defence against the infection. Previous studies have demonstrated that alveolar epithelial cells are infected during Ad infection, and that Ad7 stimulates epithelial cells to release interleukin (IL)-8 (Abbondanzo et al., 1989; Booth & Metcalf, 1999; Bruder & Kovesdi, 1997). Human alveolar macrophages (HAM) are the other type of cell that is first exposed to Ad infection. Apart from phagocytosing and killing the pathogens, macrophages also secrete chemokines to recruit cells to sites of infection (Fujiwara & Kobayashi, 2005). Thus far, there is still no clear profile of the pro-inflammatory cytokine response from HAM exposed to Ad7.
In this report, using ELISA and RNase-protection assays (RPA), we demonstrated that Ad7 induces gamma interferon (IFN-γ)-inducible protein 10 (IP-10) and macrophage inflammatory protein (MIP-1α/β) in isolated HAM. We then investigated induction of specific cytokines in a human lung tissue model by RPA and ELISA. The results showed that IL-8 and IL-6 proteins are induced at 8 h, and that IL-8, IL-6, MIP-1α/β and monocyte chemoattractant protein (MCP)-1 are all induced at 24 h after infection. The monocyte chemotaxin IP-10 was induced 30-fold 24 h after infection. Immunofluorescence staining showed that epithelial cells, but not macrophages, were the source of IL-8. In contrast, IP-10 was secreted from both macrophages and epithelial cells. IL-8 is the major neutrophil chemotaxin in the lung and is probably responsible for the initial neutrophil infiltration seen during Ad7 infection, whereas IP-10 is chemotactic for monocytes and lymphocytes and probably plays a major role in the monocytic and lymphocytic infiltration that occurs later in the disease process.
Results
Induction of cytokine and chemokine mRNA in human lung slices and HAM infected by Ad7
Previous work showed that our human lung organ culture model can be infected by Ad7 and reproduces the in vivo infectious process accurately (Booth et al., 2004). We examined the innate immune cytokine and chemokine response of human lung tissue and HAM to Ad7 using RPA (Table 1). Human lung tissue slices (three slices per data point/one slice per well) and HAM were exposed to 1×109 p.f.u. Ad7 per well in 0.5 ml medium for 8 h at 37 °C in 24-well plates. Equal volumes of virus-free buffer were used as a negative control. mRNA expression levels were determined using a custom cytokine template (Pharmingen; see Methods). We selected the eight cytokines based on screening performed using a 23-plex cytokine luminex assay (not shown). Fold increase was determined by normalization for levels of housekeeping genes present in each sample. In HAM, Ad7 exposure induced IP-10 by only 3-fold during the course of exposure. In contrast, lung slices showed that the mRNA of multiple chemokines and cytokines was induced by Ad7, including IL-6, IFN-γ, tumour necrosis factor alpha (TNF-α), IL-8, IP-10, MCP-1 and MIP-1α. The induction ranged from 1.59- to 8.45-fold.
Table 1. Ad7 induces mRNA of cytokines and chemokines in human lung tissue and HAM.
Values shown are mean±sem fold above mock.
| Cytokine/chemokine | HAM | Lung |
| IL-6 | 0.53±0.09 | 1.79±0.25 |
| IL-12p35 | 0.99±0.17 | 1.02±0.12 |
| IFN-γ | 0.99±0.02 | 3.74±0.23 |
| TNF-α | 0.79±0.08 | 3.81±0.33 |
| IL-8 | 1.29±0.11 | 2.05±0.14 |
| IP-10 | 3.34±0.22 | 7.86±0.64 |
| MCP-1 | 1.48±0.19 | 3.49±0.21 |
| MIP-1α | 1.26±0.08 | 7.16±0.72 |
Induction of cytokine and chemokine proteins in human lung tissue and HAM infected with Ad7
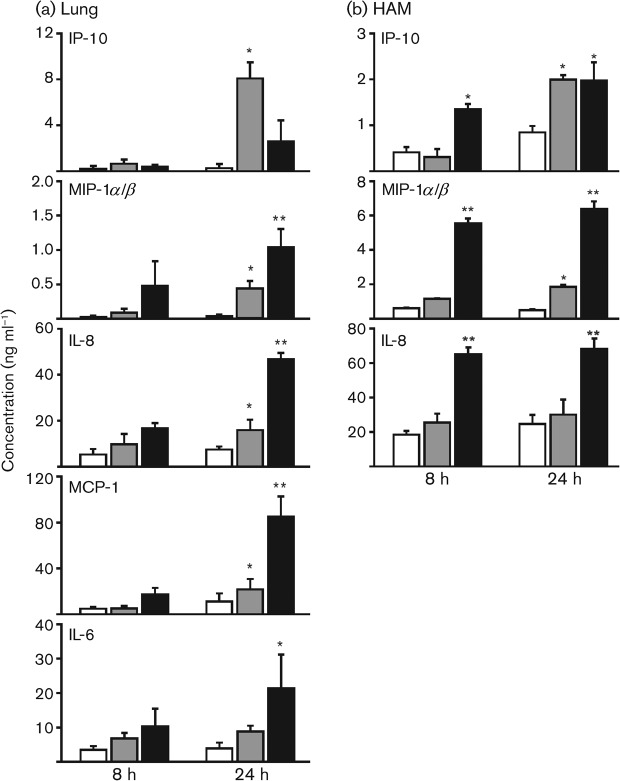
RPA data indicated an increase in the mRNA levels of several cytokines. To verify that this increase in endogenous mRNA levels was reflected at the level of translation, we tested for the relevant proteins in supernatants of lung slices and HAM exposed to Ad7 virus for 8 and 24 h using ELISA. Human lung slices were exposed to virus-free buffer (PBS/10 % glycerol), exposed to 1×109 p.f.u. Ad7 per well or to phorbol 12-myristate 13-acetate (PMA, 50 ng ml−1) in 0.5 ml medium as a positive control. Ad7 appeared to induce IL-8 and IL-6 8 h after infection, although this did not reach statistical significance (Fig. 1a). However, 24 h after infection, IL-8, IP-10, MCP-1 and MIP-1α/β were induced significantly by 2-, 30-, 2- and 12-fold, respectively (P<0.05; Fig. 1a). At both time points, Ad7 induced more IP-10 release than PMA (50 ng ml−1). Although IFN-γ and TNF-α mRNA was thought to be induced minimally by RPA, protein levels for IFN-γ and TNF-α were low (<100 pg ml−1) in lung slice supernatants and undetectable in lung slice extracts at 24 h (data not shown).
