Abstract
The fungal plant pathogen Botrytis cinerea is the causal agent of the “gray mold” disease on a broad range of hosts. As an archetypal necrotroph, B. cinerea has evolved multiple virulence strategies for inducing cell death in its host. Moreover, progress of B. cinerea colonization is commonly associated with induction of senescence in the host tissue, even in non-invaded regions. In a recent study, we showed that abscisic acid deficiency in the sitiens tomato mutant culminates in an anti-senescence defense mechanism which effectively contributes to resistance against B. cinerea infection. Conversely, in susceptible wild-type tomato a strong induction of senescence could be observed following B. cinerea infection. Building upon this earlier work, we here discuss the immune-regulatory role of a key senescence-associated protein, asparagine synthetase. We found that infection of wild-type tomato leads to a strong transcriptional upregulation of asparagine synthetase, followed by a severe depletion of asparagine titers. In contrast, resistant sitiens plants displayed a strong induction of asparagine throughout the course of infection. We hypothesize that rapid activation of asparagine synthetase in susceptible tomato may play a dual role in promoting Botrytis cinerea pathogenesis by providing a rich source of N for the pathogen, on the one hand, and facilitating pathogen-induced host senescence, on the other.
Keywords: Botrytis cinerea, asparagine synthetase, senescence, necrotrophic pathogen, gray mold, senescence-associated genes, amino acid metabolism
Results and Discussion
The amino acid asparagine is one of the major metabolic products in senescing leaves in plants.1 Asparagine has the highest N:C ratio (2:4) among all amino acids, which makes it an efficient metabolite for nitrogen transport during the process of senescence.2,3 The major route of asparagine synthesis is governed by the ATP-dependent transfer of the amino group of glutamine to a molecule of aspartate to form glutamate and asparagine, a reaction catalyzed by the enzyme asparagine synthetase (AS).3 AS is also known as a key senescence associated gene (SAG),4 with possible roles in plant-pathogen interactions.5,6 It has been proposed that AS is involved in metabolic alterations that facilitate host cell death during plant-pathogen interactions; hence promoting resistance against biotrophic pathogens. However, such metabolic alterations may also render susceptibility to necrotrophic infections.7 For instance, an early (5 hpi) induction of AS1 in pepper was shown to be involved in resistance against the hemibiotrophic bacterial pathogen Xanthomonas campestris pv vesicatoria, while later (15–20 hpi) induction of the enzyme (during the necrotrophic phase of the pathogen infection) was associated with susceptibility.8
Taking advantage of the abscisic acid (ABA)-deficient sitiens mutant of tomato, which displays high levels of immunity against the necrotrophic fungus Botrytis cinerea (B. cinerea),9-11 our research aims to decipher the molecular underpinnings of necrotroph resistance in plants. In our latest study, we showed that the observed resistance response in sitiens is dependent on timely restructuring of the central C/N metabolism, particularly the GABA-shunt.12 The GABA shunt is a cytosolic-mitochondrial pathway that connects amino acid metabolism to the tricarboxylic acid (TCA) cycle, with possible roles in plant defense mechanisms against phytopathogens.7 According to our proposed model, concurrent hyper-activation of the cytosolic glutamine synthetase (GS1) and the GABA-shunt in sitiens are involved in maintaining basic metabolism in the challenged tissue to tightly control the extent and localization of cell death.12 As a result, the extent of senescence is spatiotemporally more restricted in the resistant sitiens mutant compared with susceptible wild-type tomato plants12 (Fig. 1). In agreement with this, we also found lower levels of total protein degradation and reduced activation of the SAG, glutamate dehydrogenase (GDH), in sitiens vs. wild-type seedlings.

Figure 1. Induction of senescence in non-inoculated areas (red arrows) of an infected wild-type (WT) leaf, compared with an inoculated leaf of the sitiens (Sit) mutant at 72 hpi. Fifth or sixth leaves of 5-wk-old plants (7 leaf stage) were inoculated according to Curvers et al. (2010)11 by carefully placing 4 droplets (per leaflet) containing 10-µL of conidial suspension (5 × 105 spores mL−1 in 0.01 M KH2PO4 and 6.67 mM Glucose) on the adaxial leaf surface. Inoculated leaves were placed in enclosed trays to retain high relative humidity and incubated at 22 °C under continuous dark conditions.
