Abstract
BAK1 is a multifunctional leucine-rich repeat receptor kinase (LRR-RLK) that exerts its function by interacting with multiple ligand binding receptors and thereby influences diverse processes varying from brassinosteroid perception via PAMP and DAMP perception to cell death control. We recently identified a new BAK1 interacting protein, BIR2, that is also a LRR-RLK. In contrast to BAK1, BIR2 negatively regulates BAK1-dependent PAMP responses. While brassinosteroid responses are not affected by BIR2, cell death is negatively regulated as described for BAK1. BIR2 is released from BAK1 after ligand perception, increasing the pool of free BAK1 that is available to form complexes with activated ligand binding receptors. Individual ligands can only partially release BAK1 from BIR2. After exposition to a cocktail of ligands, almost the complete amount of BAK1 can be released indicating that BAK1 exists, together with BIR2, in subpools that can be individually addressed by specific ligands. These data support the idea that BAK1 exists in preformed complexes with its ligand binding receptor partners. Overexpression of BIR2 results in reduced complex formation of BAK1 with FLS2, showing that BIR2 negatively regulates BAK1 complex formation with ligand binding receptors.
Keywords: LRR-RLK, plant immunity, BAK1, BIR2, preformed complexes, cell death regulation, crystal structure, pseudokinase
Introduction
Complex formation of plasma membrane receptors is a common theme in plants and animals.1,2 Innate immune receptors associate with adaptor proteins after ligand induced activation and these adaptor proteins support the function of the receptors, transduce the signal over the plasma membrane and/or activate downstream responses. The most prominent example from plants is the leucine-rich repeat receptor kinase BAK1 which has an extracellular domain with 5 LRRs and interacts with several other LRR-RLKs.3 Thereby, a number of signaling pathways are affected making BAK1 a multifunctional RLK.4
BIR2 is a constitutive interactor of BAK1
We purified in vivo complexes of BAK1 and identified a number of novel BAK1 interacting proteins including BIR2 and BIR3, 2 LRR-RLKs with similarly small extracellular domains as compared with BAK1.5 Both proteins strongly and constitutively interact with BAK1 as confirmed by co-immunoprecipitation, FRET-FLIM, BIFC, and also the kinase domains alone can interact in yeast 2-hybrid assays. BIR2 shows a closely co-regulated expression with BAK1 with inducibility by pathogens. Enzymatically, BIR2 is kinase inactive but it becomes phosphorylated when incubated with active BAK1. The crystal structure of BIR2 revealed that BIR2 is indeed catalytically inactive because of an occluded ATP-binding pocket (Fig. 1), and NMR studies confirm that BIR2 is unable to bind ATP.6 The crystal structure represents the first conformation of an inactive pseudokinase from plants and provides a platform for understanding the interaction of BIR2 with BAK1. While BIR2 is a regulator of plant immunity and cell death in Arabidopsis, another pseudokinase STRUBBELIG has been shown to regulate organ development.7 Because pseudokinases likely arise through gene duplication events of active kinases, they are likely to evolve independently and act in distinct ways on their targets of regulation. It is thus to be expected that no general scheme will emerge but that individual mechanisms will be identified for different signaling modulations by the many Arabidopsis pseudokinases, BIR2 negatively regulates PAMP responses. Mutants in bir2 are hypersensitive to PAMPs, produce more ROS, show increased FRK1 gene expression and callose deposition, and are consequently more resistant to bacterial infection. They also accumulate higher levels of SA and PR1. These phenotypes are opposite to what is reported for BAK1, leading us to propose that BIR2 negatively regulates BAK1-dependent PAMP-responses. By contrast, brassinosteroid responses are not affected in bir2 mutants, and cell death induced by fungal infection is elevated in bir2 mutants, comparable to bak1 mutants. This shows that all 3 BAK1-mediated pathways are independent and can be differentially addressed by BIR2 action.
