Abstract
Epigenetic modification of the genome via cytosine methylation is a dynamic process that responds to changes in the growing environment. This modification can also be heritable. The combination of both properties means that there is the potential for the life experiences of the parental generation to modify the methylation profiles of their offspring and so potentially to “pre-condition” them to better accommodate abiotic conditions encountered by their parents. We recently identified high vapor pressure deficit (vpd)-induced DNA methylation at 2 gene loci in the stomatal development pathway and an associated reduction in leaf stomatal frequency.1 Here, we test whether this epigenetic modification pre-conditioned parents and their offspring to the more severe water stress of periodic drought. We found that 3 generations of high vpd-grown plants were better able to withstand periodic drought stress over 2 generations. This resistance was not directly associated with de novo methylation of the target stomata genes, but was associated with the cmt3 mutant’s inability to maintain asymmetric sequence context methylation. If our finding applies widely, it could have significant implications for evolutionary biology and breeding for stressful environments.
Keywords: Arabidopsis thaliana, abiotic stress, drought, methylation, transgenerational epigenetic heritability
One important function of cytosine methylation in plant genomes is to provide protection against the activation of harmful, transposable, and repeated sequence elements.2 Under certain stressful conditions, the maintenance of methylated sites can become weakened, allowing elements to become activated, leading to increased retrotransposition and newly created, environmental sensitivity.3 DNA methylation is therefore not permanently fixed, but is dynamic and reversible with abiotic stress. Cytosine methylation is usually located away from the 5 prime of Arabidopsis thaliana gene coding regions to protect important endogenes from transcriptional silencing.4 However, where methylation exists in promoter regions, presumably primarily to suppress the expression of harmful elements, the presence of this methylation can also act to silence expression of the endogenous gene.5 Release, imposition, and spread of methylation under adverse environmental conditions around harmful elements can also influence proximal gene expression6 as we recently reported for 2 gene loci in the stomatal development pathway (SPCH and FAMA) in plants grown under increased evaporative demand.1 As methylation is heritable through cell divisions, this mechanism might provide a “memory” of adverse conditions that allows for altered gene expression patterns over time and so lead to the generation of more tolerant phenotypes.7
In our plants, increased methylation in and around the SPCH and FAMA loci was associated with decreased expression of both target genes and a reduction in Stomatal Index (SI; stomata as a percentage of epidermal cells), although not in their stomatal density (stomata.mm−2). We hypothesized that these transcriptional and phenotypic changes, mediated by methylation, could increase the tolerance of our plants to more severe water stress. Epigenetic “priming” against biotic stress leads to enhanced resistance in the progeny compared with the parental generation8 and the progeny of plants salt-stressed at 25 or 75 mM NaCl also show increased tolerance to 125–150 mM NaCl.9 We found that constant exposure to high vpd protected A. thaliana ‘Landsberg erecta’ from the negative effect on leaf chlorophyll content of a 4-day periodic drought experienced 40 d post-germination (Fig. 1A) and also from the final drought-induced reduction in plant biomass at harvest (following 4 d drought and subsequent re-watering) (Fig. 1B). Surprisingly, pre-conditioning with high vpd was even associated with an increase in final dry weight following drought.
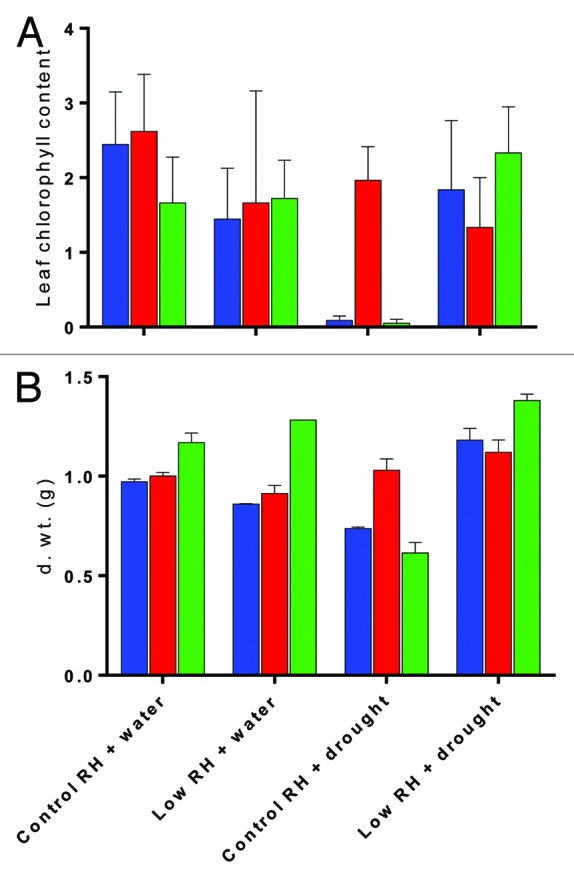
Figure 1. A The relative total chlorophyll content (measured in arbitrary units using a CL-01 chlorophyll meter (Hansatech, King’s Lynn, UK)) of the fifth true leaf of Arabidopsis thaliana ‘Landsbergerecta’. Blue bars = wild type, red bars = cmt3, and green bars = drm1/2, and (B) the final plant biomass (g) in control (65% RH) and high vapor pressure deficit (45% Low RH) when well-watered (+ water) and following 4 d drought and subsequent re-watering (+ drought). There was a significant interaction (Two-way ANOVA p = < 0.05) between RH treatment and drought for both chl. content and d. wt. such that drought only reduced chl. under control (p = < 0.05) and reduced and increased d. wt. respectively with control and high vapor pressure deficit (p = < 0.001) (Post-hoc Bonferroni multiple comparisons).
