Abstract
Arabidopsis xylan consists of a linear chain of β-1,4-linked D-xylosyl residues, about 10% of which are substituted with single residues of α-D-glucuronic acid (GlcA) or 4-O-methyl-α-D-glucuronic acid (MeGlcA) at O-2. In addition, about 60% of xylosyl residues are acetylated at O-2 and/or O-3. Previous studies have identified a number of genes responsible for elongation of the xylan backbone, addition of the GlcA substituents, and methylation of the GlcA residues. Yuan et al. (2013) have recently reported that the 2-O- and 3-O-monoacetylation of xylosyl residues in Arabidopsis xylan requires a DUF231 domain-containing protein, ESKIMO1 (ESK1), and proposed that ESK1 and its homologs are putative acetyltransferases responsible for xylan acetylation. It was noticed that the 1H nuclear magnetic resonance (NMR) spectra of the acetylated xylan from the esk1 mutant and the wild-type Arabidopsis exhibited a prominent proton signal peak at 5.42 ppm in addition to resonances corresponding to known acetylated structural groups of xylan. Here, we performed detailed structural investigation of wild-type Arabidopsis acetylated xylan using 2-dimensional 1H-1H and 1H-13C NMR spectroscopy and found that the signal peak at 5.42 ppm in the 1H NMR spectrum was attributed to GlcA residues substituted at O-2 with α-D-galactose (Gal), indicating the presence of Gal-GlcA disaccharide side chains in Arabidopsis xylan. This finding was further supported by analysis of endoxylanase-digested xylan using matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Our study demonstrates that Arabidopsis xylan contains Gal-GlcA disaccharide side chains in addition to GlcA, MeGlcA, and acetyl substitutions.
Keywords: Arabidopsis, biomass, cell wall, secondary wall, xylan
Xylan is the second most abundant polysaccharide in plant biomass. It is the predominant hemicellulose in secondary walls of xylem and fibers in angiosperms.1 Xylan consists of a linear chain of β-1,4-linked xylosyl residues with a degree of polymerization up to 120.2 The reducing end of the xylan backbone from gymnosperms and dicots also contains a distinct tetrasaccharide sequence, β-D-Xyl-(1→3)-α-l-Rha-(1→2)-α-D-GalA-(1→4)-D-Xyl.3-6 Xylan from dicots is typically substituted with single residues of α-D-glucuronic acid (GlcA) and 4-O-methyl-α-D-glucuronic acid (MeGlcA) at O-2. Xylan from lignified tissues of grasses is substituted with α-L-arabinose (Ara) at O-3 in addition to the 2-O-linked GlcA and MeGlcA, and that from cereal grains is mainly substituted with Ara residues at O-2 and O-3.1 Although xylan substituents are typically single sugar residues, those of grass xylan can be disaccharides composed of 2-O-Ara-Ara or 2-O-Xyl-Ara linked at O-3 to the xylan backbone, and the Ara substituents may be esterified by ferulic acid at O-5.1 Xylan from wood of Eucalyptus globulus was found to contain disaccharide side chains composed of MeGlcA substituted at O-2 with α-D-galactose (Gal).7 In addition to sugar substitutions, xylosyl residues in the xylan backbone may be acetylated at O-2 and/or O-3.8 Since xylan is one of the factors contributing to the recalcitrance of cellulosic biomass to saccharification,9 it is important to have a thorough understanding of xylan structure and of how genetic modification of xylan content and structure may alter lignocellulosic biomass recalcitrance in order to custom-design biomass composition tailored for biofuel production.
