Abstract
Plants maximize their chances to survive adversities by reprogramming their development according to environmental conditions. Adaptive variations in the timing to flowering reflect the need for plants to set seeds under the most favorable conditions. A complex network of genetic pathways allows plants to detect and integrate external (e.g., photoperiod and temperature) and/or internal (e.g., age) information to initiate the floral transition. Furthermore different types of environmental stresses play an important role in the floral transition. The emerging picture is that stress conditions often affect flowering through modulation of the photoperiodic pathway. In this review we will discuss different modes of cross talk between stress signaling and photoperiodic flowering, highlighting the central role of the florigen genes in this process.
Keywords: plant stress response, Florigen, drought escape, plant adaptive development, photoperiodic flowering
Photoperiodic-Dependent Activation of Flowering
After the floral transition the shoot apical meristem (SAM) changes its identity switching from vegetative to reproductive. In annual Arabidopsis ecotypes, the transition to flowering is strongly promoted by variations in day length (photoperiod). The photoperiodic pathway promotes flowering when Arabidopsis plants are exposed to long days (LDs) conditions (typical of spring and summer). Photoperiodic flowering is the result of complex interactions between the circadian clock (an endogenous timekeeping mechanism) and external cues, which ultimately results in the activation of a set of floral genes.1 Central to photoperiod-dependent flowering is the pattern of accumulation of the flowering protein CONSTANS (CO).2-4 CO expression is regulated transcriptionally by the circadian clock through the GIGANTEA (GI)-FLAVIN-BINDING, KELCH REPEAT, F-BOX (FKF1) complex.5,6 LDs also promote the stabilization of CO protein at the end of a LD via activation of the photoreceptors PHYTOCROME A, CRYPTOCHROME 1 and 2 (CRY1 and 2).3 CO protein promotes the transcriptional activation of the florigen genes FLOWERING LOCUS T (FT) and TWIN SISTER OF FT (TSF) in the phloem companion cells.7-10 FT and FT-likes proteins encode small proteins with similarity to the Raf Kinase Inhibitor Proteins (RKIP). They usually act as systemic signals, since these proteins are able to move between cells.11 FT protein moves from the leaves to the SAM where it interacts with the SAM-specific bZIP transcription factors FLOWERING LOCUS D (FD) and FD PARALOG (FDP) to initiate the floral transition.12-16 Here, the FT/FD heterodimer activates several MADS box-type transcription factors, namely SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), APETALA1, and FRUITFUL, responsible for triggering the floral transition.17,18
Florigen gene expression has been demonstrated to play a pivotal role in photoperiodic flowering in different plants including Arabidopsis, a facultative LD plant and Rice (Oryza sativa), a facultative short day (SD) plant.19 However, florigen expression is not always dependent upon photoperiod variations as in the case of the day neutral plant Tomato (Solanum lycopersicum).20 This implies that florigen upregulation can also occur in response to internal or external stimuli other than variations in day length. The data reviewed here reinforces the idea that the photoperiodic pathway and the florigen genes are central nodes of a wider network receiving a multitude of external inputs. Furthermore, mechanisms that couple photoperiodic flowering with stress acclimation are emerging.
Stress-Dependent Activation of FT Expression
LDs promote flowering via activation of the florigen genes in Arabidopsis. However, it is now apparent that the FT promoter conveys several environmental information, in some cases independent of day length. Many plant species are induced to flower following drought stress which results in a drought escape response - DE -.21-27 The onset of DE maximizes the chances to set seeds, thus “escaping” from a potentially lethal drought condition.28 We have recently shown that in Arabidopsis DE occurs under LDs but not SDs, thus revealing a strong interdependence of certain drought responses on photoperiod. Genetic screens showed that photoperiod-stimulated GI activity is necessary and sufficient to trigger a drought dependent activation of the florigen genes FT and TSF.29
The phytohormone ABA plays a pivotal role in mediating several drought adaptive mechanisms although its precise role in flowering is still poorly understood.30 Genetic and expression data suggest a role for ABA in DE response, through the activation of the florigen genes.29 aba1 mutants are impaired in ABA biosynthesis and display reduced accumulations of FT and TSF transcripts, especially under drought conditions. In addition to FT and TSF another FT-like genes MOTHER OF FT AND TFL1 (MFT) all appear to be positively regulated by ABA.31,32 Taken together these data argue in favor for a positive role for endogenous ABA in flowering via potentiation of florigen-like genes in a photoperiodic manner.
