Abstract
Meiosis is a specialized cell division program that results in the formation of haploid gametes (i.e., sperm and eggs) from diploid parental cells, and is essential for all sexually reproducing organisms. Crossover formation, the reciprocal exchange of genetic information during recombination, is critical for accurate meiotic chromosome segregation. Misregulation of crossover formation leads to genomic instability and aneuploidy (cells with the incorrect number of chromosomes), resulting in tumorigenesis, birth defects, miscarriages, and infertility in humans. Recently, a shuriken/Swiss army knife-like multi-nuclease complex has been implicated in processing various types of DNA repair intermediates. However, how these nucleases coordinate their functions during repair remained unclear. Our studies in C. elegans revealed genetic redundancies between these nucleases for meiotic crossover formation and that they promote distinct crossover control at different chromosome regions. Specifically, XPF-1 acts redundantly with both MUS-81 and SLX-1 to resolve Holliday junction recombination intermediates into crossover products at designated future crossover sites on chromosome arms. In contrast, SLX-1 is required for suppression of crossovers at the center region of chromosomes. Altogether, our studies have shed light on the interplay between structure-specific endonucleases and uncovered their ability to exert either positive or negative meiotic crossover control on a chromosome region-specific basis.
Keywords: homologous recombination, crossover control, meiosis, structure-specific endonuclease, chromosome bridge, Holliday junction resolution, double Holliday junction dissolution, crossover interference, crossover designation, chromosome domain
Introduction
Meiosis accomplishes the reduction of the chromosome number in half by following a single round of DNA replication with two consecutive rounds of cell division (meiosis I and II). The formation of crossovers via homologous recombination is essential for the production of chiasmata, physical attachments between homologous chromosomes, which secure their accurate separation at meiosis I. Failure in forming crossovers results in the missegregation of chromosomes at meiosis I and leads to infertility and miscarriages in adults as well as congenital abnormalities in newborns. Therefore, understanding the molecular mechanisms underlying the regulation of meiotic recombination is critical for human reproductive health.
Homologous recombination is initiated via induction of DNA double strand breaks (DSBs) by the conserved topoisomerase-like protein, SPO-11. DNA end resection and single-strand invasion of a homologous sequence, which serves as a repair template, leads to the formation of a recombination intermediate referred to as a double Holliday junction (dHJ). Resolution of the dHJs is the final step in homologous recombination and can result in either crossover or non-crossover formation. However, only crossovers will results in a physical attachment between homologs at meiosis.
The mechanism of homologous recombination is largely conserved from phage to humans. Recently, four kinds of HJ resolvases, MUS81 (Methyl Methanesulfonate and UV Sensitive 81)-EME1 (Essential Meiotic Endonuclease 1)/Mms4 (Methyl Methanesulfonate Sensitive 4), SLX1 (Synthetic Lethal of unknown (X) function 1)-SLX4, XPF (Xeroderma Pigmentosum group F)-ERCC1 (Excision Repair Cross-Complementation group 1), and GEN1 (XPG-like Endonuclease 1) were identified by genetic and biochemical analysis. Mus81–Mms4 (also known as Slx3–Slx2) and Slx1–Slx4 were first identified in a synthetic lethal screen in budding yeast performed in the absence of Sgs1, the BLM ortholog.1 XPF–ERCC1 is known as a nuclease required for nucleotide excision repair.2 Notably, a MEI-9/XPF mutant in Drosophila exhibited a 90% decrease in meiotic crossover formation, suggesting a role for this nuclease in HJ resolution.3 Recently, GEN1/Yen1 were identified as canonical HJ resolvases by biochemical analysis in human cells and budding yeast.4 Interestingly, SLX1, MUS81, and XPF associate with SLX4,5-9 and therefore, SLX4, the non-catalytic subunit of the SLX1–SLX4 complex, has been proposed to act as a scaffold protein for several structure-specific nucleases (Fig. 1). Coordinated action between SLX1 and MUS81 is required to resolve HJs in mice and humans.10-12 We and other groups found that XPF-1 acts redundantly with both MUS-81 and SLX-1 to promote meiotic crossover formation in C. elegans.8,13,14 Here, we discuss how structure-specific endonucleases coordinate their functions to either promote or suppress meiotic crossover formation in a chromosome region-specific manner.
