Abstract
The PEN1-SNAP33-VAMP721/722 exocytic pathway is a conserved immunity-associated secretory pathway between monocotyledonous barley and dicotyledonous Arabidopsis plants. In Arabidopsis, this secretory pathway plays an additional role in plant growth and development. However, how this pathway can be manipulated to engage in both growth/development and immunity remains to be answered. To understand its regulation, we recently analyzed the expression of VAMP721/722 genes whose products drive secretory vesicles to the target plasma membrane. By investigating their transcript and protein levels, we found that plants distinctly control the activity of this secretory pathway during biotic or abiotic stress responses. Since stress responses are in general accompanied by growth inhibition in plants and since plants in nature are simultaneously threatened by a number of environmental stresses, understanding of this growth/immunity-related secretory pathway would help to generate more efficiently growth/immunity-balancing plants.
Keywords: plant growth, plant immunity, secretory pathway, exocytosis, VAMP721/722
As in other eukaryotes, soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins are essential in plants for driving membrane fusion between different intracellular compartments.1,2 We previously identified for the first time in plants the whole set of SNARE complex components engaged in immune responses to filamentous pathogens in Arabidopsis.3,4 The plasma membrane (PM) localization of PEN1 syntaxin and SNAP33 adaptor, the localization of vesicle-associated membrane protein (VAMP) 721/722 to intracellular mobile endomembrane structures, and their specific interactions indicate that these SNARE proteins facilitate exocytosis.4 They also comprise a default secretory pathway because their depletion results in severe growth and developmental defects,4-6 suggesting that this secretory pathway is re-utilized for plant immunity. However, how this exocytic pathway can function in 2 distinct physiological processes such as plant growth and immunity is still unknown.
To understand the regulation of the VAMP721/722-mediated secretion, we recently studied their expression patterns in response to distinct growth-related stimuli. A bacterial flagellin fragment called flg22 that is recognized by its cognate receptor FLS2 induces immune responses as well as growth inhibition in plants.7,8 flg22 treatment as expected led to more growth inhibition in VAMP721/722-depleted plants (VAMP721+/− VAMP722−/− and VAMP721−/− VAMP722+/−) compared with the wild-type (WT) plants,9 which again supports the importance of VAMP721/722 for plant growth. However, flg22 unexpectedly induced more accumulation of VAMP721/722 in plant cells.9 Therefore, we also treated plants with a bacterial elongation factor Tu (EF-Tu) fragment elf18 that is additional plant immune inducer.10,11 Like flg22, elf18 also rapidly increased VAMP721/722 levels in cultured Arabidopsis cells (Fig. 1A). Taken together, our results suggest that plants in response to pathogen attack may activate the VAMP721/722 secretory pathway possibly to transport immunity-associated molecules via enhancing VAMP721/722 expression.
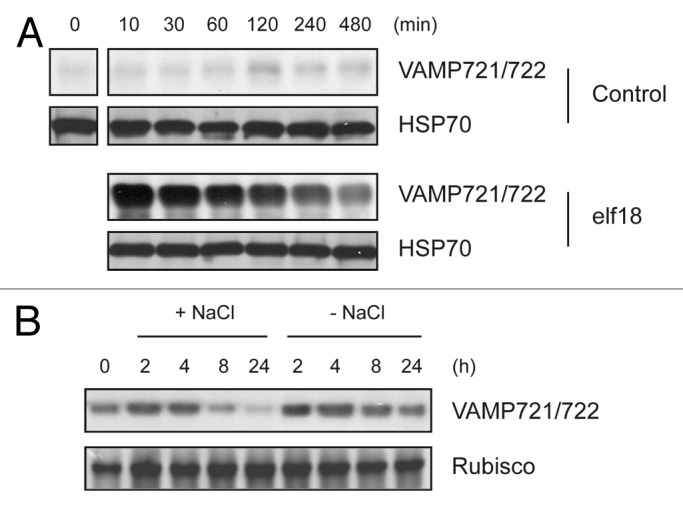
Figure 1. VAMP721/722 levels are distinctly regulated by growth-inhibiting biotic and abiotic stress inducers. Cultured Arabidopsis cells (A) or 2-wk-grown seedlings (B) were treated with 1 μM elf18 (A) or 150 mM NaCl (B) for the indicated time. Extracted proteins with 1x PBS containing 1% Triton X-100 were subject to immunoblot with the indicated antibodies. Equal loading was shown by immunoblot with the HSP70 antibody (A) or by visualizing Rubisco stained with Coomassie (B).