To understand whether this response was directly associated with the increased methylation of the 2 stomatal pathway genes, we compared the responses of mutants deficient in the methyltransferases DRM2 and CMT3 to the same treatments. In the drm1/2 mutant, there is no additional de novo methylation of SPCH or FAMA in comparison to the wild type (WT) grown under high vpd and no associated reduction in SI.1 Drought reduced both chlorophyll content and final dry biomass of drm1/2. However, in common with the WT plants, neither chlorophyll content nor final biomass was reduced by drought when the plants had been pre-exposed to high vpd (Fig. 1A and B). It follows that DRM1/2 and specifically de novo methylation (a primary function of DRM2) is not responsible for the preconditioning that increased resilience to periodic drought following exposure to high vpd. Thus, stress-induced methylation of the SPCH and FAMA genes along with resultant reductions in stomatal frequency were not directly responsible for the changed response of vpd-preconditioned plants to drought. When the cmt3 mutants were placed under high vpd, both SPCH and FAMA became hyper-methylated (similar to the WT) and the plants exhibited reduced SI. However, following drought treatment, cmt3 was able to mimic the WT response to high vpd pre-conditioning in control conditions, such that leaf chlorophyll content and final dry biomass were unaffected by drought (Fig. 1A and B). This suggests that, although de novo methylation of stomatal development genes under high vpd is not specifically required to improve subsequent drought-tolerance of pre-conditioned plants, the maintenance of asymmetric sequence methylation for which cmt3 is responsible is required in some form.
This finding also suggests 2 fairly obvious lines of future research: First, the replication of our observations in crop plants could provide new avenues in the search for genotypes and agronomic practices that enhance drought tolerance. One major cautionary note is that the epigenome of A. thaliana is relatively less extensive and complex than that of non-model plants10 but, at least with Brassica napus, it is possible to grow plants in the field that have reduced genome methylation and comparatively increased yield under moderate drought stress.11 Second, we may be able to protect plants against future stress by environmental pre-conditioning, analogous to the “hardening off” already practiced in micropropagation and transplant production and to deficit irrigation treatments.12 This would depend, to some extent, on the breeding potential of the response to pre-conditioning and its heritability.
More enticing would be the prospect of preconditioning filial generations (i.e., seed) against periodic drought. This would require the pre-conditioning effect to be maintained across meiotic, as well as mitotic, cell divisions. To investigate this possibility, we divided seeds from 3 generations of self-pollinated control and high vpd-treated plants. We then grew these plants in the 2 humidity environments and applied drought, as before. In this experiment, the effect of high evaporative demand during growth on drought response was positive for 2 generations but was outweighed by the effect of pre-conditioning the immediate parent (Fig. 2). In this relatively small-scale experiment and with a fast-growing annual plant, we did not find any significant effects on seed size, germination, or ontogeny. Environmental conditioning of the parent therefore appears to hold some promise as a treatment that protects against drought. Progeny of parents and grandparents that had both been treated with high vpd, however, did not significantly benefit from a further exposure to high vpd prior to drought. It has been speculated that the transgenerational epigenetic effects of stress will not persist beyond 1 filial generation without a repeated exposure to the stress.9 In contrast, we find that repeated exposure may actually change the response in the opposite direction. This finding could have relevance to those interested in the adaptive potential of epigenetic modifications, to those attempting epigenetic breeding, and is certainly important for plant physiological experimentation where the growth environment of parental plants may prove to be a significant factor in observed progeny responses.