Fig. 1.
Ad7 stimulates chemokines and cytokines in the human lung slice tissue model and in HAM. Lung slices (a) and HAM (b) were exposed to 1×109 p.f.u. Ad7 per well in 0.5 ml medium for the times indicated (shaded bars). Virus-free buffer was used as a negative control (empty bars) and PMA (50 ng ml−1; a) or LPS (1 µg ml−1; b) was used as a positive control (filled bars). Chemokine and cytokine protein levels were determined by ELISA on lung slice supernatants. Data are expressed as means±sem from three separate experiments. Statistical significance was determined by ANOVA. Means were compared with data from the negative-control group: *P<0.05; **P<0.01.
HAM in 24-well plates were also mock-treated with virus-free buffer, exposed to 1×109 p.f.u. Ad7 per well or stimulated with lipopolysaccharide (LPS, 1 µg ml−1) in 0.5 ml medium as a positive control (Fig. 1b). IP-10 and MIP-1α/β were both induced by exposure of these cells to Ad7. IL-8 was induced minimally by Ad7, but this did not reach statistical significance. We did not find induction of IL-6, MCP-1, IFN-γ or TNF-α by Ad7 in HAM.
These results demonstrate that there is a broad array of cytokine and chemokine induction in human lung during Ad7 infection, at least by 24 h after infection. For the most part, mRNA induction of these cytokines and chemokines, as determined by RPA, is reflected at the protein level. There is a more limited response of isolated HAM exposed to this pathogen.
Identification of the cellular source of IL-8 and IP-10 induction by Ad7 in human lung
IL-8, elevated by 8 h after infection, is the main neutrophil chemotaxin induced upon Ad7 infection of our human lung model, and may be responsible for the neutrophilic infiltration that occurs early during Ad7 pneumonia. IP-10, elevated after 24 h, is probably responsible for the monocytic and lymphocytic infiltration seen later during infection. To determine the cellular elements that participate in the lung innate immune cytokine response to Ad7, we performed immunohistochemistry for IL-8 and IP-10 on Ad7-exposed lung slices and HAM.
Lung slices were exposed to 1×109 p.f.u. Ad7 per well or virus-free buffer in 0.5 ml medium for 24 h in the presence of brefeldin A (BFA), which inhibits export of protein from the distal Golgi compartment to the cell surface and enhances intracellular detection of cytokines (early BFA). Lung slices were exposed to virus-free buffer as a negative control or PMA as a positive control. The slices were then processed for immunohistochemistry for detection of IL-8 and IP-10 using goat polyclonal antibodies as described in Methods. For IP-10 staining, BFA was also added 8 h after Ad7 infection (late BFA). An additional negative control was performed for IL-8 and IP-10 detection by using the same staining protocol, but with the IL-8 or IP-10 primary antibody omitted. BFA did not prevent initial viral infection in the tissue (Supplementary Fig. S1, panels E and F; available in JGV Online) or replication in tissue, as determined by Western blotting for Ad hexon protein (not shown).
In human lung, IL-8 detection was enhanced significantly by Ad7 exposure when BFA was added concurrently with the pathogen (Supplementary Fig. S1, panels E and F). This suggests that induction of IL-8 by Ad7 is a direct consequence of virus exposure.
With regards to IP-10 detection in human lung, this chemokine was not detected when BFA was added concurrently with Ad7 (Supplementary Fig. S2, panels E and F; available in JGV Online). However, IP-10 was detected in the lung tissue when addition of BFA was delayed until 8 h after Ad7 infection (Supplementary Fig. S2, panels H and I), suggesting that IP-10 induction was enhanced by, or even required, cytokine crosstalk that cannot occur in the presence of BFA.