Arabidopsis has been used as a model to identify genes involved in xylan biosynthesis and to study how alterations of xylan content and structure impact secondary wall biosynthesis. As a typical dicot xylan, Arabidopsis xylan consists of the xylosyl backbone, the reducing end tetrasaccharide sequence, and substitutions of xylosyl residues with GlcA/MeGlcA residues and acetyl groups.5,10 Genetic and biochemical studies of xylan biosynthesis in Arabidopsis have revealed that the elongation of the xylosyl backbone requires glycosyltransferases from both GT43 (IRX9/I9H and IRX14/I14H) and GT47 (IRX10/IRX10L) families,5,11-16 the biosynthesis of the reducing end tetrasaccharide sequence involves glycosyltransferases from GT8 (IRX8 and PARVUS) and GT47 (FRA8/F8H) families,5,6,11,17-19 the substitutions by GlcA residues is mediated by 3 GT8 glycosyltransferases (GUX1/2/3),20,21 and the methylation of GlcA residues is catalyzed by 3 DUF579 domain-containing methyltransferases (GXM1/2/3).22,23 Mutations of genes responsible for the biosynthesis of the xylan backbone and the reducing end tetrasaccharide sequence all lead to a reduction in xylan content and concomitantly a defective secondary wall thickening. Recently, Yuan et al.24 have demonstrated that a DUF231 domain-containing protein, ESKIMO1 (ESK1), is required for the acetylation of xylan during secondary wall biosynthesis in Arabidopsis. The esk1 mutation causes a specific reduction in the 2-O- and 3-O-monoacetylation of xylosyl residues in xylan and severe defects in secondary wall thickening and plant growth. It was hypothesized that ESK1 and its close homologs were putative acetyltransferases catalyzing O-acetylation of xylosyl residues in xylan.
It was noticed that the 1H nuclear magnetic resonance (NMR) spectra of the acetylated xylan from the esk1 mutant and the wild-type Arabidopsis exhibited a prominent proton resonance peak located at 5.42 ppm in addition to the resonances corresponding to 2-O- and 3-O-monacetylated xylosyl residues, 2,3-di-O-acetylated xylosyl residues, and 3-O-acetylated xylosyl residues substituted at O-2 with GlcA (Fig. 1).24 A proton resonance at 5.42 ppm has been previously observed in the 1H NMR spectrum of xylan from E. globulus and it was attributed to disaccharide side chains composed of MeGlcA substituted at O-2 with α-D-Gal.7 About 10% xylosyl residues in E. globulus xylan are substituted with MeGlcA residues and one-third of the MeGlcA substituents are attached with Gal residues. The Gal-MeGlcA disaccharide substituents in xylan have thus far only been reported in E. globulus, and it is not known whether they are also present in xylans from other species. One study has found that xylan from maize bran contains Gal-Xyl-Ara trisaccharide substituents.25
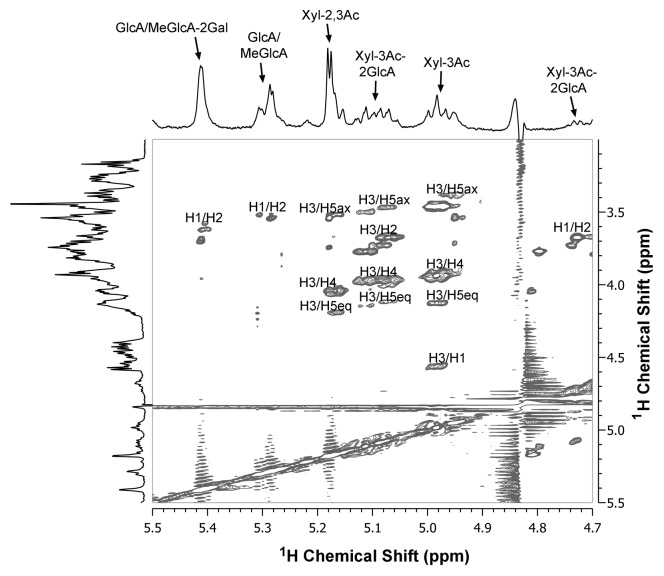
Figure 1. Two-dimensional 1H-1H TOCSY NMR spectrum of acetylated xylan from wild-type Arabidopsis stems. Acetylated xylan was extracted with DMSO28 and digested with β-endoxylanase M6 (Megazyme) to generate xylooligosaccharides,16 which were subsequently subject to structural analysis using NMR spectroscopy.29 The spectra aligned at the top and the left of the figure are the 1D 1H NMR spectra of Arabidopsis xylan. The identities of the resonance peaks in the top spectrum are marked. The following abbreviations are used: GlcA/MeGlcA-2Gal, GlcA/MeGlcA substituted at O-2 with galactose; Xyl-2,3Ac, 2,3-di-O-acetylated xylosyl residues; Xyl-3Ac-2GlcA, 3-O-acetylated xylosyl residues substituted with O-2 with GlcA/MeGlcA; Xyl-3Ac, 3-O-acetylated xylosyl residues.