Some plants use drought stress as a primary cue to flowering. Recent studies suggest that drought stress is involved in the upregulation of the florigen genes in the tropical tree Shorea beccariana.33 Moderate increases in drought index promote an increase of SbFT transcript accumulations early in bud development, preceding flower morphological changes. Shorea beccariana grows at the equator where day length and temperature are constant throughout the year. It is thus plausible that drought spells could represent a major external cue to trigger mass flowering in this species via direct activation of FT independent of photoperiod. Photoperiod-independent modes of activation of FT exist also in Arabidopsis where an increase in ambient temperature is reflected in augmented FT transcript accumulation.34 A key component of this mechanism is the bHLH transcription factor PHYTOCHROME INTERACTING FACTOR 4 (PIF4) directly activating FT expression largely independent of CO.35 It is intriguing to note that occurrence of drought episodes often coincides with an increase in ambient temperature, at least in temperate climates. Whether ambient temperature also plays a regulatory role in DE response is thus an interesting question.
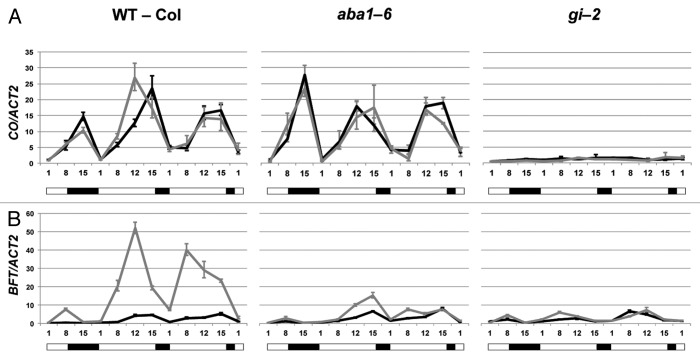
Unlike the thermosensory pathway, the mechanism through which drought stimuli affect FT activation is unknown. Drought stress results in an increase in FT expression with no evident effect on the physiological circadian oscillation of FT.29,36 Because the pattern of FT transcript accumulation depends on variations in CO protein, drought might directly affect CO expression. FLOWERING BHLH 1 (FBH1), a CO positive activator, is phosphorylated in vivo following ABA signaling activation.37,38 Although the precise role of phosphorylation on FBH1 protein function is still unknown, this finding could support a role for ABA in CO transcription under drought conditions. Also, EID1-like protein 3 (EDL3), a positive regulator of ABA signaling is an activator of CO. EDL3 transcript is upregulated following ABA applications.39 Although these findings point to a link between ABA and photoperiodic flowering via CO transcript accumulations we could find only minor variations in CO transcript in wild-type or aba1 mutant plants subjected to drought stress (Fig. 1A).
Figure 1. Real-time qPCR of CO (A) or BFT (B) transcripts in 3 wk-old wild-type (Col-0), aba1-6 or gi-2 seedlings. Plants were subjected to normal watering (black lines) or reduced watering (gray lines) regimes and harvested at the indicated time points in coincidence with the light phase (open bar) or in the dark (black bar) during a SDs to LDs shift. At each time point, values represent fold change variations of CO or BFT transcript levels relatively to Col-0 under NW. ACT2 expression was used for normalization; error bars represent SD of 2 technical replicates. A representative experiment of 2 biological replicates is shown.
