Abstract
Patatin-containing phospholipase A (pPLA) hydrolyzes membrane glycerolipids, producing free fatty acids and lysoglycerolipids. Ten pPLAs in the Arabidopsis thaliana genome are grouped into 3 subfamilies, and pPLAIIIs differ from pPLAI and IIs in their catalytic motifs and overexpression (OE) of pPLAIIIs reduces cell elongation and cellulose content. To probe the question of how pPLAIII overexpression results in the changes, comparative proteomic analyses were conducted between pPLAIIIδ-OE and WT seedlings. The data indicate a change in the microtubule-associated protein, MAP18. MAP18 is involved in destabilizing cortical microtubules and modulating directional cell growth. The result suggests that pPLAIII and their derived products may regulate cytoskeletal dynamics leading to retardation in anisotropic growth.
Keywords: cytoskeleton, microtubule-associated protein, patatin, phopsholipase A, cell elongation, lipid signaling
Patatin-containing phospholipase A (pPLA) is a multigene family of enzymes that hydrolyze membrane glycerolipids to free fatty acids (FFAs) and lysoglycerolipids. Ten pPLAs in the Arabidopsis thaliana genome have been grouped into 3 subfamilies, pPLAI, pPLAII (α, β, γ, δ, ε), and pPLAIII (α, β, γ, δ).1 The pPLAI protein has 1257 amino acid residues whereas the proteins of pPLAIIs and pPLAIIIs are much smaller, ranging from 382 to 526 amino acid residues. In terms of amino acid sequences, pPLAI is more similar to mammalian calcium-independent PLAs than to pPLAIIs and pPLAIIIs.2 pPLAI and pPLAIIs contain the GxSxG motif, which is part of the catalytic dyad S-D commonly found in other esterases, and they hydrolyze phospholipids and galactolipids.2-4 By comparison, pPLAIIIs have a non-canonical esterase box of GxGxG, and it has recently been demonstrated that pPLAIIIβ and δ are active acyl-hydrolyzing enzymes.5,6 pPLAI, IIs, and IIIs exhibit different substrate preferences. pPLAI hydrolyzes monogalactosyldiacylglycerol (MGDG) four times greater than phosphatidylglycerol (PG),2 whereas pPLAIIIβ uses PG four times greater than MGDG.5 The specific activity of pPLAIIδ is 5 times higher than that of pPLAIIIβ.3,5
In addition, pPLAIIIβ and δ have thioesterase activity, hydrolyzing acyl-CoAs to generate FFAs.5,6 pPLAIIIβ and δ are associated primarily with the plasma membrane5,6 where acyl-CoA binding proteins (ACBP1 and 2) also reside.7 In addition, ACBP2 binds lysophosphatidylcholine and a lysophospholipase, both of which may function potentially downstream of pPLAIIIs.8 The results imply that pPLAIIIs have access to acyl-CoA, and there may be plasma membrane-based protein complexes that use acyl-CoA for metabolism and for generating signaling FFAs. It remains to be determined whether the other 3 pPLAIIIs have the acyl-CoA-hydrolyzing thioesterase activity, and what cellular and physiological functions are for such activity.
The release of free fatty acids from membrane lipids has been implicated in many cellular processes, ranging from signaling messenger production, hormone synthesis, lipid remodeling, membrane repair, and cellular deterioration.1-6 pPLAI has been shown to contribute to resistance to Botrytis cinerea, possibly by mediating the basal levels of jasmonate production.2 It has recently been implicated in plant response to light and auxin.9 pPLAIIα negatively modulates both plant response to bacterial pathogens and oxylipin production.3,10 pPLAIIβ impacts root elongation during phosphate deficiency, and pPLAIIγ and pPLAIIδ have been implicated in involvement in auxin responses.11,12 No overt morphological alterations occur with pPLAI or pPLAII-altered plants under normal laboratory growth conditions. By comparison, a striking phenotype of pPLAIII-altered Arabidopsis was observed in that overexpression (OE) of 35S::pPLAIIIβ and pPLAIIIδ results in plants with shorter and smaller stature.5,6 An earlier study isolated a short and stout Arabidopsis mutant sturdy which resulted from activation-tagging of pPLAIIIδ.13 pPLAIII-OE plants are deficient in anisotropic cell growth and decreased in cellulose content.5,6 This raises intriguing questions of how pPLAIII overexpression results in retardation of cell elongation.