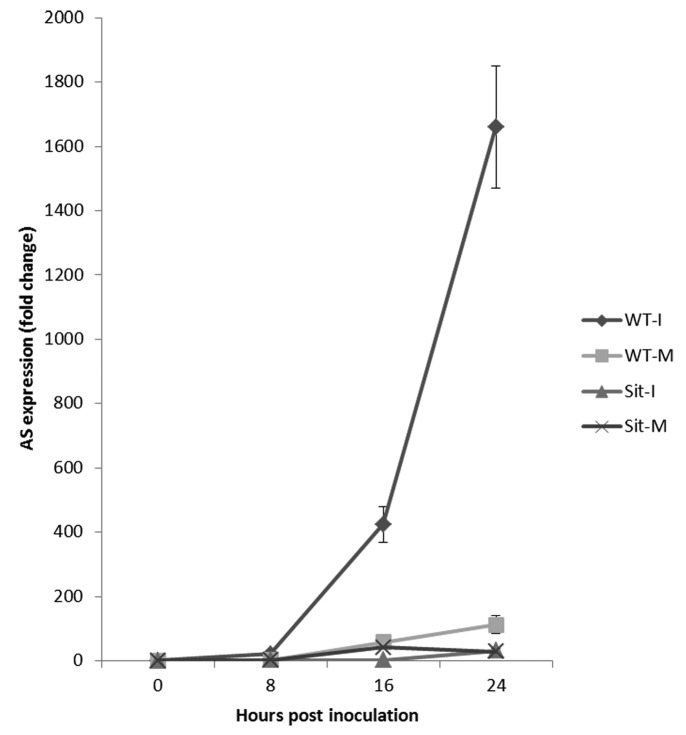
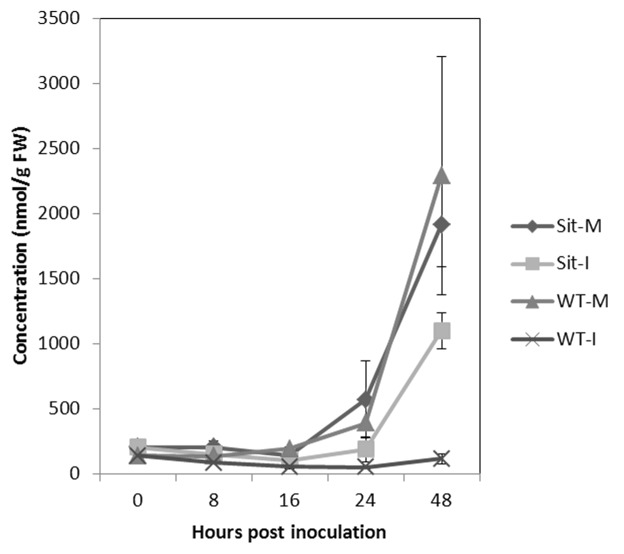
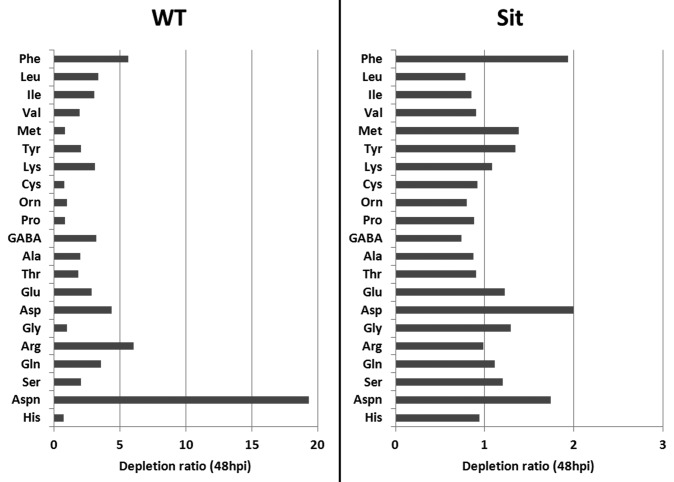
Building upon these earlier findings, we sought to extend our analysis by monitoring the expression of another key SAG, asparagine synthetase 1 (AS1; GeneBank accession number AY240926.1). As shown in Figure 2, AS1 showed a tremendous upregulation in the wild-type tomato in response to B. cinerea infection, whereas such strong induction was not seen in the sitiens mutant (Fig. 2). Figure 3 indicates that the levels of asparagine increased in all mock-treated plants, showing the effect of dark incubation on asparagine accumulation, as suggested previously.13 Moreover, asparagine titers in B. cinerea-inoculated sitiens plants increased significantly during the course of infection (Fig. 3), whereas infected wild-type seedlings showed a remarkable drop in asparagine content (Fig. 3). Particularly, the highest level of depletion of asparagine can be seen at 48 hpi in the wild-type plant, while such a severe depletion did not occur for other amino acids (Fig. 4). Finally, an in vitro assay revealed that asparagine is a perfect nitrogen (N) source for B. cinerea, resulting in rapid mycelial growth and dense fungal sporulation (Fig. 5).