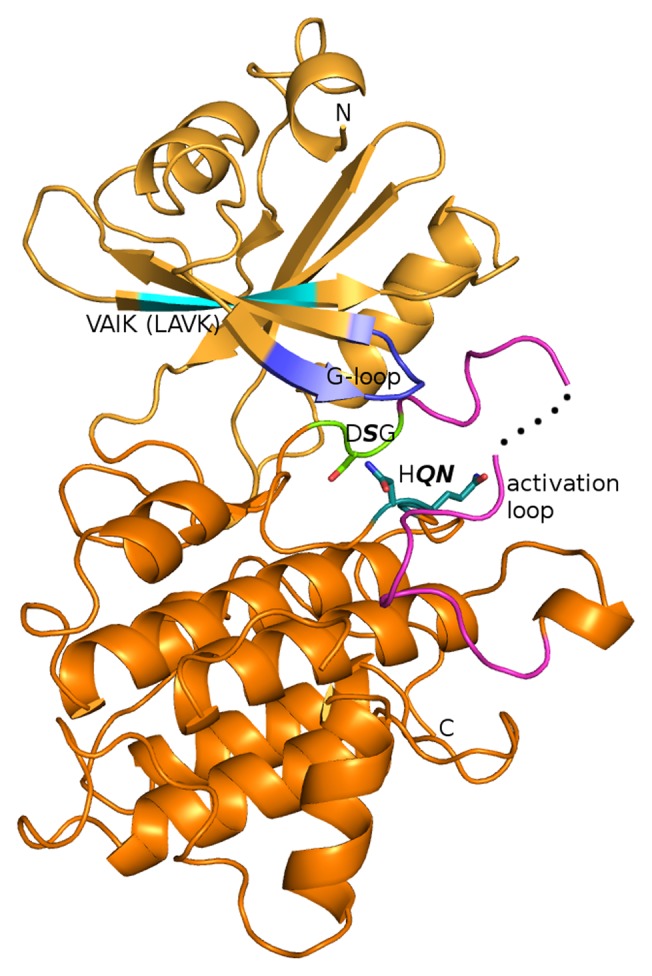
Figure 1. Crystal structure of the BIR2 kinase domain. Conserved kinase sequence motifs that display atypical variations include the glycine-rich loop (blue), the HRD motif (teal), and the DFG motif (light green). The sequence of the VAIK motif is not dramatically altered. Non-conserved residues in the HRD (BIR2: HQN) and DFG (BIR2: DSG) motifs are shown in stick display. A part of the activation loop (magenta) is missing in the electron density map, suggesting high flexibility similar to what has been observed for many active kinases.
BAK1-dependent cell death—guarding, autoimmune response or deregulated phosphorylation?
Both bir2 and bak1 mutants present spreading cell death after fungal infection. bir1 mutants show spontaneous cell death due to the constitutive activation of 2 independent resistance (R) gene-mediated pathways, one PAD4-dependent and the other SOBIR1-dependent.8 In addition, BIR1 and BAK1 interact with BON1 (BONZAI1), a C2 domain protein9 that negatively regulates another R gene, the TIR-NB-LRR SNC1 (suppressor of npr1–1, constitutive 1), suggesting that the bir1 cell death phenotype includes SNC1-dependent autoimmune responses. A guarding model might be possible for BIR2 as well where loss of BIR2 binding to BAK1 is sensed and activates a yet unknown guard system. Recently, a study using a suppressor screen identified the components leading to the cell death phenotype in mekk1, mkk1mkk2, and mpk4 mutants.10,11 This MAPK pathway negatively regulates SUMM1 (suppressor of mkk1mkk2), also known as MEKK211,12 and MEKK2 activates SUMM2, a CC-NB-LRR when MPK4 downregulation is lost.11-13 Loss of BIR1 and BIR2 might also activate a guard to trigger autoimmune responses. Interestingly, SRF3, a close relative of the atypical LRR-RLK STRUBBELIG, with a small 6-LRR-repeat extracellular domain and therefore structurally similar to BIR2, was shown to be involved in hybrid incompatibility along with the R gene RPP1 locus. Similar to bir2 cell death phenotypes, SRF3/RPP1 incompatibility is accompanied by necrotic cell death, elevated SA levels and enhanced defense responses.14 The authors conclude that SRF3 is guarded by RPP1 and, when incompatible allele combinations are sensed, defense reactions are activated in the absence of pathogens. A similar scenario seems also feasible for BIR2 complex integrity control. Overexpression of BAK1 also leads to runaway cell death.15 Simultaneous overexpression of BRI1 and BAK1 suppresses this cell death suggesting that recruitment of BAK1 by interacting partners, such as ligand binding receptors or BIR1 and BIR2, is necessary to keep the autoimmune cell death pathway inactive.15 Further analysis of cell death in RLK mutants, which share similar effects as autoimmune responses16 and hybrid incompatibility14,17 will shed more light on this interesting phenomenon.