To investigate what structure the observed 5.42-ppm proton resonance in Arabidopsis acetylated xylan is attributed to, we employed 2-dimensional (2D) total correlation NMR spectroscopy (TOCSY) to analyze the structural units of wild-type Arabidopsis xylan. It has previously been shown that in the 2D 1H-1H TOCSY spectrum of E. globulus xylan, the H-1 signal of the Gal-MeGlcA disaccharide substituents at 5.42 ppm has correlation cross peaks with H-2 proton at 3.77 ppm.26 The H-1 signal of Arabidopsis xylan at 5.42 ppm also has correlation with H-2 proton at 3.7 ppm (Fig. 1), indicating that the cross peak corresponds to the Gal-MeGlcA disaccharide substituents. Unlike E. globulus xylan that has only MeGlcA substituents,26 Arabidopsis xylan contains both GlcA and MeGlcA substituents. Since the proton resonances for GlcA and MeGlcA are overlapped in the 2D NMR spectrum,27 it is not discernable whether the cross peak at H-1 of 5.42 ppm and H-2 of 3.7 ppm in the 2D TOCSY spectrum of Arabidopsis xylan contains both Gal-GlcA and Gal-MeGlcA, and thus it is designated as Gal-GlcA/MeGlcA (Fig. 1). Other correlations in the 2D TOCSY spectrum of Arabidpsis xylan correspond to GlcA/MeGlcA (the H-1/H-2 cross peak at 5.3/3.55 ppm), 2,3-di-O-acetylated xylosyl residues (H-3/H-5ax at 5.17/3.53 ppm, H-3/H-4 at 5.17/4.05 ppm, and H-3/H-5eq at 5.17/4.2 ppm), 3-O-acetylated xylosyl residues substituted at O-2 with GlcA (H-3/H-5ax at 5.08/3.48 ppm, H-3/H-2 at 5.08/3.7 PM, H-3/H-4 at 5.08/3.98 ppm, H-3/H-5eq at 5.08/4.2 ppm, and H-1/H-2 at 4.73/3.69 ppm), and 3-O-monoacetylated xylosyl residues (H-3/H-5ax at 4.98/3.48 ppm, H-3/H-4 at 4.98/3.93 ppm, H-3/H-5eq at 4.98/4.13 ppm, and H-3/H-1 at 4.98/4.58 ppm). The spectral positions of these proton cross peaks are in good agreement with those reported for acetylated xylans from E. globulus and aspen,8,26 thus validating the reliability of the 2D TOCSY spectrum of Arabidopsis xylan.