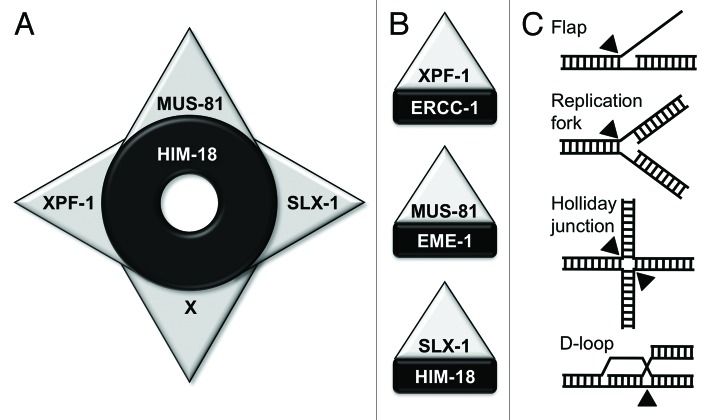
Figure 1. The HIM-18 complex: a molecular “shuriken” for DNA repair. (A) HIM-18 interacts with multiple nucleases including XPF-1, MUS-81 and SLX-1. X corresponds to SNM1B/Apollo in mammals. (B) Each unit of Shuriken: XPF-1-ERCC1, MUS-81-EME1 and SLX-1-HIM-18. (C) Representative DNA structures that arise during repair via homologous recombination. Black triangles indicate the sites of nicks induced by structure-specific endonucleases.
Conserved Protein–Protein Interactions Form a Molecular “Shuriken,” Which is a Multinuclease Complex for DNA Repair
A series of studies have recently identified various SLX4-interacting proteins (Table 1).1,5-7,9,15,16 In budding yeast, Slx4 was shown to interact with Slx1 and Rad1, a human XPF homolog. In flies, mice, and humans, SLX4 interacts with SLX1, MUS81, and XPF. In C. elegans, we found that HIM-18 interacts with SLX-1, MUS-81, and XPF-1.8 More recently, SNM1B/Apollo, a member of the highly conserved metallo-β-lactamase super family of nucleases, which plays a central role in interstrand crosslink repair, has also been identified as an SLX4 interactor in human cells.17 These protein–protein interactions are reminiscent of the multipronged shuriken, a traditional weapon used by Ninjas (Fig. 1). An important remaining question is whether these proteins form a single complex or heterodimers with SLX4. In yeast, Slx1–Slx4 and Rad1–Slx4 exist in a mutually exclusive manner, while these same proteins form a single complex in mammals.7,9,18 Further studies will reveal the state of HIM–18/SLX4-associated nucleases, especially what combinations are formed between subunits and their substrate specificities in C. elegans.
Table 1. Comparison between model organisms for HIM-18/SLX4-associated nucleases.
| S. pombe | S. cerevisiae | C. elegans | D. melanogaster | M. musculus | H. sapiens | |
|---|---|---|---|---|---|---|
| SLX1-SLX4 | YES | YES | YES | YES | YES | YES |
| MUS81-EME1 | YES | YES | YES | YES | YES | YES |
| XPF-ERCC1 | YES | YES | YES | YES | YES | YES |
| MUS81-SLX4 | - | - | YES | - | YES | YES |
| XPF-SLX4 | - | YES | YES | YES | YES | YES |
Yes indicates positive interactions. – indicates no detected interactions.
MUS-81 and SLX-1, but not XPF-1 and GEN-1, Have Overlapping Roles with HIM-6/BLM for DNA Repair
The Sgs1 helicase disassembles early meiotic recombination intermediates, both to generate non-crossovers and to prevent formation of aberrant multi-chromatid recombination intermediates in budding yeast.19,20 It is known that there is a functional overlap between Sgs1 and the Slx proteins in budding yeast.1 Similar to yeast, we found that mus-81 and slx-1, but not xpf-1 and gen-1, exhibit synthetic germline defects with him-6, the C. elegans BLM homolog.8 Specifically, more than 95% embryonic lethality was observed in mus-81;him-6 and slx-1;him-6 double mutants compared with 7.0%, 7.3%, and 59.1% in mus-81, slx-1, and him-6 single mutants, respectively.8 These results suggest that MUS-81 and SLX-1, but not XPF-1 and GEN-1, have overlapping roles with HIM-6, probably in processing recombination intermediates. HIM-18/SLX4 also exhibits synthetic germline defects with him-6, as evidenced by the elevated levels of chromosome bridges with associated RAD-51, a protein involved in strand invasion/exchange during repair, detected in mitotically proliferating germ cells.16 Therefore, the accumulation of unresolved recombination intermediates can result in mitotic catastrophe, further highlighting the important function of HIM-18 and its associated nucleases in maintaining genomic integrity.