BIR2 affects complex formation of BAK1 with ligand binding receptors
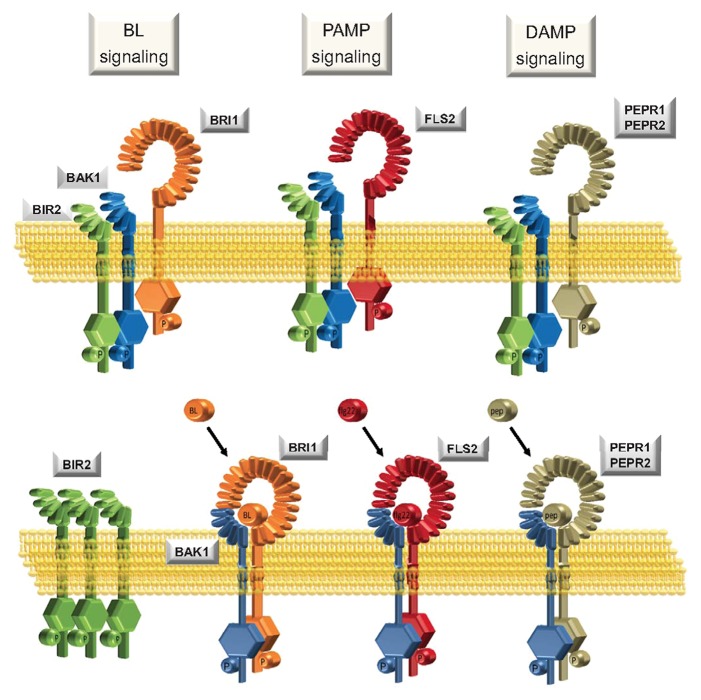
But how does BIR2 affect BAK1-dependent PAMP responses? As described above, BIR2 interacts constitutively with BAK1. After ligand binding to the respective receptor, BIR2 is released from BAK1 enabling BAK1 to join the activated ligand-binding receptors. Only recently the co-complex crystal structure of BAK1-flg22-FLS2 has revealed that BAK1 binds not only to the receptor FLS2 but also to the receptor bound ligand flg22.18 Binding of the ligand creates an additional binding site for BAK1 and might increase the affinity of BAK1 to FLS2. In the absence of the ligand, no interaction of BAK1 with FLS2 can be detected, though the interaction happens within seconds after ligand perception, indicating that the 2 receptors must exist in close vicinity in the membrane. Further evidence supporting the existence of preformed complexes was provided by visualization of the BRI1-BAK1-heteromer by extended microscopic imaging.19 We observed that treatment of plants with individual ligands leads only to a partial release of BIR2 from BAK1. Exposure to a cocktail of ligands increased the pool of free BAK1 available for binding to distinct ligand binding receptors such as FLS2, EFR, PEPR1, and BRI1. These results suggest that in the absence of ligand, BAK1 is kept in complex with BIR2 in subpools with specific receptors. These subpools can be specifically addressed by a specific ligand - flg22, for example, would be unable to induce the release of BIR2 from a specific BAK1 molecule that exists in a preformed complex with BRI1 (Fig. 2).