We also performed a more detailed examination of the cell types infected with Ad7 and the cells expressing IL-8 and IP-10. Tissue was exposed to Ad7 as described in Methods. For detection of IL-8, BFA was added concurrently with virus; for detection of IP-10, BFA was added 8 h after infection. Ad7 infection was detected by the use of an anti-hexon antibody, and cytokines by anti-cytokine antibodies. Type I alveolar epithelial cells were identified by using an anti-caveolin-1 antibody (Kathuria et al., 2004; Newman et al., 1999) and type II alveolar epithelial cells were identified by using an anti-prosurfactant protein C antibody (Jain-Vora et al., 1997; Reynolds et al., 2003).
As to Ad infection, both type I and type II alveolar epithelial cells were infected by the virus, as detected by co-localization of the markers for type I and type II cells and the adenovirus hexon (Figs 2, 3, 4 and 5f).
Fig. 2.
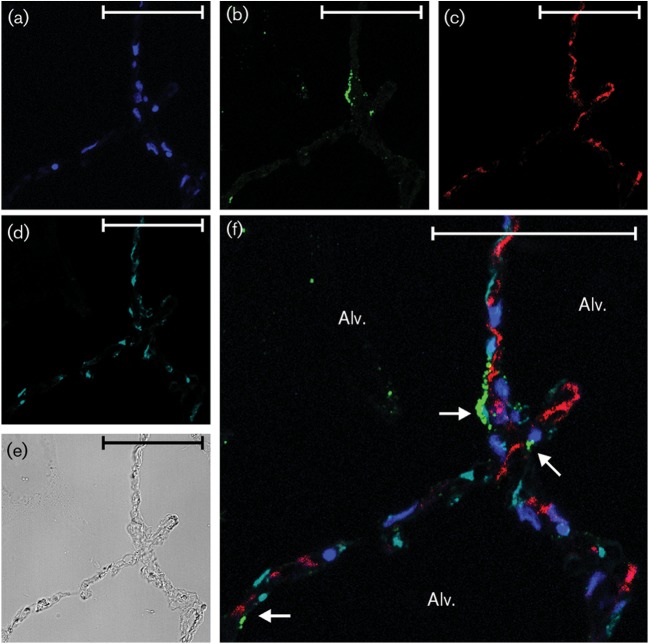
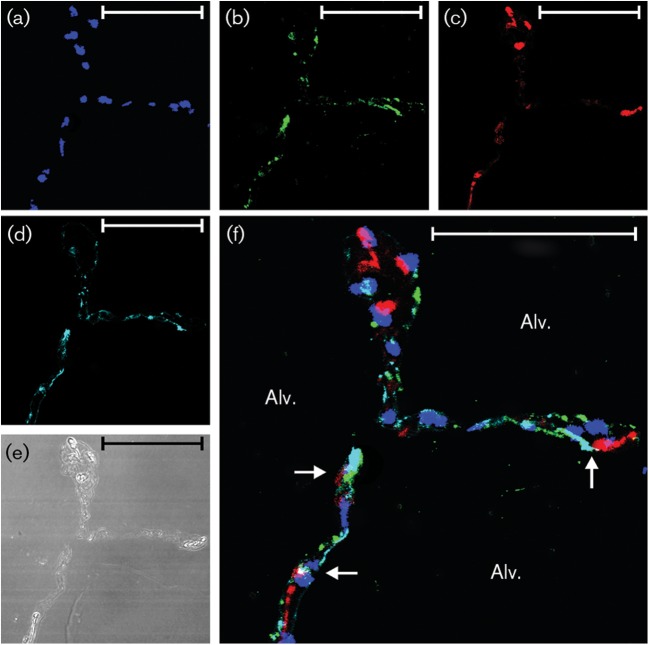
IL-8 induction by Ad7 in type I epithelial cells. Lung slices (one slice per well containing 0.5 ml medium) were exposed to 1×109 p.f.u. Ad7 per well or virus-free buffer for 24 h. (a–c) The slices were then processed for immunohistochemistry for detection of the chemokine IL-8 (red), Ad7 virus (green) and nuclei (DAPI staining; blue). (d) Type I alveolar epithelial cells (cyan) were identified by using an anti-caveolin-1 antibody. (e) Bright-field image demonstrating that lung architecture is preserved during the experiment. (f) Overlay of the fluorescent images. Arrows point to cells that are positive for IL-8, Ad7 and caveolin-1. Alv., Alveolus. Bars, 50 µm.
Fig. 3.