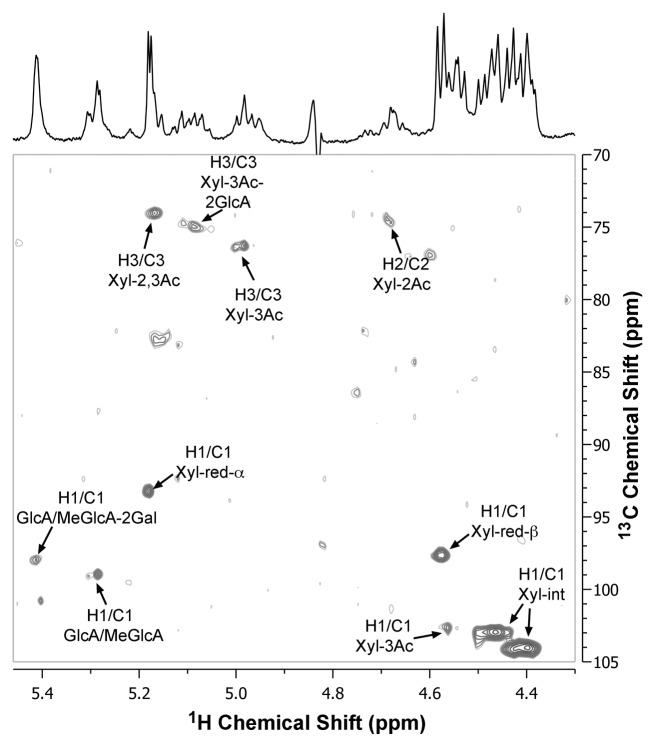
To confirm the identity of the 5.42-ppm proton resonance in Arabidopsis acetylated xylan as Gal-GlcA/MeGlcA disaccharide substituents, we next analyzed the structural units of Arabidopsis xylan using 2D heteronuclear single-quantum correlation NMR spectroscopy (HSQC). The 2D 1H-13C HSQC spectrum of Arabidopsis xylan showed a cross peak of H-1 and C-1 signals at 5.42 and 98 ppm (Fig. 2) corresponding to the resonances of Gal-MeGlcA disaccharide substituents, which is the same as that observed in E. globulus xylan.26 Congruent with the 2D HSQC spectra of acetylated xylans from E. globulus and aspen,8,26 other cross peaks of the 1H and 13C chemical shifts in the spectrum of Arabidopsis xylan correspond to GlcA/MeGlcA (the H-1/C-1 signals at 5.28/98.8 ppm), 2,3-di-O-acetylated xylosyl residues (H-3/C-3 at 5.17/74 ppm), 3-O-acetylated xylosyl residues substituted at O-2 with GlcA (H-3/C-3 at 5.08/75 ppm), 3-O-monoacetylated xylosyl residues (H-3/C-3 at 4.98/76.3 ppm and H-1/C-1 at 4.58/102.5 ppm), and non-acetylated internal xylosyl residues (H-1/C-1 at 4.42/104 and 4.48/103 ppm). Because xylooligomers released from xylanase digestion of acetylated xylan were used for NMR spectroscopy, cross peaks of H-1 and C-1 signals for reducing end α-xylose (H-1/C-1 at 5.18/93.2 ppm) and reducing end β-xylose (H-1/C-1 at 4.58/97.6 ppm) were prominent.
Figure 2. Two-dimensional 1H-13C HSQC NMR spectrum of acetylated xylan from wild-type Arabidopsis stems. Acetylated xylan was extracted with DMSO28 and digested with β-endoxylanase M6 (Megazyme) to generate xylooligosaccharides,16 which were subsequently subject to structural analysis using NMR spectroscopy.29 The spectrum aligned at the top of the figure is the 1D 1H NMR spectra of Arabidopsis xylan. See the abbreviations in Figure 1. Xyl-red-α, reducing end α-Xyl; Xyl-red-β, reducing end β-Xyl; Xyl-int, non-acetylated internal Xyl.
