Abstract
The methylesterification status of cell wall pectins, mediated through the interplay of pectin methylesterases (PMEs) and pectin methylesterase inhibitors (PMEIs), influences the biophysical properties of plant cell walls. We found that the overexpression of a PMEI gene in Arabidopsis thaliana plants caused the stems to develop twists and loops, most strongly around points on the stem where leaves or inflorescences failed to separate from the main stem. Altered elasticity of the stem, underdevelopment of the leaf cuticle, and changes in the sugar composition of the cell walls of stems were evident in the PMEI overexpression lines. We discuss the mechanisms that potentially underlie the aberrant growth phenotypes.
Keywords: Arabidopsis thaliana, pectin methylesterification, stem growth, organ separation, cuticle integrity
Introduction
Homogalacturonan, the most abundant pectin of the plant cell wall, can be methylesterified at the C-6 position of the galacturonic acid residues.1 Demethylesterification of cell wall homogalacturonan is catalyzed by pectin methylesterases (PMEs); this process can promote the formation of Ca2+cross-links along the stretches of the demethylesterified galacturonic acid residues.1 Demethylesterification also increases the susceptibility of the modified homogalacturonans to polygalacturonase.2 Evidence is accumulating that biomechanical properties affecting growth potential are modified by these changes. Recently, a connection between the pectin methylesterification status and the biomechanical properties of Arabidopsis stems was established.3 This report focuses on some aspects of the growth and morphological phenotypes associated with PME modulation in Arabidopsis.
Results
Arabidopsis stems mostly grow by cell elongation in the internode regions.4 When cell elongation ceases, secondary cell walls are established. Axillary branching can produce lateral shoots from the meristems in the leaf axils. These meristems can either directly carry an inflorescence, or repeat the pattern of the main shoot including secondary branching.5
To study how changes in the extent of methylesterification of cell wall pectins affect Arabidopsis growth and development, we overexpressed a gene encoding the pectin methylesterase inhibitor, PMEI56,7 by use of the Cauliflower mosaic virus 35S promoter (PMEI5 OE plants). A range of phenotypes are affected in the PMEI5 OE plants, including decreased fertility, faster seed germination, and larger seed size.7 Here we report on other strong morphological phenotypes that were apparent in these lines. Contrary to the wild-type (WT) plants, which had straight stems (Fig. 1A and C), the stems of PMEI5 OE plants grew in waves, twists and loops (Fig. 1B and D). These aberrant growth patterns appeared to occur almost exclusively at branching points, where a side stem carrying a leaf (Fig. 1D) or an inflorescence (Fig. 1G and H) failed to separate properly from the main stem. This failed separation often led to a thickened fasciated stem (e.g., Figure 1G), and to the stem curling inwards around the site of unsuccessful separation to a varying extent. We observed that fusion always took place, but to a different extent in individual plants, with some of them showing a fusion of all side branches and inhibited internode elongation, and some showing only a few side branches or flowers fused to the stem.
Figure 1. Growth phenotypes and leaf cuticle characteristics of Arabidopsis thaliana plants overexpressing the PMEI5 gene. WT plants (A) exhibited straight stems, while PMEI OE plants (B) had twisted stems and stunted growth. The WT plants (C, F) showed clear organ separation; in PMEI OE plants (D, G, and H) the side stems carrying a leaf or inflorescence failed to separate from the main stem. This led to a thickened stem and twisting growth around the areas of failed separation. Note that the stem regions associated with an unseparated leaf or inflorescence were always on the inside of a twist or loop. (E) Comparison of toluidine blue staining of leaves of WT- (left) and PMEI5 OE- plants (right) to evaluate the integrity of the cuticle. Toluidine blue cannot penetrate an intact cuticle; this dye was readily washed off from the surfaces of the WT leaves (E, left). In PMEI OE leaves (E, right), the staining remained after washing, indicating a cuticle defect. Also note that the cuticle of the WT leaves appeared as a whitish layer under the dissection microscope light.
The organ fusion phenotype that characterized the PMEI5 OE lines resembles the phenotypes associated with mutants having a defective cuticle.8 Toluidine blue staining is a good indicator of cuticle integrity as this dye is readily washed off from plant cell surfaces with an intact cuticle, but is able to penetrate the tissue of plant surfaces with an underdeveloped or otherwise defective cuticle.9 Staining of leaves showed clear differences in cuticle integrity between the WT and PMEI5 OE plants (Fig. 1E): in contrast to those of the WT plants, the dye remained associated with the leaf tissues of the PMEI5 OE plants even after extensive washing, indicative of a cuticle defect. The distribution of the non-removable dye on the leaf surface was patchy, indicating a discontinuous cuticle.9 Microscopy revealed no cracks or tears in the epidermis through which the dye could have entered. We observed a similar but not as strong difference in stems.