Drought (via ABA) could affect CO protein activity or stability. For example, besides the well-established role in seed germination the ABA signaling protein ABA INSENSITIVE 3 (ABI3) is involved in the control of flowering time. abi3 mutants are early flowering under both SDs and LDs while ABI3 overexpression results in an increased vegetative phase under LDs.40 ABI3 binds to the CO CCT (CO, CO-like, TOC1) domain involved in the recruitment of the CO protein to the promoter of FT.41,42 Thus, interaction with ABI3 may interfere with CO (and perhaps other CCT domain - containing proteins) binding to DNA. Intriguingly, following spray with ABA, abi3 mutants display high levels of TSF, suggesting a repressive role for ABI3 on TSF expression.43 In germinating seeds, the expression of MFT is downregulated by ABI3.31 ABI3 may thus act as a negative regulator of flowering through downregulation of florigen-like genes.
Despite the GI-CO module being responsible for most of the activation of FT, FT upregulation may occur independently of either CO or GI. For example, warm temperatures results in an acceleration of flowering in the absence of GI and CO activities.34 In contrast, a DE response can be induced in co but not gi mutants, although it is unknown whether drought can stimulate FT upregulation in the absence of CO activity.29 Nonetheless this observation suggests that drought signals can overcome CO action to trigger flowering, provided that GI is photoperiod-stimulated. In support of the key role of GI in DE, ABA hypersensitive mutants are early flowering under LDs, but not under SDs. Thus ABA hyper-activation cannot override the requirement of photoperiod-stimulated GI in flowering.29 Examples of GI dependent but CO-independent mechanisms for FT activation have been described.35,44-48 However it is currently unclear how drought might affect GI-derived signals upon the FT promoter. Other pathways could facilitate the responsiveness of FT to photoperiod-stimulated GI. For example, similarly to gi, cry2 mutants have a defective DE response, despite constitutively accumulating increased ABA levels compared with wild type.29,49 Therefore, one could speculate that also CRY2 may participate in the GI- and ABA-dependent activation of FT.
Arabidopsis has 3 florigen genes, of which 2 (FT and TSF) act redundantly to mediate photoperiodic flowering.8,50,51 Despite this functional redundancy, FT and TSF transcripts are found in a non-overlapping pattern of expression.8 Also, TSF expression (but not FT) can be activated under SDs following exogenous applications of a synthetic Cytokinin (CK).52 Thus, unlike ABA, CKs do not require a photoperiodic input for the activation of TSF. Because of this reduced dependence on photoperiod, TSF upregulation might also occur in the absence of CO (although still in a GI-dependent manner) under drought conditions and contribute to the DE response observed in co mutants. In conclusion, more work is needed to clarify the mode of FT and FT-like genes activations under drought conditions and their specific interdependence with the photoperiodic pathway machinery.
Stress Dependent Downregulation of FT Expression
Not all abiotic stresses are interpreted as an escape signal. For example, cold stress delays flowering and alters the diurnal oscillation of FT expression even under inductive photoperiodic conditions. It has been shown that cold temperatures induce the degradation of CO protein via an ubiquitin/proteasome pathway that involves the E3 ubiquitin ligase HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1 (HOS1).53 Under normal growth temperature HOS1 acts as a general component of photoperiodic flowering by destabilizing CO protein in response to daylight signals.54 Modulation of HOS1 activity by light and cold temperature plays a crucial role in the daily pattern of CO accumulation, thus revealing yet another example of interplay between environmental cues and day length perception via florigen regulation.