To further substantiate the existence of the disaccharide side chain Gal-GlcA/MeGlcA in Arabidopsis xylan, we next applied matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF-MS) to examine xylooligomers released from xylanase digestion of Arabidopsis xylan. The MALDI-TOF spectrum showed the expected prominent ion peaks [M+Na]+ at mass-to-charge ratio (m/z) 745, 759, 877, and 891 that are attributed to GlcA-substituted Xyl4, MeGlcA-substituted Xyl4, GlcA-substituted Xyl5, MeGlcA-substituted Xyl5, respectively (Fig. 3).17 Noticeably, the spectrum also had ion peaks at m/z 775 and 907, which correspond to the expected masses of (Gal-GlcA)-substituted Xyl3 and (Gal-GlcA)-substituted Xyl4, respectively. No ion peaks corresponding to the expected masses of (Gal-MeGlcA)-substituted Xyl3 (m/z 789) and (Gal-MeGlcA)-substituted Xyl4 (m/z 921) were detected. The MALDI-TOF-MS data showing the presence of ions corresponding to (Gal-GlcA)-substituted xylooligomers not only is consistent with the 2D NMR data but also demonstrate that the disaccharide side chain in Arabidopsis xylan is mainly composed of GlcA substituted with Gal and that MeGlcA substituted with Gal, if present, is a minor component.
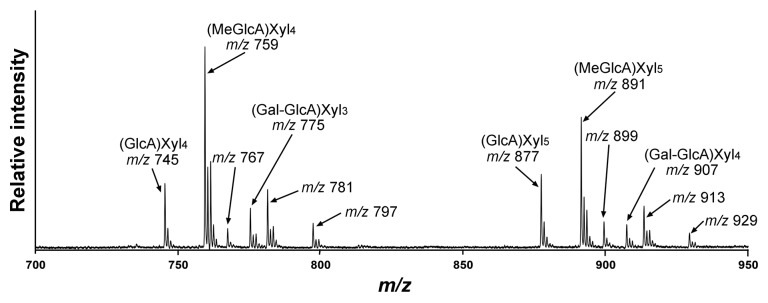
Figure 3. MALDI-TOF spectrum of xylooligomers generated by xylanase digestion of KOH-extracted Arabidopsis xylan. The ions [M+Na]+ at m/z 745 and 759 are attributed to xylotetrasaccharides bearing a GlcA residue [(GlcA)Xyl4] and a methylated GlcA residue [(MeGlcA)Xyl4], respectively, and those at m/z 877 and 891 are attributed to xylopentasaccharides bearing a GlcA residue [(GlcA)Xyl5] and a methylated GlcA residue [(MeGlcA)Xyl5], respectively. Note the presence of ions at m/z 775 and 907 that correspond to the masses of xylotrisaccharides bearing Gal and GlcA residues [(Gal-GlcA)Xyl3] and xylotetrasaccharides bearing Gal and GlcA residues [(Gal-GlcA)Xyl4], respectively. The ions at m/z 767, 781, and 797 are attributed to the doubly sodiated species [M+2Na]+ of (GlcA)Xyl4, (MeGlcA)Xyl4 and (Gal-GlcA)Xyl3, respectively. The ions at m/z 899, 913, and 929 are attributed to the doubly sodiated species [M+2Na]+ of (GlcA)Xyl5, (MeGlcA)Xyl5, and (Gal-GlcA)Xyl4, respectively.
In summary, our results from the 2D 1H-1H and 1H-13C NMR spectroscopy and the MALDI-TOF-MS provide unequivocal evidence demonstrating that Arabidopsis xylan contains Gal-GlcA disaccharide side chains in addition to substitutions with GlcA, MeGlcA and acetyl groups (Fig. 4). Since GlcA substituents are common structural units of xylans from different species, it will be interesting to investigate whether Gal-GlcA substitutions of xylan also occur commonly in various plant species. Further studies on the functional roles of the Gal-GlcA substitutions in xylan properties and identification of glycosyltransferases responsible for Gal-GlcA substitutions of xylan will enrich our understanding of the mechanisms controlling xylan biosynthesis.
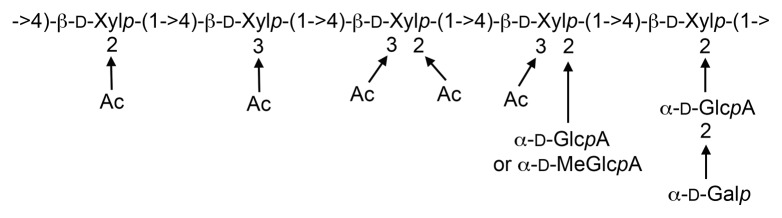
Figure 4. Diagram of Arabidopsis xylan structure with all known substituents. The reducing end tetrasaccharide sequence, β-d-Xyl-(1→3)-α-l-Rha-(1→2)-α-d-GalA-(1→4)-d-Xyl, is not shown.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy through Grant DE-FG02–03ER15415.