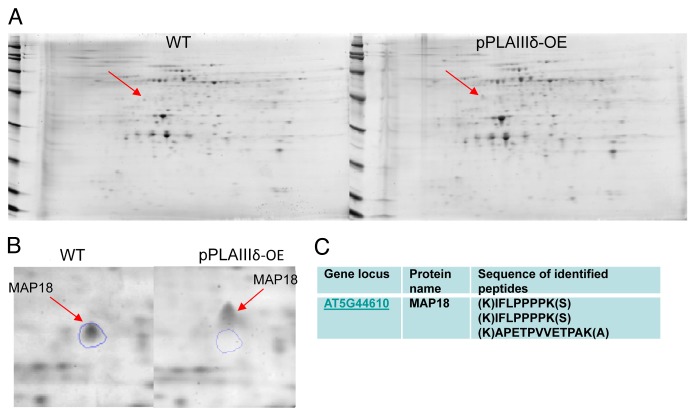
To probe the question, we conducted proteomic analyses comparing proteins isolated from seedlings of pPLAIIIδ-OE and WT. Since pPLAIIIδ was mostly associated with the plasma membrane,6 plasma membrane proteins were isolated by 2-phase partitioning, followed by 2-dimensional gel electrophoresis (Fig. 1). More than 500 protein spots were detected by a gel imaging system and the spot intensity and position on the gel were compared between wild-type and pPLAIIIδ-OE samples after gel alignment, spot averaging, and normalization. The comparison of gel images indicates that a dozen of protein spots were altered in intensity by at least 1.5-fold (P < 0.05) between wild-type and pPLAIIIδ-OE proteins. One protein has found to be shifted in mobility on SDA-PAGE between WT and pPLAIIIδ-OE seedlings. The protein spot in WT is approximately 43 kD whereas that in OE is about 5 kD greater. Amino acid sequencing of the spot identified the protein as the microtubule-associated protein MAP18. The calculated molecular weight of MAP18 is 18.5 kD, but MAP18 migrates much slower on SDS-polyacrylamide gels due to its high content of Pro (Fig. 1), and the reduced mobility is consistent with what was reported previously.14 The difference in size could mean that the protein is posttranslationally modified. MAP18 has multiple phosphorylation sites and thus may be phosphorylated,15 but evidence for posttranslational modifications of MAP18 has not been reported. In this study, MAP18 migrated similarly under isoelectric focusing electrophoresis, suggesting that MAP18s from WT and OE have similar pI. Whether protein phosphorylation, which often results in changing pI, or other types of modifications, such as glycosylation are involved require further investigation.
Figure 1. Comparative analysis of membrane proteins isolated from WT and OE Arabidopsis seedlings. (A) 2-D gel images of membrane proteins from one-week-old liquid-grown Arabidopsis seedlings. The plasma membrane fraction was isolated by 2-phase partitioning with 6% polyethylene glycol 3350, 6% Dextran T-500, and 8 mM KCl. The lower conductivity plasma membrane protein was prepared using the kit of perfect-FOCUS (G-BIOSCIENCE). Isoelectric focusing electrophoresis (IEF) was performed using a Bio-Rad Protean IEF cell, followed by SDS-PAGE on Bio-Rad Criterion pre-cast gels (8–16%). Gel images were acquired using a Typhoon 9410 variable mode imager (Amersham Biosciences, Pittsburgh, PA). Each gel was imaged sequentially at excitation/emission filter wavelengths. Image analysis was performed using SameSpots software (Nonlinear Dynamics, Durham, NC) for gel alignment, spot averaging and normalization. (B) One representative of 3 gel images indicating the mobility shift of a protein spot that subsequently identified as MAP18. Protein spots of interest were proteolytically digested and sequenced using an ABI QSTAR XL (Applied Biosystems/MDS Sciex) hybrid QTOF MS/MS mass spectrometer equipped with a nanoelectrospray source.
The alteration of MAP18 shed light onto the cellular effect resulting from pPLAIII overexpression. Map18 destabilizes cortical microtubules by inhibiting tubulin polymerization, affecting directional cell growth, and overexpressing MAP18 resulted in altered cortical microtubule arrays in cells and abnormal cell expansion.14 MAP18-OE resulted in decreased cell elongation growth and shorter hypocotyls,14 a phenotype similar to that of pPLAIII-OE Arabidopsis.5,6 pPLAIIIs and MAP18 are primarily associated with the plasma membrane.5,6,15 The plasma membrane and its associated microtubule cytoskeleton are vital to cellulose synthase delivery and the cellulose synthase complex trajectories on the plasma.16 MAPs have been reported to impact cellulose synthesis and deposition.17 In addition, a recent study indicates that MAP18 in pollen tubes affects actin organization with a Ca2+ -dependent F-actin-severing activity, and thus MAP18 may play a role in coordinating the microtubule and actin cytoskeletons.18 Furthermore, MAP18 can directly interact with phosphatidylinositol phosphates in a Ca2+ dependent manner.15 These data suggest that lipid mediators and membrane lipid environments affect MAP18 activity and thus cytoskeletal organization. Our data of activity assays in vitro showed that pPLAIIIδ hydrolyzed PC,6 but the in vivo substrate lipids for the enzyme remain to be determined. It will be of great interest in future studies to determine whether lipid mediators generated by pPLAIIIδ interact with MAP18 and how the membrane lipid changes in pPLAIII-OE alter the function of MAP18 and the microtubule and actin cytoskeletal organization.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the National Science Foundation (MCB-0920681; IOS-0818740).