A different osmotic stress, salinity, delays flowering in Arabidopsis by interfering with the photoperiodic pathway. Interestingly salt affects FT at 2 different levels, transcriptional and post-transcriptional. Salt stress promotes GI protein degradation through an unknown ubiquitin/proteasome pathway.55 Consequently, salt negatively regulates CO and FT transcripts accumulation. Salt stress delays flowering by activating the floral repressor BROTHER OF FT (BFT), a florigen-like protein with opposite function to FT.56 BFT competes with FT for the binding to FD, thus delaying the switch to flowering. BFT is strongly responsive to drought stress and ABA.57 We also confirmed that BFT can be transcriptionally activated under drought conditions in an ABA dependent manner and this regulation is dependent on GI (Fig. 1B). Thus, BFT expression is subject to a similar regulatory mechanism that orchestrates the activation of FT and TSF and is responsible for the DE response. However, the physiological role of BFT in DE is unclear since under drought conditions the positive regulation of flowering (i.e., via FT and TSF) clearly prevails over BFT. One could hypothesize that the balance between florigen and anti-florigen proteins is necessary to generate an optimal duration of reproductive development according to environmental stress. In this sense BFT may buffer FT action and prevent a premature interruption of inflorescence development. Deciphering the regulatory logic of the different florigen genes is thus an important goal to gain insights into the different flowering adaptations to stress as well as the mechanisms that govern crop seed yield under adverse conditions.
Future challenges: coordination of escape and tolerance strategies
A question arise as to how plants might coordinate flowering networks with tolerance responses, which allow individual cells to survive under stress conditions. GI is emerging as a key node connecting different abiotic responses with flowering time. gi mutants display different phenotypes including an increased salt tolerance.55 GI directly binds to SALT OVERLY SENSITIVE 2 (SOS2) protein and prevents its action under normal growth condition. Salt stress triggers the degradation of GI, thus releasing SOS2 and activating a salt-stress tolerance pathway. Besides salt, GI affects several developmental transitions (e.g., seedling photomorphogenesis and flowering time) as well as different environmental responses (starch accumulation, sucrose metabolism, sensitivity to light and oxidative stress).48,58-62 Furthermore GI controls guard cell activity.63 GI could coordinate different responses through a process of sequestration and release of interacting partners.55 In this model GI stability plays a key role through which plants can coordinately regulate independent processes with flowering.
In conclusion, plant adaptation to stress is complex and involves different strategies. In Arabidopsis the escape strategy requires a positive integration between photoperiodic and drought-dependent signals. A floral delay strategy takes place upon conditions where growth restraint provides an adaptive advantage over an escape, namely on salt.64 In all these cases, modulation of florigen genes represents the common central thread for how differential flowering strategies are enacted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Frantisek Baluska for kindly inviting this review and an anonymous reviewer for comments. This work was supported by a starting grant “piano di sviluppo di ateneo 2014” from the University of Milan to LC and the AGRISOST project from Fondazione Umberto Veronesi per il Progresso delle Scienze, Milano, to MG. ART is supported by a PhD studentship from the University of Milan.