The analysis of publically available microarray data reveals that both flg22 and elf18 induce the transcription of VAMP722 gene (https://www.genevestigator.com).12 Since VAMP721 and VAMP722 are functionally redundant in both plant growth and immunity,4 the enhanced VAMP721/722 protein levels by flg22 and elf18 are most likely attributed to their transcriptional upregulation. Interestingly, we recently found that VAMP721/722 levels can also be post-translationally controlled, because the single treatment of a 26S proteasome inhibitor MG132 upregulated their levels.9 In addition, the very rapid (within 10 min) increase of VAMP721/722 levels in cultured cells by flg22 and elf18 cannot be explained solely by transcription-derived translation (Fig. 1A).9 Therefore, it is likely that plants both transcriptionally and post-translationally regulate the VAMP721/722 exocytic pathway for more rapid and prolonged defense-associated secretion in response to pathogen attack. An important question is then why plant growth is inhibited by flg22 and elf187,11 in spite of more VAMP721/722 protein accumulation. A possible explanation is the priority of defense to growth likely for survival by allocating more VAMP721/722 vesicles to immune responses, which results in their less contribution to growth-related secretion.
Abscisic acid (ABA) is the hormone to induce resistance responses to abiotic stresses such as drought, salt, and heat, which is in general accompanied by growth retardation in plants.13 We found that ABA treatment resulted in gradual reduction of VAMP721/722 levels as well as growth inhibition in Arabidopsis plants.14 Since the VAMP721/722-mediated secretion is essential for plant growth,4 more growth inhibition by ABA in VAMP721/722-depleted plants compared with WT14 can be easily expected by less number of VAMP721/722 vesicles likely containing growth-related cargo. Interestingly, VAMP721/722 levels was no more decreased by ABA in the presence of MG132.14 Since our previous data and the analysis of publically available microarray data show that the transcript levels of both VAMP721/722 genes are not changed by ABA (https://www.genevestigator.com),14 this indicates that ABA post-translationally regulates the expression of VAMP721/722 to control plant growth at least in part at the level of secretion. We here additionally investigated a change of VAMP721/722 levels by high salt to extend our knowledge on the regulation of VAMP721/722 expression. Like ABA, NaCl treatment gradually diminished VAMP721/722 levels (Fig. 1B). Because NaCl, similar to ABA, has little effect on the transcriptional change of VAMP721/722 genes (https://www.genevestigator.com), this suggests that NaCl also post-translationally controls VAMP721/722 levels. Since ABA mediates resistance to abiotic stresses in plants, it seems that the NaCl-driven downregulation of VAMP721/722 levels might be an indirect consequence by NaCl-induced ABA.
Based on our previous and present analysis of VAMP721/722 expression, it is likely that plants employ distinct strategies to control the VAMP721/722 exocytic pathway for responses to abiotic or biotic stresses. To resist to abiotic stresses or respond to ABA, plants transiently shut down the VAMP721/722 secretory pathway by degrading VAMP721/722 proteins but maintaining their transcription.14 Plants through this can more rapidly resume the growth-related secretion via VAMP721/722 vesicles when an abiotic stress disappears. To defend against pathogens, plants increase VAMP721/722 levels by inducing VAMP721/722 transcription as well as by inhibiting the 26S proteasome-associated basal degradation of VAMP721/722 proteins.9,12 By this dual upregulation mechanism, plants can synergistically raise VAMP721/722 levels possibly for faster delivery of immune molecules. However, plant growth is inhibited during immune responses despite more accumulation of VAMP721/722 proteins, because the majority of VAMP721/722 vesicles are used for immune responses rather than growth. This defense priority to growth can be seen in plant cells attacked by pathogens, in which the randomly moving VAMP721/722 vesicles are re-directed to pathogen attacking sites for focal secretion.4