XPF-1 Acts Redundantly With Both MUS-81 and SLX-1 to Promote Crossover Formation During Meiosis
To investigate whether the structure-specific endonucleases have an overlapping role during crossover formation, we measured crossover frequencies along three chromosome regions (left arm, center, and right arm), encompassing approximately 97% of the whole lengths of chromosomes V and X. The boundaries between these chromosome regions have been defined by utilizing single-nucleotide polymorphisms (SNPs) present in the C. elegans Bristol and Hawaiian strains.8,21 Crossover frequencies were not affected in any of the mus-81, xpf-1, slx-1, and gen-1 single mutants. However, crossover frequencies were significantly reduced in mus-81;xpf-1 and slx-1;xpf-1 double mutants on both chromosome V (65% and 81% of wild-type; P = 0.0041 and 0.0013, respectively, by the Fisher's Exact Test) and the X chromosome (40% and 68% of wild-type; P = 4.85E-08 and 3.04E-05, respectively).8 Therefore, this analysis revealed that XPF-1 acts redundantly with both MUS-81 and SLX-1 to promote crossover formation during C. elegans meiosis (Fig. 2). Our conclusion is also supported by the recent finding that MUS81-EME1 and SLX1-SLX4 act in the same pathway for HJ resolution in mice and human cells.10-12 In yeast, flies, and humans, a genetic interaction has been shown between GEN1 and MUS81-EME1.22-25 However, we could not find any evidence of a genetic interaction between these factors in C. elegans. Further studies will determine whether there are proteins compensating for the role of GEN1 in C. elegans. Interestingly, we observed that crossover frequencies were more reduced on the X chromosome compared with chromosome V.8 Gene expression is repressed along the X compared with the autosomes in the germline due to both meiosis-specific transcriptional silencing as well as dosage compensation that serves to halve transcription from both X chromosomes in hermaphrodites, equating it to the transcript levels stemming from the single X chromosome present in the X0 males.26-28 Therefore, there is higher nucleosome occupancy at X-linked gene promoters29 and an enrichment for histone modifications associated with transcriptional silencing detected on the X chromosome compared with the autosomes. This raises the interesting question of how chromatin state/architecture may influence the resolution of recombination intermediates.
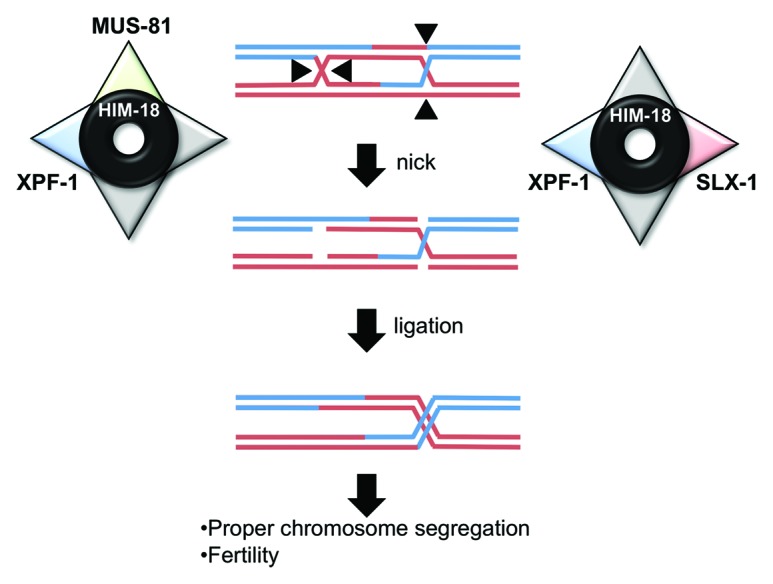
Figure 2. A model for crossover formation. XPF-1 has redundant roles with MUS-81 and SLX-1 to resolve dHJs in crossover formation.