References
- 1.Ebringerova A, Heinze T. Xylan and xylan derivatives-biopolymers with valuable properties. Macromol Rapid Commun. 2000;21:542–56. doi: 10.1002/1521-3927(20000601)21:9<542::AID-MARC542>3.0.CO;2-7. [DOI] [Google Scholar]
- 2.Jacobs A, Dahlman O. Characterization of the molar masses of hemicelluloses from wood and pulps employing size exclusion chromatography and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Biomacromolecules. 2001;2:894–905. doi: 10.1021/bm010050b. [DOI] [PubMed] [Google Scholar]
- 3.Johansson MH, Samuelson O. Reducing end groups in birch xylan and their alkaline degradation. Wood Sci Technol. 1977;11:251–63. doi: 10.1007/BF00356924. [DOI] [Google Scholar]
- 4.Andersson S-I, Samuelson O, Ishihara M, Shimizu K. Structure of the reducing end-groups in spruce xylan. Carbohydr Res. 1983;111:283–8. doi: 10.1016/0008-6215(83)88312-8. [DOI] [Google Scholar]
- 5.Peña MJ, Zhong R, Zhou G-K, Richardson EA, O’Neill MA, Darvill AG, York WS, Ye ZH. Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell. 2007;19:549–63. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C, Teng Q, Huang W, Zhong R, Ye Z-H. Down-regulation of PoGT47C expression in poplar results in a reduced glucuronoxylan content and an increased wood digestibility by cellulase. Plant Cell Physiol. 2009;50:1075–89. doi: 10.1093/pcp/pcp060. [DOI] [PubMed] [Google Scholar]
- 7.Shatalov AA, Evtuguin DV, Pascoal Neto C. (2-O-α-D-galactopyranosyl-4-O-methyl-α-D-glucurono)-D-xylan from Eucalyptus globulus Labill. Carbohydr Res. 1999;320:93–9. doi: 10.1016/S0008-6215(99)00136-6. [DOI] [PubMed] [Google Scholar]
- 8.Teleman A, Lundqvist J, Tjerneld F, Stålbrand H, Dahlman O. Characterization of acetylated 4-O-methylglucuronoxylan isolated from aspen employing 1H and 13C NMR spectroscopy. Carbohydr Res. 2000;329:807–15. doi: 10.1016/S0008-6215(00)00249-4. [DOI] [PubMed] [Google Scholar]
- 9.Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–7. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 10.Lee C, Teng Q, Zhong R, Ye Z-H. The four Arabidopsis reduced wall acetylation genes are expressed in secondary wall-containing cells and required for the acetylation of xylan. Plant Cell Physiol. 2011;52:1289–301. doi: 10.1093/pcp/pcr075. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Goubet F, Wong VW, Goodacre R, Stephens E, Dupree P, Turner SR. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J. 2007;52:1154–68. doi: 10.1111/j.1365-313X.2007.03307.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, O’Neill MA, Tsumuraya Y, Darvill AG, Ye Z-H. The irregular xylem9 mutant is deficient in xylan xylosyltransferase activity. Plant Cell Physiol. 2007;48:1624–34. doi: 10.1093/pcp/pcm135. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Teng Q, Huang W, Zhong R, Ye Z-H. The Arabidopsis family GT43 glycosyltransferases form two functionally nonredundant groups essential for the elongation of glucuronoxylan backbone. Plant Physiol. 2010;153:526–41. doi: 10.1104/pp.110.155309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C, Zhong R, Ye Z-H. Arabidopsis family GT43 members are xylan xylosyltransferases required for the elongation of the xylan backbone. Plant Cell Physiol. 2012;53:135–43. doi: 10.1093/pcp/pcr158. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Zhang Z, Stephens E, Dupree P, Turner SR. Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J. 2009;57:732–46. doi: 10.1111/j.1365-313X.