Measurements of the elasticity of stems revealed that those of the PMEI5 OE plants stood up less rigorously to different stress loads as compared with the stems of WT plants (Fig. 2). Notably the OE stems were more difficult to clamp into the devise for measuring elasticity. However, the difference in the stress behavior between the WT and PMEI5 OE stems was striking, and a calculation of the elastic modulus showed a difference of 2 orders of magnitude (WT: 596.2+/− 7.4MPa vs. PMEI OE: 5.5+/− 0.05 MPa).
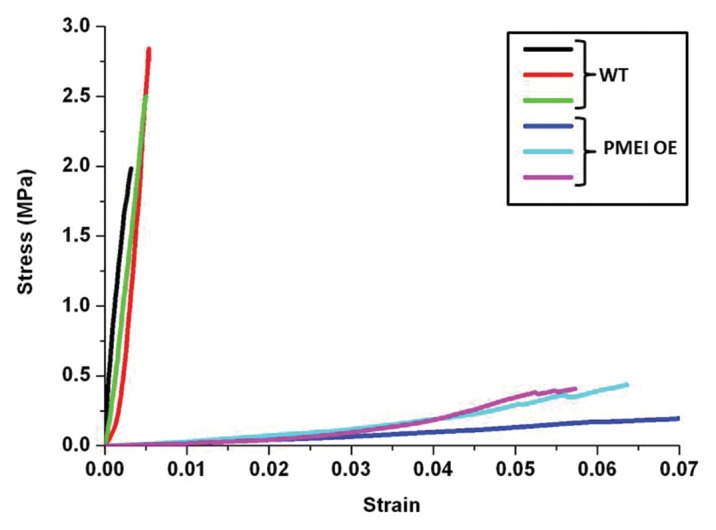
Figure 2. Stress-strain-curves for stem segments of 3 WT lines as compared with the stem segments of 3 PMEI5 OE lines.
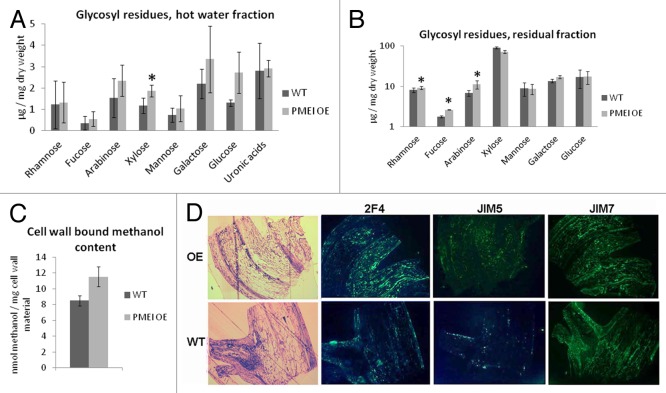
A comparative analysis of the sugars and sugar derivatives of the cell walls of PMEI5 OE stems and WT stems revealed that xylose (p < 0.05) was more abundant in the former (Fig. 3A, pectin-enriched - hot water extracted - fraction). The levels of uronic acids, which would largely be galacturonic acids of pectins, were not significantly changed. Significantly higher amounts of neutral sugars in the residual fraction, i.e., rhamnose, fucose and arabinose, were found in the cell walls of the OE plants as compared with the WT plants (all p < 0.05) (Fig. 3B). Thus changes in sugar composition were evident, but the pectic uronic acid fraction was unchanged as a result of PMEI5 overexpression.
Figure 3. Sugar composition of cell walls of WT- and PMEI5 OE- stems and immunostaining of stem sections to detect homogalacturonans of differing methylesterification. (A) Glycosyl residues in the hot water soluble fraction and (B) residual fraction. The data are based on the average of 5 replicates (+/− SD). An asterisk over a column indicates a significant (p < 0.05, paired t-test) difference between PMEI5 OE and WT. (C) Estimate of esterification levels through quantification of methanol released on saponification. The average of 4 biological replicates (+/− SE) are shown. (D) Representative immuno-staining of sections of stem branching points of the PMEI5 overexpressor (OE) and wild-type (WT) plants. Sections stained with toluidine blue were immunostained with the antibodies 2F4 (binds to homogalacturonan stretches that are largely demethylesterified and cross linked by calcium bridges), JIM5 (binds to highly methylesterified pectin with a loose sequence of methyl groups), and JIM7 (binds to highly methylesterified homogalacturonans with dense stretches of methylesters).
In our previous work we determined that PME activity is reduced in all organs (including stems) as a result of PMEI5 overexpression.7 Here we determined that the decreased PME activity directly leads to an increased degree of cell wall pectin methylesterification in the stem. This was assessed by estimating the amount of methanol released from the cell wall materials by saponification and subsequent oxidation (Fig. 3C). The methanol would be derived solely from methylesters associated with the homogalacuronans of the cell wall fraction.