SLX-1 is Required for Suppression of Crossover Formation at the Center Region of the Autosomes
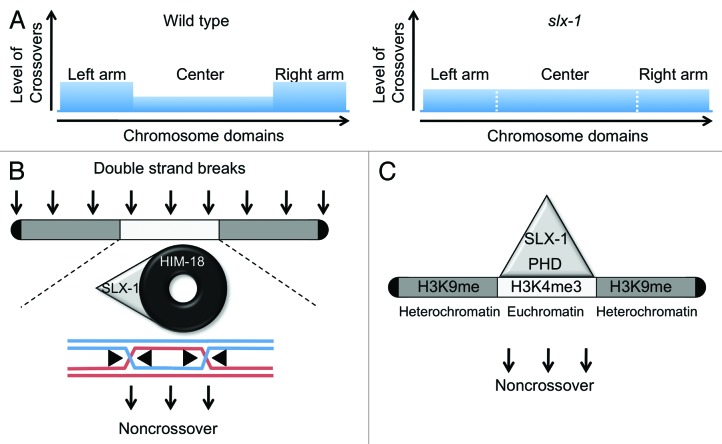
Crossover formation does not occur randomly along chromosomes. For example, crossovers are formed at the arm regions, but are rarely formed at centromeres and telomeres, in many species.30-32 It is known that crossover formation is suppressed at the center region of chromosomes in C. elegans (Fig. 3).21,33 However, the molecular mechanism underlying this chromosome region-dependent difference in crossover regulation is not understood.
Figure 3. Two non-mutually exclusive hypotheses for how SLX-1 suppresses crossovers at the center of the chromosomes. (A) While crossover formation is suppressed at the center region in wild type, it is not suppressed in slx-1 mutants. (B) SLX-1 may act as a non-crossover specific resolvase in a HIM-18-dependent manner. (C) SLX-1 may act as an epigenetic reader, via its PHD finger, recognizing boundaries between the arms and the center region of the chromosomes delimited in part by their differences in histone methylation.
Among the structure-specific endonucleases, we found that only SLX-1 is required for suppression of crossover formation at the center region of chromosome V, which encompasses 51% of its whole length. Specifically, 36% of total crossovers are observed at the center region in slx-1 mutants (1.7 cM/Mb), compared with only 21% in wild-type (1.1 cM/Mb; P = 0.0312). Nevertheless, the crossover frequency observed for the whole chromosome V is similar between slx-1 mutants (50 cM) and wild-type (48 cM). Interestingly, there are some distinct features between the arm and center regions of the chromosomes. First, while DNA repeat sequences and transposons are enriched at the arm regions, a high gene density is observed in the center region.34 Second, histone H3 lysine 9 methylation (H3K9me1/2/3), which is associated with heterochromatin, and the nuclear transmembrane protein LEM-2, are both enriched at the arm regions, while H3K4me3, which is associated with euchromatin, is enriched in the center regions during early embryogenesis and the L3 larval stage.35-37 While it remains to be determined whether these epigenetic marks and their distribution are maintained in the adult germline, we propose two possible models for how suppression of crossover formation is exerted by SLX-1 (Fig. 3). One possibility is that SLX-1 acts as a non-crossover-specific HJ resolvase at the center region of chromosome V, and presumably other autosomes. The second model is that SLX-1 may act as an epigenetic reader given that it has a PHD finger domain that is largely known to recognize modified histones such as H3K4me. This recognition would in turn recruit yet unknown non-crossover-promoting factors, resulting in non-crossover formation at the center region of the chromosomes.