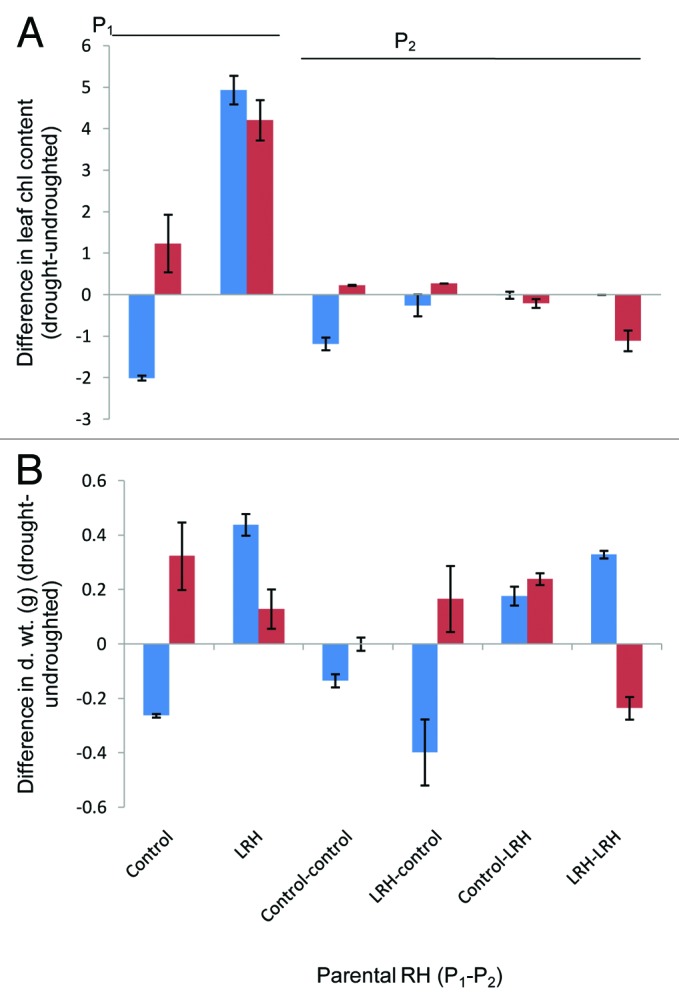
Figure 2. The difference in response of (A) leaf chlorophyll content (as before) and (B) final plant biomass (g) to drought (in comparison with the undroughted control) of generations of plant grown in high vapor pressure deficit or control conditions dependent on parental humidity treatment (x-axis; parental generation 1 (P1)–parental generation 2 (P2)) and pre-exposure of the current generation to high vpd (45% RH (LRH) red bars) or control (65% RH, blue bars) prior to drought. Data are means of measurements of 6 replicate plants (± SE) in each parent*treatment combination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Cocoa Research (UK) Ltd. and the Dutch Ministry of Agriculture, Nature, and Food Quality (Ministerie van LNV) for the funding that made this work possible and M Richardson for his excellent technical support of the controlled environment experiments.
Glossary
Abbreviations
- SI
stomatal index
- WT
wild type
- vpd
vapour pressure deficit
- RH
relative humidity
- chl.
chlorophylls
- d. wt.
dry weight
References
- 1.Tricker PJ, Gibbings JG, Rodríguez López CM, Hadley P, Wilkinson MJ. Low relative humidity triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. J Exp Bot. 2012;63:3799–813. doi: 10.1093/jxb/ers076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisch D. Epigenetic regulation of transposable elements in plants. Annu Rev Plant Biol. 2009;60:43–66. doi: 10.1146/annurev.arplant.59.032607.092744. [DOI] [PubMed] [Google Scholar]
- 3.Ito H, Gaubert H, Bucher E, Mirouze M, Vaillant I, Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature. 2011;472:115–9. doi: 10.1038/nature09861. [DOI] [PubMed] [Google Scholar]
- 4.Finnegan EJ. DNA methylation: a dynamic regulator of genome organization and gene expression in plants. In: Pua E-C, Davey MR, eds. Plant Developmental Biology – Biotechnological Perspectives: Volume 2. Heidelberg: Springer-Verlag, 2010:295-323. [Google Scholar]
- 5.Kinoshita Y, Saze H, Kinoshita T, Miura A, Soppe WJJ, Koornneef M, Kakutani T. Control of FWA gene silencing in Arabidopsis thaliana by SINE-related direct repeats. Plant J. 2007;49:38–45. doi: 10.1111/j.1365-313X.2006.02936.x. [DOI] [PubMed] [Google Scholar]
- 6.Huettel B, Kanno T, Daxinger L, Aufsatz W, Matzke AJ, Matzke M. Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO J. 2006;25:2828–36. doi: 10.1038/sj.emboj.7601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyko A, Kovalchuk I. Genome instability and epigenetic modification--heritable responses to environmental stress? Curr Opin Plant Biol. 2011;14:260–6. doi: 10.1016/j.pbi.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012;158:835–43. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, Hollunder J, Meins F, Jr., Kovalchuk I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LJ, Tricker PJ. Epigenomic plasticity within populations: its evolutionary significance and potential. Heredity (Edinb) 2010;105:113–21. doi: 10.1038/hdy.2010.25. [DOI] [PubMed] [Google Scholar]
- 11.Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, Van Breusegem F, Guisez Y, Bots M, Lambert B, et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc Natl Acad Sci U S A. 2009;106:20109–14. doi: 10.1073/pnas.0908755106. 10.1073_pnas.0908755106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang S, Zhang J. Controlled alternate partial root-zone irrigation: its physiological consequences and impact on water use efficiency. J Exp Bot. 2004;55:2437–46. doi: 10.1093/jxb/erh249. [DOI] [PubMed] [Google Scholar]