Figure 2. Model of BAK1-BIR2 complex formation prior and after ligand perception. BAK1 is a multifunctional co-receptor that interacts with different ligand binding receptors and therefore acts in diverse signaling pathways in the plant. BAK1 is involved in brassinosteroid signaling by interacting with the brassinosteroid receptor BRI1 and in PAMP signaling by interacting with the flg22 receptor FLS2 but also acts in DAMP signaling by interaction with the Atpep receptors PEPR1 and PEPR2.4 BIR2 constitutively interacts with BAK1 in the absence of ligands. Release of BAK1 from BIR2 after activation of BAK1-dependent pathways by their ligands would allow recruitment of BAK1 into ligand binding receptor complexes. Treatment with individual ligands such as flg22, Atpep or BL leads to a partial release of BAK1 from the BIR2 complex. Treatment with a ligand cocktail leads to a strongly increased release of BAK1 from BIR2 than treatment with individual ligands.5 The rapid association of BAK1 with ligand binding receptors suggests that BAK1 resides in the membrane in preformed complexes with different ligand binding receptors. Our data provide additional evidence that BAK1 exists in distinct subpools with individual ligand binding receptors that can be specifically addressed by their individual ligands resulting in specific release of BIR2.
FLS2 and BAK1 do not interact in the absence of the ligand flg22. But why is that the case? The bak1–5 mutation is a point mutation that renders BAK1 hypoactive and that dissects the known signaling pathways by specifically showing only PAMP insensitivity while cell death and BL signaling are left unaltered. In the bak1–5 mutants, interaction of BAK1–5 with FLS2 already occurs in the absence of flg22.20 Also the interaction with other ligand binding receptors such as EFR and BRI1 is increased. Interestingly, interaction of BAK1–5 with BIR2 (and most likely other BIR proteins) is strongly reduced. This strong correlation in affinity changes support the idea that BIR2 is needed to prevent unwanted interaction of BAK1 with ligand binding receptors. In BIR2 overexpressing plants, interaction of BAK1 with FLS2 is strongly reduced showing that BIR2 has indeed the capacity to prevent this interaction.5
Conclusions and Perspectives
Our results show that BIR2 is a negative regulator of BAK1-dependent PAMP responses and acts by interfering with complex formation of BAK1 with ligand binding receptors. Our functional and structural characterization of BIR2 strongly suggests that its kinase domain is permanently catalytically inactive. Pseudokinases make up about 20% of Arabidopsis LRR-RLKs21 and fulfill regulatory functions in all kingdoms of life. The set of pseudokinases whose regulatory functions have been elucidated comprehensively serve as scaffolding proteins (for the assembly of multi-protein complexes) or as allosteric regulators of kinase signaling pathways.22 The future will reveal how BIR2 mechanistically exerts its function. In the absence of ligand, BAK1 is bound to BIR2 with an affinity of BAK1 that is higher than to unactivated ligand-binding receptors. Upon ligand binding, the BAK1 relative affinities shift to preferentially binding ligand-bound receptors such as FLS2-flg22.