Figure 2. Expression of asparagine synthetase in wild type (WT) and sitiens (Sit) plants following infection with B. cinerea (M: mock; I: infected). The qPCR analysis was performed following the protocol described previously12 using AS1-specific primers; Forward: TCCCCTTTGG TGTTCTGCTC TCG, Reverse: TGCTCCCCAT TGCTTAGCAG C. Error bars represent ± SE of 3 biological replicates.
Figure 3. Alterations in asparagine levels in mock-treated (M) and Botrytis-inoculated (I) sitiens (Sit) and wild-type (WT) tomato plants at different time points post inoculation. Metabolic analysis of free amino acid levels was conducted as described before.12 Error bars represent ± SE of 3 biological replicates.
Figure 4. Amino acid depletion ratios (levels in mock vs. infected samples) in Botrytis-infected sitiens (Sit) and wild-type (WT) tomato at 48 hpi. Among all amino acids, asparagine displays the most marked depletion in the wild-type plant.
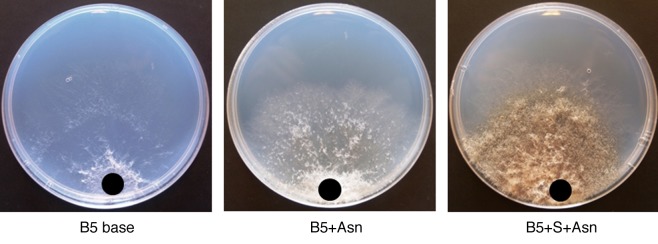
Figure 5. In vitro effect of asparagine (Asn, 5mM) on the growth and sporulation of B. cinerea grown on Gamborg B5 salt base medium (B5 base: with no carbon and nitrogen sources). Sucrose (S) was used as carbon source. The black circles indicate the place of the inoculation plaque.
Induction of senescence in the host is a well-studied pathogenicity mode for the necrotrophic pathogen B. cinerea.14 Accordingly, it appears that in wild-type tomato susceptibility to B. cinerea is strongly correlated with induction of classic SAGs, including AS. AS has also been shown to be an ABA-responsive gene that is upregulated in response to different abiotic stresses.15,16 This might explain why in the ABA-deficient sitiens mutant, compared with the wild-type plant, the AS gene is only slightly induced upon B. cinerea infection. Moreover, the relatively low level of AS activation in sitiens might consequently prevent the cell N reservoir (glutamine and aspartate) from being converted to transportable forms of amino acid (asparagine), thus providing more cytosolic supply for the GABA-shunt-mediated, anti-senescence defense response seen in the mutant.12
Previous studies revealed that, depletion in sugar levels in tomato plants subjected to prolonged dark conditions also result in strong activation of the AS gene.17 Therefore, the strong induction of AS in B. cinerea-infected wild-type tomato (Fig. 2) could be explained through both catabolization of the host apoplastic sugar content by the pathogen, and continuous incubation of tomato leaves in darkness. Interestingly, B. cinerea-induced upregulation of AS in the wild-type plant seems to be followed by strong depletion of asparagine content in the infected leaves, suggesting a causal link between both phenomena. Having the highest N content among all amino acids, asparagine may serve as a rich N source for B. cinerea in vitro (Fig. 5), and possibly also in planta. In view of these findings, it is tempting to speculate that strong induction of AS in susceptible tomato plants plays a dual role in the pathogenicity of B. cinerea by both facilitating pathogen-initiated host senescence and providing a rich N reservoir to support fungal growth in planta. Future studies should be focused on exploring the molecular mechanism(s) by which B. cinerea targets AS and manipulates the plant’s primary metabolism for its own benefit.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the Fund for Scientific Research Flanders (FWO grants 3G052607 and 3G000210) and by a postdoctoral fellowship of the Research Foundation–Flanders (to D.D.V.).