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu AM, Rihouey C, Seveno M, Hörnblad E, Singh SK, Matsunaga T, Ishii T, Lerouge P, Marchant A. The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J. 2009;57:718–31. doi: 10.1111/j.1365-313X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhong R, Peña MJ, Zhou G-K, Nairn CJ, Wood-Jones A, Richardson EA, Morrison WH, 3rd, Darvill AG, York WS, Ye ZH. Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell. 2005;17:3390–408. doi: 10.1105/tpc.105.035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Zhong R, Richardson EA, Himmelsbach DS, McPhail BT, Ye Z-H. The PARVUS gene is expressed in cells undergoing secondary wall thickening and is essential for glucuronoxylan biosynthesis. Plant Cell Physiol. 2007;48:1659–72. doi: 10.1093/pcp/pcm155. [DOI] [PubMed] [Google Scholar]
- 19.Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, Hahn MG, Mohnen D, Somerville C. The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell. 2007;19:237–55. doi: 10.1105/tpc.106.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, Yu X, Seffen KA, Stephens E, Turner SR, et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci U S A. 2010;107:17409–14. doi: 10.1073/pnas.1005456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C, Teng Q, Zhong R, Ye Z-H. Arabidopsis GUX proteins are glucuronyltransferases responsible for the addition of glucuronic acid side chains onto xylan. Plant Cell Physiol. 2012;53:1204–16. doi: 10.1093/pcp/pcs064. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Teng Q, Zhong R, Yuan Y, Haghighat M, Ye Z-H. Three Arabidopsis DUF579 domain-containing GXM proteins are methyltransferases catalyzing 4-o-methylation of glucuronic acid on xylan. Plant Cell Physiol. 2012;53:1934–49. doi: 10.1093/pcp/pcs138. [DOI] [PubMed] [Google Scholar]
- 23.Urbanowicz BR, Peña MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, Foston M, Li H, O’Neill MA, Ragauskas AJ, et al. 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc Natl Acad Sci U S A. 2012;109:14253–8. doi: 10.1073/pnas.1208097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Teng Q, Zhong R, Ye Z-H. The Arabidopsis DUF231 domain-containing protein ESK1 mediates 2-O- and 3-O-acetylation of xylosyl residues in xylan. Plant Cell Physiol. 2013;54:1186–99. doi: 10.1093/pcp/pct070. [DOI] [PubMed] [Google Scholar]
- 25.Saulnier L, Vigouroux J, Thibault J-F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr Res. 1995;272:241–53. doi: 10.1016/0008-6215(95)00053-V. [DOI] [PubMed] [Google Scholar]
- 26.Evtuguin DV, Tomás JL, Silva AM, Neto CP. Characterization of an acetylated heteroxylan from Eucalyptus globulus Labill. Carbohydr Res. 2003;338:597–604. doi: 10.1016/S0008-6215(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves VM, Evtuguin DV, Domingues MR. Structural characterization of the acetylated heteroxylan from the natural hybrid Paulownia elongata/Paulownia fortunei. Carbohydr Res. 2008;343:256–66. doi: 10.1016/j.carres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Teleman A, Tenkanen M, Jacobs A, Dahlman O. Characterization of O-acetyl-(4-O-methylglucurono)xylan isolated from birch and beech. Carbohydr Res. 2002;337:373–7. doi: 10.1016/S0008-6215(01)00327-5. [DOI] [PubMed] [Google Scholar]
- 29.Teng Q, Ekman DR, Huang W, Collette TW. Push-through direct injection NMR: an optimized automation method applied to metabolomics. Analyst. 2012;137:2226–32. doi: 10.1039/c2an16251b. [DOI] [PubMed] [Google Scholar]