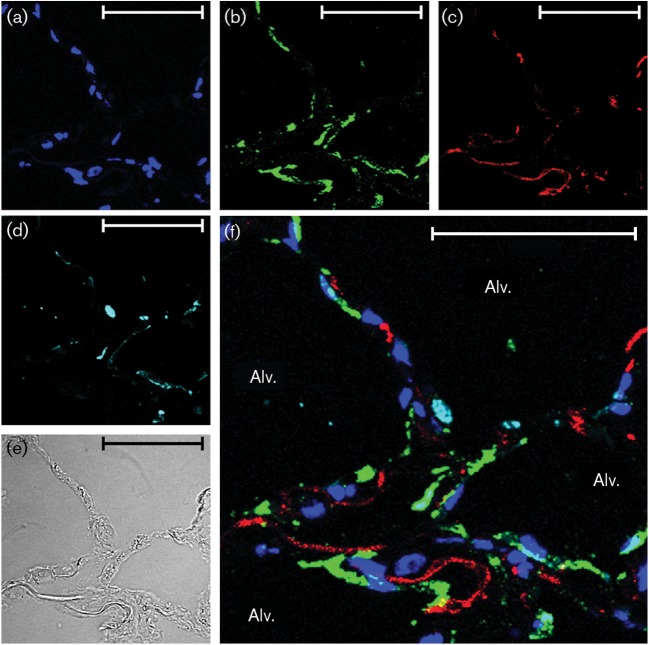
IL-8 induction by Ad7 does not involve type II epithelial cells. Lung slices (one slice per well containing 0.5 ml medium) were exposed to 1×109 p.f.u. Ad7 per well for 24 h. (a–c) The slices were then processed for immunohistochemistry for detection of the chemokine IL-8 (red), Ad7 virus (green) and nuclei (DAPI staining; blue). (d) Type II alveolar epithelial cells (cyan) were identified by using an anti-prosurfactant protein C antibody. (e) Bright-field image demonstrating that lung architecture is preserved during the experiment. (f) Overlay of the fluorescent images. Alv., Alveolus. Bars, 50 µm.
Fig. 4.
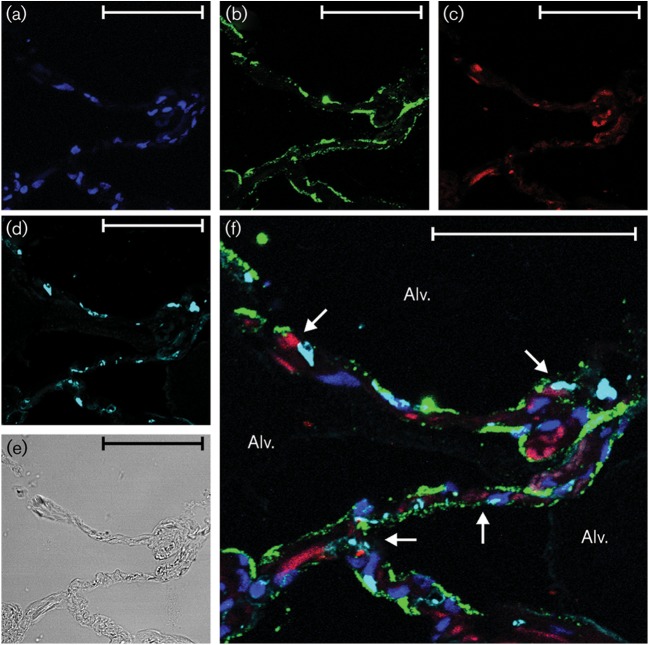
IP-10 induction by Ad7 in type I epithelial cells. Lung slices (one slice per well containing 0.5 ml medium) were exposed to 1×109 p.f.u. Ad7 per well for 24 h. BFA was added 8 h after Ad7 infection to enhance detection of cytokines. (a–c) The slices were then processed for immunohistochemistry for detection of the chemokine IP-10 (red), Ad7 virus (green) and nuclei (DAPI staining; blue). (d) Type I alveolar epithelial cells (cyan) were identified by using an anti-caveolin-1 antibody. (e) Bright-field image demonstrating that lung architecture is preserved during the experiment. (f) Overlay of the fluorescent images. Arrows point to cells that are positive for IP-10, Ad7 and caveolin-1. Alv., Alveolus. Bars, 50 µm.
Fig. 5.
IP-10 induction by Ad7 in type II epithelial cells. Lung slices (one slice per well containing 0.5 ml medium) were exposed to 1×109 p.f.u. Ad7 per well for 24 h. BFA was added 8 h after Ad7 infection to enhance detection of cytokines. (a–c) Fluorescent images that demonstrate nuclei by DAPI staining (blue), Ad7 virus (green) and IP-10 (red). (d) Type II epithelial cells (cyan) were identified by using an anti-prosurfactant protein C antibody. (e) Bright-field image demonstrating that lung architecture is preserved during the experiment. (f) Overlay of the fluorescent images. Arrows point to cells that are positive for IP-10, Ad7 and prosurfactant protein C. Alv., Alveolus. Bars, 50 µm.
In terms of cellular sources of the cytokines, IP-10 was detected in both type I and type II alveolar epithelial cells (Figs 2f, 3f, 4f and 5f), whereas IL-8 was only clearly detected in type I cells. Additional interstitial cells also appeared to be making both cytokines (Figs 2f, 3f, 4f and 5f; Supplementary Figs S1 and S2). These data suggest that the lung epithelium is not only infected by the pathogen, but also participates in the innate immune response to Ad7 through elaboration of cytokines. Interstitial cells also participate in the cytokine response to the virus.
Further examination of the lung tissue and additional experimentation using freshly isolated human macrophages were performed to determine whether alveolar macrophages were infected by Ad7 and participated in the innate immune cytokine response. In terms of infection, macrophages in lung tissue and in isolated culture were infected by, or at least internalized, the pathogen (Fig. 6). Of interest, despite the fact that the macrophages internalized the virus, only IP-10, but not IL-8, was made by these cells, whether these macrophages were associated with lung tissue or were in isolated culture (Fig. 6).
Fig. 6.