Structure-Specific Endonucleases Play a Role in Crossover Interference
Crossover distribution is tightly regulated in most organisms including budding yeast, flies, worms, and mammals, as indicated by the fact that crossovers exhibit “interference” since a crossover in one location of the genome discourages the formation of another crossover nearby.38,39 C. elegans is an ideal system to understand the mechanism of crossover interference given that the number of crossover is tightly regulated during meiosis such that only and always one crossover occurs between each pair of homologous chromosomes.40,41 However, 4.1% and 7.1% of total crossover events are double crossovers in slx-1;xpf-1;gen-1 triple and mus-81 slx-1;xpf-1;gen-1 quadruple mutants, respectively.8 This raises two possibilities: (1) structure-specific endonucleases are redundantly required for crossover interference; or (2) if recombination intermediates are not properly resolved at the designated future crossover site, crossover interference is attenuated to accommodate multiple crossovers. Therefore, understanding the mechanisms underlying crossover interference, which remain a mystery for over more than a century, is an issue of critical importance in the field and will further clarify which of the possibilities outlined above might apply.
Unresolved Holliday Junctions Result in Chromosome Bridges Between Homologous Chromosomes
If recombination intermediates are not properly resolved, they are detected as chromatin bridges at anaphase of mitosis.42 In C. elegans meiosis, this can also be observed as chromosome bridges at late diakinesis and prometaphase I.16 Resolution of a HJ into a crossover, results in the formation of mature bivalents in wild-type. It is thought that unresolved HJs trapped as interhomolog connections result in the intrabivalent bridges observed in the resolvase mutants. Consistent with the reduction in crossover frequencies observed in mus-81;xpf-1 and slx-1;xpf-1 mutants, a high frequency of chromosome bridges in oocytes at the late diakinesis stage were also observed in these genetic backgrounds compared with wild-type and each single mutant.8 These results further support the model that XPF-1 functions in a redundant manner with both MUS-81 and SLX-1 for HJ resolution in order to promote the formation of functional or intact chiasmata.
Structure-Specific Endonucleases Act Downstream of Crossover Designation
Studies in budding yeast and worms suggest that the positions of the crossovers along chromosomes are designated prior to the resolution of recombination intermediates, which is the final step of crossover formation.16,43-46 We examined the localization of ZHP-3, the budding yeast Zip3 homolog containing a ring finger motif, which has been implicated as a pro-crossover factor,47 and determined that crossover designation was not affected in any of the nuclease mutants (single or combinatorial mutants).8 These results indicate that structure-specific endonucleases act downstream of crossover designation.
Hypothetical Model
Based on our results and recent chromosome-wide epigenetic analyses, we provide a hypothetical model for crossover control by structure-specific endonucleases. XPF-1, MUS-81, and SLX-1, which interact with HIM-18, promote crossover formation at the arm regions that are epigenetically marked by histone H3K9me in somatic cells. Repeat sequences are also enriched in these regions. Probably these nucleases are recruited to the arm regions and work coordinately. While H3K9me is enriched at the arm regions, it is low at the center region of autosomes and the right arm of the X chromosome, where instead there is enrichment for H3K4me. Given that SLX-1-dependent crossover suppression is observed at the center region of the autosomes,8,48 we propose that the PHD finger motif of SLX-1 might act as an epigenetic reader, thereby recognizing the H3K4me and promoting non-crossover formation at the center region (Fig. 3).
Concluding Remarks
We found that MUS-81 and SXL-1 act in the same pathway, while XPF-1 acts in a parallel pathway to promote meiotic crossover formation. Moreover, SLX-1 has the additional function of suppressing crossover formation at the center of the autosomes. Important future directions in this research field will include identifying additional resolvases for recombination intermediates and determining how SLX-1-dependent suppression of crossover occurs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a National Institutes of Health (NIH) grant R01GM072551, and a Milton Fund and Dr. Harold and Golden Lamport Research awards to Colaiácovo MP.