References
- 1.Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr Opin Plant Biol. 2010;13:83–9. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–57. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 3.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–6. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 4.Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–20. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293–7. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- 6.Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–5. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–26. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005;46:1175–89. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- 9.Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–5. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–2. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 11.Wigge PAFT. FT, a mobile developmental signal in plants. Curr Biol. 2011;21:R374–8. doi: 10.1016/j.cub.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–4. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis. Plant Cell. 2013;25:820–33. doi: 10.1105/tpc.113.109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008;49:1645–58. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu J, Warthmann N, Küttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–60. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–3. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 17.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–6. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 18.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–9. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 19.Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature. 2003;422:719–22. doi: 10.1038/nature01549. [DOI] [PubMed] [Google Scholar]
- 20.Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci U S A. 2006;103:6398–403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu JL, Lafitte HR, Gao YM, Fu BY, Torres R, Li ZK. QTLs for drought escape and tolerance identified in a set of random introgression lines of rice. Theor Appl Genet. 2005;111:1642–50. doi: 10.1007/s00122-005-0099-8. [DOI] [PubMed] [Google Scholar]
- 22.Lafitte HR, Li ZK, Vijayakumar CHM, Gao YM, Shi Y, Xu JL, Fu BY, Yu SB, Ali AJ, Domingo J, et al. Improvement of rice drought tolerance through backcross breeding: Evaluation of donors and selection in drought nurseries. Field Crops Res. 2006;97:77–86. doi: 10.1016/j.fcr.2005.08.017. [DOI] [Google Scholar]
- 23.Franks SJ, Sim S, Weis AE. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci U S A. 2007;104:1278–82. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franks SJ. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 2011;190:249–57. doi: 10.1111/j.1469-8137.2010.03603.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharp RG, Else MA, Cameron RW, Davies WJ. Water deficits promote flowering in Rhododendron via regulation of pre and post initiation development. Sci Hortic (Amsterdam) 2009;120:511–7. doi: 10.1016/j.scienta.2008.12.008. [DOI] [Google Scholar]
- 26.Ivey CT, Carr DE. Tests for the joint evolution of mating system and drought escape in Mimulus. Ann Bot. 2012;109:583–98. doi: 10.1093/aob/mcr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherrard ME, Maherali H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution. 2006;60:2478–89. doi: 10.1111/j.0014-3820.2006.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 28.Verslues PE, Juenger TE. Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Curr Opin Plant Biol. 2011;14:240–5. doi: 10.1016/j.pbi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Riboni M, Galbiati M, Tonelli C, Conti L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiol. 2013;162:1706–19. doi: 10.1104/pp.113.217729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot. 2007;58:221–7. doi: 10.1093/jxb/erl164. [DOI] [PubMed] [Google Scholar]
- 31.Xi W, Liu C, Hou X, Yu H. MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell. 2010;22:1733–48. doi: 10.1105/tpc.109.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo SY, Kardailsky I, Lee JS, Weigel D, Ahn JH. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1) Mol Cells. 2004;17:95–101. [PubMed] [Google Scholar]
- 33.Kobayashi MJ, Takeuchi Y, Kenta T, Kume T, Diway B, Shimizu KK. Mass flowering of the tropical tree Shorea beccariana was preceded by expression changes in flowering and drought-responsive genes. Mol Ecol. 2013;22:4767–82. doi: 10.1111/mec.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2006;2:e106. doi: 10.1371/journal.pgen.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484:242–5. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Z, Ma X, Guo H, Sukiran NL, Guo B, Assmann SM, Ma H. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell. 2013;25:3785–807. doi: 10.1105/tpc.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito S, Song YH, Josephson-Day AR, Miller RJ, Breton G, Olmstead RG, Imaizumi T. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proc Natl Acad Sci U S A. 2012;109:3582–7. doi: 10.1073/pnas.1118876109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Xue L, Batelli G, Lee S, Hou Y-J, Van Oosten MJ, Zhang H, Tao WA, Zhu J-K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci U S A. 2013;110:11205–10. doi: 10.1073/pnas.1308974110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koops P, Pelser S, Ignatz M, Klose C, Marrocco-Selden K, Kretsch T. EDL3 is an F-box protein involved in the regulation of abscisic acid signalling in Arabidopsis thaliana. J Exp Bot. 2011;62:5547–60. doi: 10.1093/jxb/err236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Garreton V, Chua N-H. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 2005;19:1532–43. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurup S, Jones HD, Holdsworth MJ. Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J. 2000;21:143–55. doi: 10.1046/j.1365-313x.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 42.Tiwari SB, Shen Y, Chang H-C, Hou Y, Harris A, Ma SF, McPartland M, Hymus GJ, Adam L, Marion C, et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010;187:57–66. doi: 10.1111/j.1469-8137.2010.03251.x. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M, Ketterling MG, Li Q-B, McCarty DR. Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol. 2003;132:1664–77. doi: 10.1104/pp.103.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawa M, Kay SA. GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2011;108:11698–703. doi: 10.1073/pnas.1106771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iñigo S, Alvarez MJ, Strasser B, Califano A, Cerdán PD. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J. 2012;69:601–12. doi: 10.1111/j.1365-313X.2011.04815.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim W-Y, Hicks KA, Somers DE. Independent roles for EARLY FLOWERING 3 and ZEITLUPE in the control of circadian timing, hypocotyl length, and flowering time. Plant Physiol. 2005;139:1557–69. doi: 10.1104/pp.105.067173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung J-H, Seo Y-H, Seo PJ, Reyes JL, Yun J, Chua N-H, Park C-M. The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell. 2007;19:2736–48. doi: 10.1105/tpc.107.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, et al. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–70. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boccalandro HE, Giordano CV, Ploschuk EL, Piccoli PN, Bottini R, Casal JJ. Phototropins but not cryptochromes mediate the blue light-specific promotion of stomatal conductance, while both enhance photosynthesis and transpiration under full sunlight. Plant Physiol. 2012;158:1475–84. doi: 10.1104/pp.111.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol. 2005;137:149–56. doi: 10.1104/pp.104.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009;60:614–25. doi: 10.1111/j.1365-313X.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 52.D’Aloia M, Bonhomme D, Bouché F, Tamseddak K, Ormenese S, Torti S, Coupland G, Périlleux C. Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 2011;65:972–9. doi: 10.1111/j.1365-313X.2011.04482.x. [DOI] [PubMed] [Google Scholar]
- 53.Jung J-H, Seo PJ, Park C-M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. J Biol Chem. 2012;287:43277–87. doi: 10.1074/jbc.M112.394338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazaro A, Valverde F, Piñeiro M, Jarillo JA. The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell. 2012;24:982–99. doi: 10.1105/tpc.110.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim W-Y, Ali Z, Park H-J, Park SJ, Cha J-Y, Perez-Hormaeche J, Quintero FJ, Shin G, Kim MR, Qiang Z, et al. Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat Commun. 2013;4:1352. doi: 10.1038/ncomms2357. [DOI] [PubMed] [Google Scholar]
- 56.Ryu JY, Lee HJ, Seo PJ, Jung JH, Ahn JH, Park CM. The Arabidopsis floral repressor BFT delays flowering by competing with FT for FD binding under high salinity. Mol Plant. 2014;7:377–87. doi: 10.1093/mp/sst114. [DOI] [PubMed] [Google Scholar]
- 57.Chung KS, Yoo SY, Yoo SJ, Lee JS, Ahn JH. BROTHER OF FT AND TFL1 (BFT), a member of the FT/TFL1 family, shows distinct pattern of expression during the vegetative growth of Arabidopsis. Plant Signal Behav. 2010;5:1102–4. doi: 10.4161/psb.5.9.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurepa J, Smalle J, Van Montagu M, Inzé D. Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J. 1998;14:759–64. doi: 10.1046/j.1365-313x.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- 59.Cao S, Ye M, Jiang S. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep. 2005;24:683–90. doi: 10.1007/s00299-005-0061-x. [DOI] [PubMed] [Google Scholar]
- 60.Seo E, Lee H, Jeon J, Park H, Kim J, Noh Y-S, Lee I. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell. 2009;21:3185–97. doi: 10.1105/tpc.108.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18:4679–88. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huq E, Tepperman JM, Quail PH. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci U S A. 2000;97:9789–94. doi: 10.1073/pnas.170283997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ando E, Ohnishi M, Wang Y, Matsushita T, Watanabe A, Hayashi Y, Fujii M, Ma JF, Inoue S, Kinoshita T. TWIN SISTER OF FT, GIGANTEA, and CONSTANS have a positive but indirect effect on blue light-induced stomatal opening in Arabidopsis. Plant Physiol. 2013;162:1529–38. doi: 10.1104/pp.113.217984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–4. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]