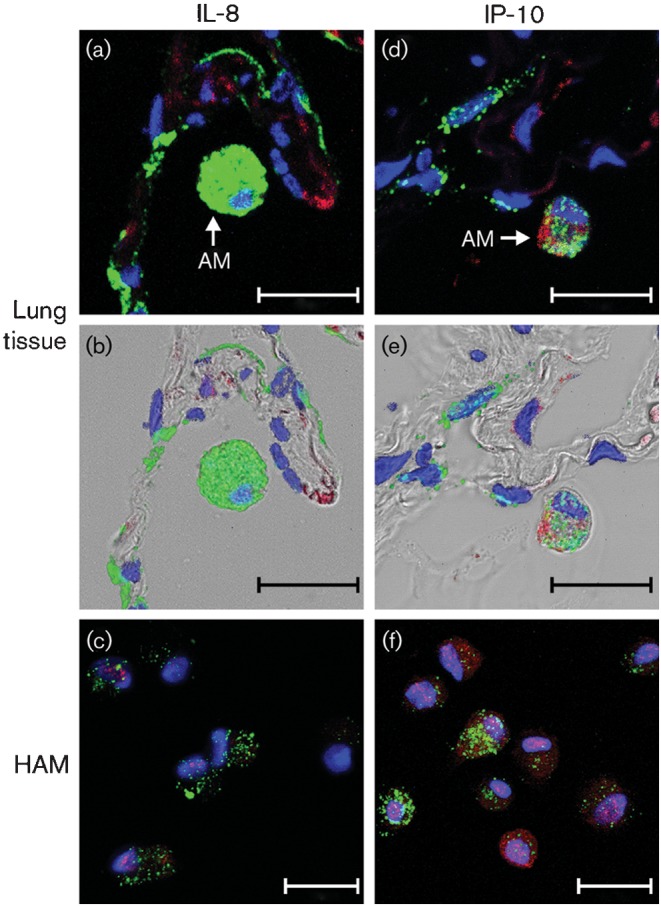
Alveolar macrophages contribute to IP-10 but not IL-8 induction by Ad7. In (a), (b), (d) and (e), human lung slices (one slice per well containing 0.5 ml medium) were exposed to 1×109 p.f.u. Ad7 per well for 24 h, and are higher-magnification images obtained from experiments performed as described in the legends to Figs 2–5. (a, d) Fluorescent images only; (b, e) overlays of the bright-field and fluorescent images. IL-8 (a, b) and IP-10 (d, e) were stained in red and Ad7 virus particles were stained in green. (c, f) Isolated HAM were exposed to 1×109 p.f.u. Ad7 per well in 0.5 ml medium for 24 h in the presence of BFA. Fluorescent images that demonstrate nuclei by DAPI staining (blue) and IL-8 (c) or IP-10 (f) detection by Alexa Fluor 546 (red) secondary antibody are shown. AM, Alveolar macrophage. Bars, 20 µm.
The results indicate that both lung epithelium and alveolar macrophages contribute to the innate immune response through induction of cytokines and chemokines. There also appears to be compartmentalization of the response, with release of IL-8 coming primarily from the epithelium, whereas IP-10 is released from both alveolar macrophages and the lung epithelium. Interstitial cells may also contribute to the release of both chemokines.
Discussion
In this report, we performed an expanded study comparing the cytokines and chemokines released in human lung and HAM infected by Ad7. Ad7 induced a more diverse cytokine response in human lung than in isolated HAM. In human lung, IL-6 and IL-8 were the first two cytokines induced, increasing as early as 8 h after infection. IL-6 is one of the most important cytokines that mediate fever and the acute-phase response (Conti et al., 2004; Sundgren-Andersson et al., 1998). IL-8 is probably responsible for the recruitment and sequestration of neutrophils observed in early infection with Ad7. Later, MIP-1α/β was elevated, and this chemokine may contribute to the neutrophilic infiltration that occurs. At 24 h after infection, MCP-1 and IP-10 induction became dominant and these chemokines may be responsible for the monocytic infiltration that occurs at this time. IP-10 may also contribute to the lymphocytic infiltration that occurs later in the disease process.
We detected a much broader cytokine and chemokine response in intact lung exposed to Ad7 than in isolated HAM exposed to this pathogen. This suggests that, whilst macrophages may play some role in the innate immune response to Ad7 by elaboration of specific chemokines such as IP-10 and MIP-1α/β, the more important role of these cells may be in triggering the transition from innate to adaptive immunity through antigen presentation.
IP-10 induction occurs in a very characteristic way. When BFA and Ad7 were added to the lung slices concurrently, no IP-10 was detected in human lung. When BFA was added 8 h after Ad7 infection, IP-10 was detected in multiple cells. This result could be due to intercellular cytokine communication that is inhibited by early addition of BFA. We did not see inhibition of IL-8 detection with early addition of BFA. This is consistent with a direct effect of Ad7 on cytokine induction, and is also consistent with our previous work showing that IL-8 induction in lung cells occurs during a very early stage of infection, as UV/psoralen-inactivated virus still induces this cytokine (Wu et al., 2006).