Glossary
Abbreviations:
- AS
asparagine synthetase
- GDH
glutamate dehydrogenase
- SAG
senescence-associated gene
- hpi
hours post inoculation
References
- 1.Herrera-Rodríguez MB, Maldonado JM, Pérez-Vicente R. Role of asparagine and asparagine synthetase genes in sunflower (Helianthus annuus) germination and natural senescence. J Plant Physiol. 2006;163:1061–70. doi: 10.1016/j.jplph.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Kim HB, Lee SH, An CS. Isolation and characterization of a cDNA clone encoding asparagine synthetase from root nodules of Elaeagnus umbellata. Plant Sci. 1999;149:85–94. doi: 10.1016/S0168-9452(99)00124-7. [DOI] [Google Scholar]
- 3.Lea PJ, Azevedo RA. Nitrogen use efficiency. 2. Amino acid metabolism. Ann Appl Biol. 2007;151:269–75. doi: 10.1111/j.1744-7348.2007.00200.x. [DOI] [Google Scholar]
- 4.Gaufichon L, Reisdorf-Cren M, Rothstein SJ, Chardon F, Suzuki A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010;179:141–53. doi: 10.1016/j.plantsci.2010.04.010. [DOI] [Google Scholar]
- 5.AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich RA, Mengiste T. Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 2006;48:28–44. doi: 10.1111/j.1365-313X.2006.02849.x. [DOI] [PubMed] [Google Scholar]
- 6.Pageau K, Reisdorf-Cren M, Morot-Gaudry JF, Masclaux-Daubresse C. The two senescence-related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicotiana tabacum L. leaves. J Exp Bot. 2006;57:547–57. doi: 10.1093/jxb/erj035. [DOI] [PubMed] [Google Scholar]
- 7.Seifi HS, Van Bockhaven J, Angenon G, Höfte M. Glutamate metabolism in plant disease and defense: friend or foe? Mol Plant Microbe Interact. 2013;26:475–85. doi: 10.1094/MPMI-07-12-0176-CR. [DOI] [PubMed] [Google Scholar]
- 8.Hwang IS, An SH, Hwang BK. Pepper asparagine synthetase 1 (CaAS1) is required for plant nitrogen assimilation and defense responses to microbial pathogens. Plant J. 2011;67:749–62. doi: 10.1111/j.1365-313X.2011.04622.x. [DOI] [PubMed] [Google Scholar]
- 9.Audenaert K, De Meyer GB, Höfte MM. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asselbergh B, Achuo AE, Höfte M, Van Gijsegem F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol Plant Pathol. 2008;9:11–24. doi: 10.1111/j.1364-3703.2007.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curvers K, Seifi H, Mouille G, de Rycke R, Asselbergh B, Van Hecke A, Vanderschaeghe D, Höfte H, Callewaert N, Van Breusegem F, et al. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 2010;154:847–60. doi: 10.1104/pp.110.158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifi HS, Curvers K, De Vleesschauwer D, Delaere I, Aziz A, Höfte M. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the ABA-deficient sitiens mutant of tomato leads to resistance against Botrytis cinerea. New Phytol. 2013;199:490–504. doi: 10.1111/nph.12283. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira IC, Brenner E, Chiu J, Hsieh MH, Kouranov A, Lam HM, Shin MJ, Coruzzi G. Metabolite and light regulation of metabolism in plants: lessons from the study of a single biochemical pathway. Braz J Med Biol Res. 2001;34:567–75. doi: 10.1590/S0100-879X2001000500003. [DOI] [PubMed] [Google Scholar]
- 14.Swartzberg D, Kirshner B, Rav-David D, Elad Y, Granot D. Botrytis cinerea induces senescence and is inhibited by autoregulated expression of the IPT gene. Eur J Plant Pathol. 2008;120:289–97. doi: 10.1007/s10658-007-9217-6. [DOI] [Google Scholar]
- 15.Wang H, Liu D, Sun J, Zhang A. Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J Plant Physiol. 2005;162:81–9. doi: 10.1016/j.jplph.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann P, Hennig L, Gruissem W. Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci. 2005;10:407–9. doi: 10.1016/j.tplants.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Devaux C, Baldet P, Joubès J, Dieuaide-Noubhani M, Just D, Chevalier C, Raymond P. Physiological, biochemical and molecular analysis of sugar-starvation responses in tomato roots. J Exp Bot. 2003;54:1143–51. doi: 10.1093/jxb/erg113. [DOI] [PubMed] [Google Scholar]