As BIR2 exists together with BAK1 in subpools that can be specifically addressed by individual ligands, BIR2 might be the docking station that keeps BAK1 in preformed but signaling inactive complexes with specific ligand binding receptors. When these preformed complexes are disturbed by mutating either BIR2 or BAK1, cell death is induced. The same is true for BIK1, a cytoplasmic component of BAK1 receptor complexes.23,24 How all these receptors assemble still remains unclear and needs to be analyzed in the future. The complexes might be guarded by resistance proteins that lead to activation of cell death in the absence of pathogens as described for autoimmune responses and hybrid incompatibility. All these phenomena are resulting in the activation of constitutive SA-dependent defense responses coming along with dwarfism in a quantitative manner, the stronger the defense responses the smaller the mutants. The future will show which molecular patterns influence the gradual differences between BIR1 and BIR2 in cell death control.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monaghan J, Zipfel C. Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol. 2012;15:349–57. doi: 10.1016/j.pbi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: the receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–41. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemmerling B, Halter T, Mazzotta S, Mosher S, Nürnberger T. A genome-wide survey for Arabidopsis leucine-rich repeat receptor kinases implicated in plant immunity. Front Plant Sci. 2011;2:88. doi: 10.3389/fpls.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bücherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, et al. The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol. 2014;24:134–43. doi: 10.1016/j.cub.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 6.Blaum BS, Mazzotta S, Nöldeke ER, Halter T, Madlung J, Kemmerling B, Stehle T. Structure of the pseudokinase domain of BIR2, a regulator of BAK1-mediated immune signaling in Arabidopsis. J Struct Biol. 2014;186:112–21. doi: 10.1016/j.jsb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier D, Batoux M, Fulton L, Pfister K, Yadav RK, Schellenberg M, Schneitz K. STRUBBELIG defines a receptor kinase-mediated signaling pathway regulating organ development in Arabidopsis. Proc Natl Acad Sci U S A. 2005;102:9074–9. doi: 10.1073/pnas.0503526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe. 2009;6:34–44. doi: 10.1016/j.chom.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Meng P, Zhang X, Ren D, Yang S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 2011;67:1081–93. doi: 10.1111/j.1365-313X.2011.04659.x. [DOI] [PubMed] [Google Scholar]
- 10.Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008;18:1190–8. doi: 10.1038/cr.2008.300. [DOI] [PubMed] [Google Scholar]
- 11.Kong Q, Qu N, Gao M, Zhang Z, Ding X, Yang F, Li Y, Dong OX, Chen S, Li X, et al. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell. 2012;24:2225–36. doi: 10.1105/tpc.112.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su SH, Bush SM, Zaman N, Stecker K, Sussman MR, Krysan P. Deletion of a tandem gene family in Arabidopsis: increased MEKK2 abundance triggers autoimmunity when the MEKK1-MKK1/2-MPK4 signaling cascade is disrupted. Plant Cell. 2013;25:1895–910. doi: 10.1105/tpc.113.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe. 2012;11:253–63. doi: 10.1016/j.chom.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Alcázar R, García AV, Kronholm I, de Meaux J, Koornneef M, Parker JE, Reymond M. Natural variation at Strubbelig Receptor Kinase 3 drives immune-triggered incompatibilities between Arabidopsis thaliana accessions. Nat Genet. 2010;42:1135–9. doi: 10.1038/ng.704. [DOI] [PubMed] [Google Scholar]
- 15.Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci U S A. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Meng P, Zhang X, Ren D, Yang S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 2011;67:1081–93. doi: 10.1111/j.1365-313X.2011.04659.x. [DOI] [PubMed] [Google Scholar]
- 17.Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 2007;5:e236. doi: 10.1371/journal.pbio.0050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013;342:624–8. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 19.Bücherl CA, van Esse GW, Kruis A, Luchtenberg J, Westphal AH, Aker J, van Hoek A, Albrecht C, Borst JW, de Vries SC. Visualization of BRI1 and BAK1(SERK3) membrane receptor heterooligomers during brassinosteroid signaling. Plant Physiol. 2013;162:1911–25. doi: 10.1104/pp.113.220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castells E, Casacuberta JM. Signalling through kinase-defective domains: the prevalence of atypical receptor-like kinases in plants. J Exp Bot. 2007;58:3503–11. doi: 10.1093/jxb/erm226. [DOI] [PubMed] [Google Scholar]
- 22.Zeqiraj E, van Aalten DM. Pseudokinases-remnants of evolution or key allosteric regulators? Curr Opin Struct Biol. 2010;20:772–81. doi: 10.1016/j.sbi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell. 2006;18:257–73. doi: 10.1105/tpc.105.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]