IP-10 was found in both epithelial cells and macrophages, suggesting that both types of cell participate and cooperate in the innate immune response that is responsible for the influx of inflammatory cells seen in Ad7 infection, and both cell types produce IP-10 in other systems (Pechkovsky et al., 2005; Tibbles et al., 2002; Zaiss et al., 2009). It has been shown that interactions between macrophages and epithelial cells promote inflammatory responses to hyperoxia, particulates and other toxins (Drumm et al., 2000; Hjort et al., 2003; Lee & Rannels, 1996; Sharma et al., 2004; Tao & Kobzik, 2002). Elegant work by D’Angio and co-workers demonstrated that cell–cell contact of THP-1 monocyte/macrophages and A549 epithelial cells is important for extracellular and intracellular IL-8 induction by hyperoxia in co-cultured THP-1 cells (Hjort et al., 2003). A dose-dependent increase in TNF-α and MIP-2 release was induced by airborne particulates in rat alveolar macrophages co-cultured with rat alveolar type II epithelial cells, but not in isolated cultures of either cell type. In contrast, no enhancement was found in macrophages co-cultured with fibroblasts (Tao & Kobzik, 2002). Therefore, there are some limited data that macrophage–epithelial cell interactions are important in the innate immune response to various stimuli, and this interaction may be important in IP-10 induction in our model. This human lung tissue model has advantages for infectious disease studies over those using cultured epithelial cells. Structural integrity of lung tissue is maintained and this allows for cell–cell interaction in a more complex three-dimensional system. Detailed mechanistic studies of intercellular processes such as inflammatory cell recruitment and therapeutics can be examined in human tissue without risk to the host.
Overall, our results indicate that the human lung responds to Ad7 infection by producing a broad range of cytokines and chemokines. This induction is probably responsible for recruitment of neutrophils, monocytes and lymphocytes that participate in the innate immune response. Isolated HAM alone do not produce many cytokines and chemokines in response to Ad7, but they might interact with epithelial cells to promote the inflammatory response, and they are important in the transition from the innate immune response to adaptive immunity (Holt et al., 1993; Strickland et al., 1993). The source of the neutrophil chemokine IL-8 is mainly epithelial cells, whereas the monocyte and lymphocyte chemokine IP-10 is produced by both macrophages and epithelial cells.
Methods
Preparation of Ad7 stock.
Ad7, obtained from the ATCC (VR-7), was propagated and purified by CsCl gradient ultracentrifugation as described previously (Booth et al., 2004; Wu et al., 2006). The titre of viable virus was quantified by plaque assay and the purified Ad7 preparations were stored at −80 °C. All reagents, including virus stocks and buffers, were verified to be free of significant endotoxin contamination by Limulus amebocyte assay (Cambrex).
Lung explant culture and collection of HAM.
Human lung tissue was obtained from patients undergoing resection for lung cancer in accordance with protocols approved by the Institutional Review Boards of the University of Oklahoma, Veterans Administration Hospital, Baptist-Integris Hospital, St Anthony’s Hospital and Mercy Health Center, all of Oklahoma City, OK, USA. Only tissue that did not contain tumour was used for experiments. The tumour-free lung tissue was transported in sterile PBS (pH 7.2) containing 200 µg gentamicin ml−1, 100 U penicillin ml−1, 100 µg streptomycin ml−1 and 2.5 µg amphotericin B ml−1 (PBS+antibiotics) and the tissue was subsequently stored at 4 °C in PBS+antibiotics for no longer than 4 h. The lung segments were inflated with lung slice medium [LSM; minimal essential medium (Sigma) supplemented with (ml−1): 1.0 µg bovine insulin, 0.1 µg hydrocortisone, 0.1 µg retinyl acetate, 200 µg gentamicin, 100 U penicillin, 100 µg streptomycin and 1.25 µg amphotericin B] containing 1.5 % agarose and tissues were sliced into 500 µm thick sections as described previously (Chakrabarty et al., 2007). Each slice was placed in 0.5 ml LSM in a single well of a 24-well plate, then placed in a humidified incubator at 37 °C in 5 % CO2. The LSM was replaced prior to subjecting the slices to the experimental treatments.
HAM were obtained by bronchoscopy as described previously (Chakrabarty et al., 2006). Cells (1 ml per well) were plated into 24-well plates and allowed to incubate for 2–4 h to facilitate attachment. Subsequently, the medium was removed and fresh medium containing 2 % fetal calf serum (FCS) and gentamicin was added. The cells were incubated overnight at 37 °C with 5 % CO2.
Infection of human lung slices and HAM with Ad7 and determination of cytokine and chemokine induction.
After overnight incubation of the lung slices, the culture medium was replaced with 0.5 ml fresh LSM. For each data point, lung slices in separate wells were exposed to 1×109 p.f.u. Ad7 per well and allowed to incubate at 37 °C, 5 % CO2 for the indicated periods. We have shown previously that this dose elicits an immune response in this model (Booth et al., 2004). PBS/10 % glycerol served as a negative control and PMA (50 ng ml−1) was used as a positive control. Following stimulation for various times, medium supernatants and tissue extracts were harvested and stored at −20 °C prior to ELISA. For HAM, after overnight incubation of the macrophages, the medium was removed and 0.5 ml fresh RPMI/2 % FCS was added. Macrophages were exposed to 1×109 p.f.u. Ad7 per well in triplicate wells of 24-well plates and allowed to incubate at 37 °C for 8 and 24 h. PBS/10 % glycerol was used as a negative control and LPS (1 µg ml−1) was used as a positive control. Ad7 exposure did not affect HAM viability. After incubation, the supernatants were collected, centrifuged at 10 000 g for 2 min, transferred to a new tube and stored at −20 °C. The positive controls, PMA for lung slices and LPS for HAM, were selected for their ability to elicit a cytokine response in the material used.