In addition to PEN1, SYP132 is also required for plant growth and immunity.15 Intriguingly, SYP132 is involved solely in defense against bacterial pathogens, whereas PEN1 is only in resistance to fungal pathogens.3,4,15 We recently found that VAMP721/722 can specifically interact with SYP132 in addition to PEN1 in plant cells.9 Although how VAMP721/722 can change their interacting PM syntaxins is not clear yet, the elevation of VAMP721/722 levels by bacteria-derived elicitors suggests a possibility that they may interact with SYP132 in response to bacterial infection but with PEN1 during fungal attack. Interestingly, PEN1 was reported to be involved in ABA-related trafficking of ion and water channels.16-18 Since VAMP721/722 levels are decreased by ABA,14 it is likely that PEN1 may interact with other VAMP protein(s) than VAMP721/722 during ABA responses. A recent finding that reduced expression of VAMP71 genes resulted in delayed ABA-associated stomatal closure19 suggests a possibility that PEN1 may interact with VAMP721/722 for defense responses but with VAMP71 proteins for ABA responses. Although a set of SNARE proteins involve in a specific membrane fusion in vivo, they promiscuously form the SNARE complex in vitro.20 Therefore, the exchange of partner proteins by PEN1 or VAMP721/722 might be assisted by accessory proteins such as Rab proteins, Sec1/Munc18-like (SM) proteins, and synaptotagmins, which are known mammalian proteins to control the localization and complex-forming/fusion activities of SNARE proteins.1,21
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from Rural Development Administration (PJ009763) and National Research Foundation (2011–0013248 and 2013R1A1A2058295), Korea
References
- 1.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Lipka V, Kwon C, Panstruga R. SNARE-ware: the role of SNARE-domain proteins in plant biology. Annu Rev Cell Dev Biol. 2007;23:147–74. doi: 10.1146/annurev.cellbio.23.090506.123529. [DOI] [PubMed] [Google Scholar]
- 3.Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;425:973–7. doi: 10.1038/nature02076. [DOI] [PubMed] [Google Scholar]
- 4.Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, et al. Co-option of a default secretory pathway for plant immune responses. Nature. 2008;451:835–40. doi: 10.1038/nature06545. [DOI] [PubMed] [Google Scholar]
- 5.Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G. Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol. 2001;155:239–49. doi: 10.1083/jcb.200107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Feechan A, Pedersen C, Newman MA, Qiu JL, Olesen KL, Thordal-Christensen H. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J. 2007;49:302–12. doi: 10.1111/j.1365-313X.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–11. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–7. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 9.Yun HS, Kwaaitaal M, Kato N, Yi C, Park S, Sato MH, Schulze-Lefert P, Kwon C. Requirement of vesicle-associated membrane protein 721 and 722 for sustained growth during immune responses in Arabidopsis. Mol Cells. 2013;35:481–8. doi: 10.1007/s10059-013-2130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16:3496–507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–60. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Humphry M, Bednarek P, Kemmerling B, Koh S, Stein M, Göbel U, Stüber K, Pislewska-Bednarek M, Loraine A, Schulze-Lefert P, et al. A regulon conserved in monocot and dicot plants defines a functional module in antifungal plant immunity. Proc Natl Acad Sci U S A. 2010;107:21896–901. doi: 10.1073/pnas.1003619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(Suppl):S15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi C, Park S, Yun HS, Kwon C. Vesicle-associated membrane proteins 721 and 722 are required for unimpeded growth of Arabidopsis under ABA application. J Plant Physiol. 2013;170:529–33. doi: 10.1016/j.jplph.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kalde M, Nühse TS, Findlay K, Peck SC. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci U S A. 2007;104:11850–5. doi: 10.1073/pnas.0701083104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyman B, Geelen D, Quintero FJ, Blatt MR. A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science. 1999;283:537–40. doi: 10.1126/science.283.5401.537. [DOI] [PubMed] [Google Scholar]
- 17.Honsbein A, Sokolovski S, Grefen C, Campanoni P, Pratelli R, Paneque M, Chen Z, Johansson I, Blatt MR. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell. 2009;21:2859–77. doi: 10.1105/tpc.109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besserer A, Burnotte E, Bienert GP, Chevalier AS, Errachid A, Grefen C, Blatt MR, Chaumont F. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell. 2012;24:3463–81. doi: 10.1105/tpc.112.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leshem Y, Golani Y, Kaye Y, Levine A. Reduced expression of the v-SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid-dependent stomatal closure. J Exp Bot. 2010;61:2615–22. doi: 10.1093/jxb/erq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem. 1999;274:15440–6. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 21.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–7. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]