References
- 1.Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–18. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weeda G, Hoeijmakers JH. Genetic analysis of nucleotide excision repair in mammalian cells. Semin Cancer Biol. 1993;4:105–17. [PubMed] [Google Scholar]
- 3.Sekelsky JJ, McKim KS, Chin GM, Hawley RS. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics. 1995;141:619–27. doi: 10.1093/genetics/141.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, West SC. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–61. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- 5.Andersen SL, Bergstralh DT, Kohl KP, LaRocque JR, Moore CB, Sekelsky J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol Cell. 2009;35:128–35. doi: 10.1016/j.molcel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, Dong MQ, Ruse C, Yates JR, 3rd, Russell P, et al. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz IM, Hain K, Déclais AC, Gardiner M, Toh GW, Sanchez-Pulido L, Heuckmann JM, Toth R, Macartney T, Eppink B, et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol Cell. 2009;35:116–27. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Saito TT, Lui DY, Kim HM, Meyer K, Colaiácovo MP. Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9:e1003586. doi: 10.1371/journal.pgen.1003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svendsen JM, Smogorzewska A, Sowa ME, O’Connell BC, Gygi SP, Elledge SJ, Harper JW. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castor D, Nair N, Déclais AC, Lachaud C, Toth R, Macartney TJ, Lilley DM, Arthur JS, Rouse J. Cooperative control of holliday junction resolution and DNA repair by the SLX1 and MUS81-EME1 nucleases. Mol Cell. 2013;52:221–33. doi: 10.1016/j.molcel.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner E, Kim Y, Lach FP, Kottemann MC, Smogorzewska A. Human GEN1 and the SLX4-associated nucleases MUS81 and SLX1 are essential for the resolution of replication-induced Holliday junctions. Cell Rep. 2013;5:207–15. doi: 10.1016/j.celrep.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyatt HD, Sarbajna S, Matos J, West SC. Coordinated actions of SLX1-SLX4 and MUS81-EME1 for Holliday junction resolution in human cells. Mol Cell. 2013;52:234–47. doi: 10.1016/j.molcel.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Agostinho A, Meier B, Sonneville R, Jagut M, Woglar A, Blow J, Jantsch V, Gartner A. Combinatorial regulation of meiotic holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet. 2013;9:e1003591. doi: 10.1371/journal.pgen.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neil NJ, Martin JS, Youds JL, Ward JD, Petalcorin MI, Rose AM, Boulton SJ. Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet. 2013;9:e1003582. doi: 10.1371/journal.pgen.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulon S, Gaillard PH, Chahwan C, McDonald WH, Yates JR, 3rd, Russell P. Slx1-Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol Biol Cell. 2004;15:71–80. doi: 10.1091/mbc.E03-08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito TT, Youds JL, Boulton SJ, Colaiácovo MP. Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet. 2009;5:e1000735. doi: 10.1371/journal.pgen.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salewsky B, Schmiester M, Schindler D, Digweed M, Demuth I. The nuclease hSNM1B/Apollo is linked to the Fanconi anemia pathway via its interaction with FANCP/SLX4. Hum Mol Genet. 2012;21:4948–56. doi: 10.1093/hmg/dds338. [DOI] [PubMed] [Google Scholar]
- 18.Flott S, Alabert C, Toh GW, Toth R, Sugawara N, Campbell DG, Haber JE, Pasero P, Rouse J. Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast. Mol Cell Biol. 2007;27:6433–45. doi: 10.1128/MCB.00135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Muyt A, Jessop L, Kolar E, Sourirajan A, Chen J, Dayani Y, Lichten M. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol Cell. 2012;46:43–53. doi: 10.1016/j.molcel.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakharyevich K, Tang S, Ma Y, Hunter N. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell. 2012;149:334–47. doi: 10.1016/j.cell.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000419. doi: 10.1371/journal.pgen.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen SL, Kuo HK, Savukoski D, Brodsky MH, Sekelsky J. Three structure-selective endonucleases are essential in the absence of BLM helicase in Drosophila. PLoS Genet. 2011;7:e1002315. doi: 10.1371/journal.pgen.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco MG, Matos J, Rass U, Ip SC, West SC. Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae. DNA Repair (Amst) 2010;9:394–402. doi: 10.1016/j.dnarep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Tay YD, Wu L. Overlapping roles for Yen1 and Mus81 in cellular Holliday junction processing. J Biol Chem. 2010;285:11427–32. doi: 10.1074/jbc.M110.108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wechsler T, Newman S, West SC. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642–6. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ercan S, Lieb JD. C. elegans dosage compensation: a window into mechanisms of domain-scale gene regulation. Chromosome Res. 2009;17:215–27. doi: 10.1007/s10577-008-9011-0. [DOI] [PubMed] [Google Scholar]