Cytokine ELISAs were performed using anti-cytokine monoclonal primary antibodies and biotinylated anti-cytokine polyclonal secondary antibodies (R&D Systems). Because the MIP-1α monoclonal antibody cross-reacts with MIP-1β, the results were expressed as MIP-1α/β levels. Plates were developed using TMB reagent (BD Biosciences).
RNA preparation and RPA.
Total RNA was extracted from lung slices, and HAM and RPA were performed as described previously (Chakrabarty et al., 2007). Fold increase for each RNA species over control samples prepared at the same time points was determined after correction for loading using ribosomal protein L32 and glyceraldehyde-3-phosphate dehydrogenase standards.
Cytokine immunohistochemistry on lung tissue explants and HAM.
To examine which cell types in the lung tissue produced IL-8 and IP-10 after Ad infection, lung slices or isolated HAM were exposed to 1×109 p.f.u. Ad7 per well or PBS/10 % glycerol in 0.5 ml fresh lung slice medium containing 50 µg gentamicin ml−1 and incubated at 37 °C for 24 h. BFA (L C Laboratories) was added at a concentration of 5 µg ml−1 to block protein export, in order to enhance cytokine detection. BFA was added either concurrently with Ad7 (early BFA) or 8 h after Ad7 infection (late BFA). Following the incubation, the lung slices were fixed with 4 % paraformaldehyde in PBS at room temperature for 30 min and were then embedded in paraffin. Sections (3–5 µm) were mounted on glass slides and immuno-probed overnight at 4 °C with a rabbit anti-human polyclonal antibody for IP-10 (Abcam) or a goat anti-human polyclonal antibody for IL-8 (R&D Systems). After washing, the sections were probed with a goat anti-rabbit or a donkey anti-goat secondary antibody conjugated to Alexa Fluor 546 (Molecular Probes), and the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Ad was stained with an anti-Ad hexon monoclonal antibody (Chemicon) and a goat anti-mouse secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes). Type I and II alveolar epithelial cells were identified by using an anti-caveolin-1 antibody and an anti-prosurfactant protein C antibody, respectively, and Alexa Fluor 750-labelled secondary antibodies. Fluorescent confocal laser-scanning microscopy was conducted using a Zeiss LSM-510 META laser-scanning confocal microscope. In Supplementary Figs S1 and S2, transmitted light and fluorescent microscopy images were obtained by using an Olympus BX51 microscope equipped with a Spot 4.2 digital camera.
Statistical analysis.
Where applicable, the data have been expressed as means±sem. Statistical significance was determined by one-way ANOVA with Student–Newman–Keuls post hoc correction for multiple comparisons. Significance was considered as P<0.05 (Zar, 1996).
Acknowledgements
This work is partly supported by a Grant-in-Aid from the American Heart Association Heartland Affiliate (to J. P. M.) and a Clinical Innovator Award of Flight Attendant Medical Research Institute (to W. W.). We wish to acknowledge the assistance of the staff at the Oklahoma Medical Research Foundation Imaging Analysis core facility.
Footnotes
Two supplementary figures showing the cellular source of IL-8 and IP-10 induction by Ad7 are available with the online version of this paper.