Glossary
Abbreviations:
- FFA
free fatty acid
- MAP
microtubule associated protein
- MGDG, monogalactosyldiacylglycerol
pPLA, patatin-containing phospholipase A
- PG
phosphatidylglycerol
References
- 1.Scherer GF, Ryu SB, Wang X, Matos AR, Heitz T. Patatin-related phospholipase A: nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010;15:693–700. doi: 10.1016/j.tplants.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Devaiah SP, Pan X, Isaac G, Welti R, Wang X. AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. J Biol Chem. 2007;282:18116–28. doi: 10.1074/jbc.M700405200. [DOI] [PubMed] [Google Scholar]
- 3.La Camera S, Geoffroy P, Samaha H, Ndiaye A, Rahim G, Legrand M, Heitz T. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 2005;44:810–25. doi: 10.1111/j.1365-313X.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- 4.Rietz S, Dermendjiev G, Oppermann E, Tafesse FG, Effendi Y, Holk A, Parker JE, Teige M, Scherer GF. Roles of Arabidopsis patatin-related phospholipases a in root development are related to auxin responses and phosphate deficiency. Mol Plant. 2010;3:524–38. doi: 10.1093/mp/ssp109. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Bahn SC, Guo L, Musgrave W, Berg H, Welti R, Wang X. Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell. 2011;23:1107–23. doi: 10.1105/tpc.110.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Bahn SC, Fan C, Li J, Phan T, Ortiz M, Roth MR, Welti R, Jaworski J, Wang X. Patatin-related phospholipase pPLAIIIδ increases seed oil content with long-chain fatty acids in Arabidopsis. Plant Physiol. 2013;162:39–51. doi: 10.1104/pp.113.216994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao S, Chye ML. New roles for acyl-CoA-binding proteins (ACBPs) in plant development, stress responses and lipid metabolism. Prog Lipid Res. 2011;50:141–51. doi: 10.1016/j.plipres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Gao W, Li HY, Xiao S, Chye ML. Acyl-CoA-binding protein 2 binds lysophospholipase 2 and lysoPC to promote tolerance to cadmium-induced oxidative stress in transgenic Arabidopsis. Plant J. 2010;62:989–1003. doi: 10.1111/j.1365-313X.2010.04209.x. [DOI] [PubMed] [Google Scholar]
- 9.Effendi Y, Radatz K, Labusch C, Rietz S, Wimalasekera R, Helizon H, Zeidler M, Scherer GF. Mutants of phospholipase A (pPLA-I) have a red light and auxin phenotype. Plant Cell Environ. 2014;••• doi: 10.1111/pce.12278. [DOI] [PubMed] [Google Scholar]
- 10.Yang WY, Zheng Y, Bahn SC, Pan XQ, Li MY, Vu HS, Roth MR, Scheu B, Welti R, Hong YY, et al. The patatin-containing phospholipase A pPLAIIα modulates oxylipin formation and water loss in Arabidopsis thaliana. Mol Plant. 2012;5:452–60. doi: 10.1093/mp/ssr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rietz S, Holk A, Scherer GF. Expression of the patatin-related phospholipase A gene AtPLA IIA in Arabidopsis thaliana is up-regulated by salicylic acid, wounding, ethylene, and iron and phosphate deficiency. Planta. 2004;219:743–53. doi: 10.1007/s00425-004-1275-9. [DOI] [PubMed] [Google Scholar]
- 12.Labusch C, Shishova M, Effendi Y, Li M, Wang X, Scherer GF. Patterns and timing in expression of early auxin-induced genes imply involvement of phospholipases A (pPLAs) in the regulation of auxin responses. Mol Plant. 2013;6:1473–86. doi: 10.1093/mp/sst053. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Cerny RE, Bhat DS, Brown SM. Cloning of an Arabidopsis patatin-like gene, STURDY, by activation T-DNA tagging. Plant Physiol. 2001;125:573–84. doi: 10.1104/pp.125.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhu L, Liu B, Wang C, Jin L, Zhao Q, Yuan M. Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell. 2007;19:877–89. doi: 10.1105/tpc.106.048579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Nagasaki-Takeuchi N, Ide Y, Maeshima M. An Arabidopsis hydrophilic Ca2(+) -binding protein with a PEVK-rich domain, PCaP2, is associated with the plasma membrane and interacts with calmodulin and phosphatidylinositol phosphates. Plant Cell Physiol. 2010;51:366–79. doi: 10.1093/pcp/pcq003. [DOI] [PubMed] [Google Scholar]
- 16.Endler A, Persson S. Cellulose synthases and synthesis in Arabidopsis. Mol Plant. 2011;4:199–211. doi: 10.1093/mp/ssq079. [DOI] [PubMed] [Google Scholar]
- 17.Oda Y, Iida Y, Kondo Y, Fukuda H. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol. 2010;20:1197–202. doi: 10.1016/j.cub.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Zhang Y, Kang E, Xu Q, Wang M, Rui Y, Liu B, Yuan M, Fu Y. MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell. 2013;25:851–67. doi: 10.1105/tpc.113.110528. [DOI] [PMC free article] [PubMed] [Google Scholar]