- 27.Kelly WG, Aramayo R. Meiotic silencing and the epigenetics of sex. Chromosome Res. 2007;15:633–51. doi: 10.1007/s10577-007-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer BJ, Casson LP. Caenorhabditis elegans compensates for the difference in X chromosome dosage between the sexes by regulating transcript levels. Cell. 1986;47:871–81. doi: 10.1016/0092-8674(86)90802-0. [DOI] [PubMed] [Google Scholar]
- 29.Ercan S, Lubling Y, Segal E, Lieb JD. High nucleosome occupancy is encoded at X-linked gene promoters in C. elegans. Genome Res. 2011;21:237–44. doi: 10.1101/gr.115931.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Y, Barton AB, Kaback DB. Decreased meiotic reciprocal recombination in subtelomeric regions in Saccharomyces cerevisiae. Chromosoma. 2000;109:467–75. doi: 10.1007/s004120000098. [DOI] [PubMed] [Google Scholar]
- 31.Lambie EJ, Roeder GS. Repression of meiotic crossing over by a centromere (CEN3) in Saccharomyces cerevisiae. Genetics. 1986;114:769–89. doi: 10.1093/genetics/114.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choo KH. Why is the centromere so cold? Genome Res. 1998;8:81–2. doi: 10.1101/gr.8.2.81. [DOI] [PubMed] [Google Scholar]
- 33.Barnes TM, Kohara Y, Coulson A, Hekimi S. Meiotic recombination, noncoding DNA and genomic organization in Caenorhabditis elegans. Genetics. 1995;141:159–79. doi: 10.1093/genetics/141.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.C. elegans Sequencing Consortium Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–8. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 35.Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 2010;11:R120. doi: 10.1186/gb-2010-11-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Rechtsteiner A, Egelhofer TA, Vielle A, Latorre I, Cheung MS, Ercan S, Ikegami K, Jensen M, Kolasinska-Zwierz P, et al. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 2011;21:227–36. doi: 10.1101/gr.115519.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, Yip KY, Robilotto R, Rechtsteiner A, Ikegami K, et al. modENCODE Consortium Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–87. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller HJ. The Mechanism of Crossing-over. Am Nat. 1916;50:193–221. doi: 10.1086/279534. [DOI] [Google Scholar]
- 39.Sturtevant AH. The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. J Exp Zool. 1913;14:43–59. doi: 10.1002/jez.1400140104. [DOI] [Google Scholar]
- 40.Meneely PM, Farago AF, Kauffman TM. Crossover distribution and high interference for both the X chromosome and an autosome during oogenesis and spermatogenesis in Caenorhabditis elegans. Genetics. 2002;162:1169–77. doi: 10.1093/genetics/162.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villeneuve AM. A cis-acting locus that promotes crossing over between X chromosomes in Caenorhabditis elegans. Genetics. 1994;136:887–902. doi: 10.1093/genetics/136.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan KL, Hickson ID. New insights into the formation and resolution of ultra-fine anaphase bridges. Semin Cell Dev Biol. 2011;22:906–12. doi: 10.1016/j.semcdb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Bishop DK, Zickler D. Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell. 2004;117:9–15. doi: 10.1016/S0092-8674(04)00297-1. [DOI] [PubMed] [Google Scholar]
- 44.Börner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/S0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- 45.Hunter N, Kleckner N. The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/S0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 46.Kleckner N, Zickler D, Jones GH, Dekker J, Padmore R, Henle J, Hutchinson J. A mechanical basis for chromosome function. Proc Natl Acad Sci U S A. 2004;101:12592–7. doi: 10.1073/pnas.0402724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhalla N, Wynne DJ, Jantsch V, Dernburg AF. ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet. 2008;4:e1000235. doi: 10.1371/journal.pgen.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito TT, Mohideen F, Meyer K, Harper JW, Colaiácovo MP. SLX-1 is required for maintaining genomic integrity and promoting meiotic noncrossovers in the Caenorhabditis elegans germline. PLoS Genet. 2012;8:e1002888. doi: 10.1371/journal.pgen.1002888. [DOI] [PMC free article] [PubMed] [Google Scholar]