References
- Abbondanzo S. L., English C. K., Kagan E., McPherson R. A. (1989). Fatal adenovirus pneumonia in a newborn identified by electron microscopy and in situ hybridization. Arch Pathol Lab Med 113, 1349–1353 [PubMed] [Google Scholar]
- Booth J. L., Metcalf J. P. (1999). Type-specific induction of interleukin-8 by adenovirus. Am J Respir Cell Mol Biol 21, 521–527 [DOI] [PubMed] [Google Scholar]
- Booth J. L., Coggeshall K. M., Gordon B. E., Metcalf J. P. (2004). Adenovirus type 7 induces interleukin-8 in a lung slice model and requires activation of Erk. J Virol 78, 4156–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. L., Metzger M., Danel C., Rosenfeld M. A., Crystal R. G. (1994). Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther 5, 821–836 [DOI] [PubMed] [Google Scholar]
- Bruder J. T., Kovesdi I. (1997). Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol 71, 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballal G., Videla C., Misirlian A., Requeijo P. V., Aguilar M. (2002). Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr 2, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty K., Wu W., Booth J. L., Duggan E. S., Coggeshall K. M., Metcalf J. P. (2006). Bacillus anthracis spores stimulate cytokine and chemokine innate immune responses in human alveolar macrophages through multiple mitogen-activated protein kinase pathways. Infect Immun 74, 4430–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty K., Wu W., Booth J. L., Duggan E. S., Nagle N. N., Coggeshall K. M., Metcalf J. P. (2007). Human lung innate immune response to Bacillus anthracis spore infection. Infect Immun 75, 3729–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B., Tabarean I., Andrei C., Bartfai T. (2004). Cytokines and fever. Front Biosci 9, 1433–1449 [DOI] [PubMed] [Google Scholar]
- Diamond G., Legarda D., Ryan L. K. (2000). The innate immune response of the respiratory epithelium. Immunol Rev 173, 27–38 [DOI] [PubMed] [Google Scholar]
- Drumm K., Attia D. I., Kannt S., Micke P., Buhl R., Kienast K. (2000). Soot-exposed mononuclear cells increase inflammatory cytokine mRNA expression and protein secretion in cocultured bronchial epithelial cells. Respiration 67, 291–297 [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Kobayashi K. (2005). Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 4, 281–286 [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. (1991). A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A 88, 1651–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort M. R., Brenyo A. J., Finkelstein J. N., Frampton M. W., LoMonaco M. B., Stewart J. C., Johnston C. J., D'Angio C. T. (2003). Alveolar epithelial cell–macrophage interactions affect oxygen-stimulated interleukin-8 release. Inflammation 27, 137–145 [DOI] [PubMed] [Google Scholar]
- Holt P. G., Oliver J., Bilyk N., McMenamin C., McMenamin P. G., Kraal G., Thepen T. (1993). Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 177, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain-Vora S., Wert S. E., Temann U. A., Rankin J. A., Whitsett J. A. (1997). Interleukin-4 alters epithelial cell differentiation and surfactant homeostasis in the postnatal mouse lung. Am J Respir Cell Mol Biol 17, 541–551 [DOI] [PubMed] [Google Scholar]
- Kathuria H., Cao Y. X., Ramirez M. I., Williams M. C. (2004). Transcription of the caveolin-1 gene is differentially regulated in lung type I epithelial and endothelial cell lines. A role for ETS proteins in epithelial cell expression. J Biol Chem 279, 30028–30036 [DOI] [PubMed] [Google Scholar]
- Klinger J. R., Sanchez M. P., Curtin L. A., Durkin M., Matyas B. (1998). Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med 157, 645–649 [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Rannels D. E. (1996). Alveolar macrophages modulate the epithelial cell response to coal dust in vitro. Am J Physiol 270, L123–L132 [DOI] [PubMed] [Google Scholar]
- Newman G. R., Campbell L., von Ruhland C., Jasani B., Gumbleton M. (1999). Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res 295, 111–120 [DOI] [PubMed] [Google Scholar]
- Otake K., Ennist D. L., Harrod K., Trapnell B. C. (1998). Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum Gene Ther 9, 2207–2222 [DOI] [PubMed] [Google Scholar]
- Pechkovsky D. V., Goldmann T., Ludwig C., Prasse A., Vollmer E., Muller-Quernheim J., Zissel G. (2005). CCR2 and CXCR3 agonistic chemokines are differently expressed and regulated in human alveolar epithelial cells type II. Respir Res 6, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mucenski M. L., Whitsett J. A. (2003). Thyroid transcription factor (TTF)-1 regulates the expression of midkine (MK) during lung morphogenesis. Dev Dyn 227, 227–237 [DOI] [PubMed] [Google Scholar]
- Sharma N., He Q., Sharma R. P. (2004). Augmented fumonisin B1 toxicity in co-cultures: evidence for crosstalk between macrophages and non-parenchymatous liver epithelial cells involving proinflammatory cytokines. Toxicology 203, 239–251 [DOI] [PubMed] [Google Scholar]
- Strickland D. H., Thepen T., Kees U. R., Kraal G., Holt P. G. (1993). Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology 80, 266–272 [PMC free article] [PubMed] [Google Scholar]
- Sundgren-Andersson A. K., Östlund P., Bartfai T. (1998). IL-6 is essential in TNF-α-induced fever. Am J Physiol 275, R2028–R2034 [DOI] [PubMed] [Google Scholar]
- Tao F., Kobzik L. (2002). Lung macrophage–epithelial cell interactions amplify particle-mediated cytokine release. Am J Respir Cell Mol Biol 26, 499–505 [DOI] [PubMed] [Google Scholar]
- Tibbles L. A., Spurrell J. C., Bowen G. P., Liu Q., Lam M., Zaiss A. K., Robbins S. M., Hollenberg M. D., Wickham T. J., Muruve D. A. (2002). Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J Virol 76, 1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Varsanyi T. M., Lord A., Sutton R. N. (1980). Epidemic outbreaks of adenovirus 7 with special reference to the pathogenicity of adenovirus genome type 7b. Am J Epidemiol 112, 619–628 [DOI] [PubMed] [Google Scholar]
- Wu W., Booth J. L., Coggeshall K. M., Metcalf J. P. (2006). Calcium-dependent viral internalization is required for adenovirus type 7 induction of IL-8 protein. Virology 355, 18–29 [DOI] [PubMed] [Google Scholar]
- Zaiss A. K., Vilaysane A., Cotter M. J., Clark S. A., Meijndert H. C., Colarusso P., Yates R. M., Petrilli V., Tschopp J., Muruve D. A. (2009). Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J Immunol 182, 7058–7068 [DOI] [PubMed] [Google Scholar]
- Zar J. H. (1996). Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]