To further investigate the spatial aspects of the degree of methylesterification of cell wall pectins, WT and PMEI5 OE stems at branching regions were analyzed by immunfluorescence, in which they were challenged with different antibodies specific for homogalacturonans of differing degrees and patterns of methylesterification. These included a JIM7-antibody, which binds exclusively to highly methylesterified homogalacturonans with dense stretches of methylesters;10 JIM5, which recognizes pectins with lower degrees of methylesterification and more sporadic methylesters, and the 2F4 antibody, which recognizes homogalacturonan stretches that are largely demethylesterified and cross-linked by calcium bridges.11 The differential binding of the antibodies to the OE vs. WT sections did not show much discrimination with respect to general fluorescence intensity. However, the OE stem sections showed less organization in the distribution of antibody binding (i.e., less spatial specificity) as compared with the WT. For example, in contrast to the OE, the WT showed JIM5 binding only in very specific domains around the epidermal areas of the side branch. The 2F4 antibody also bound in a differential fashion to the WT sections, with the highest fluorescence signals again occurring in the epidermal regions of the side branch, and also in the stem. JIM7 binding to WT sections was most tenacious in the stem and the side branch, with a decreased intensity of fluorescence in the inner tissues of the side branch. Thus the inhibition of PME activity by overexpression of PMEI5 caused an increase in methylesterification of cell wall homogalacturonans (Fig. 3C), but the changes to cell walls were spatially regulated (Fig. 3D).
Discussion
Our findings support the emerging perception that the pectin matrix plays a critical and dynamic role in plant development and morphology. The regulatory mechanisms underlying changes in cell growth and organogenesis that are evident upon PME modulation, may be direct, e.g., by changing the biomechanical properties of organs, and thus the driving forces for cell growth by expansion. Indirect changes may also be relevant, such as eliciting changes in non-pectin cell wall components or altering lignin deposition (and thus cell wall extensibility), or interfering with the deposition of cuticular waxes.
There is evidence for a role of pectin methylesterification in the mechanical characteristics of the stem. For example, an Arabidopsis mutant with a defective PME gene (the loss-of-function mutant pme35) has a “floppy” stem phenotype.3 Yet interestingly, the biomechanical properties of the stems of our PMEI5 OE plants are clearly the opposite of what is observed for the stems of pme35 plants. The overexpression of PMEI5 likely has more widespread effects than the loss-of-function mutant (i.e., it likely affects the activities of several PMEs with different spatial characteristics) and thus the overall effect on the biomechanical properties of the stem is different. In addition, the anatomy of the side branches will be affected in areas where no separation has occurred. For example, the vasculature might remain fused to a certain extent, and the distribution of lignins could be altered, leading to altered biomechanical properties. Our experiments do not allow us to pinpoint the exact biochemical and anatomical changes that lead to the altered elasticity and morphology of the PMEI5 OE plants.
We observed statistically significant differences in the content of 4 neutral sugars. The xylose content was elevated in PMEI OE- compared with WT- stems in the hot water extractable pectic fraction, and rhamnose, fucose, and arabinose were elevated in the TCA residual fraction, which is derived mainly from hemicelluloses and some remaining pectins. Fucose could be derived from xyloglucans,12 but as the levels of xylose and other xyloglucan components were unchanged in the residual fraction, it is unlikely that higher fucose is an indication of higher xyloglucan levels. Arabinose could be associated with cell wall pectic arabinans, which have recently been shown to influence the mechanical properties of the stem: arabinan-deficient mutants exhibit enhanced rigidity in response to compression stress, and altered responsiveness to mechanical stress.13 The changes in sugars may be a result of the anatomical changes (e.g., the fusion phenotype), as the proportions of different tissues comprising the stem may be consequently altered (e.g., vascular vs. cortical tissues). Alternatively or additionally, they may reflect an altered distribution of methylesterified pectins, which may further influence the deposition of other cell wall components.
Pectin methylesterases also participate in organ initiation,14,15 and this has been attributed to changes in the viscoelastic properties of the meristem cells as a result of altered pectin demethylesterification, which might act as a mechanical signal. Such a mechanism may account for the failed separation of side branches from the main stem that was evident in our PMEI OE plants. It may also account for the failure of the meristems to develop proper boundaries between primordia, and thus failed separation from the main stem. We did not observe an altered phyllotaxis as Peaucelle et al. have found in plants that have an altered expression of PME35 in the internodes.16 This may indicate that PME35 is not a target of PMEI5. However, we did observe an overall shortened phenotype.
At another level of control, brassinosteroids have been shown to mediate the plant cell’s attempts to re-establish cell wall homeostasis and thus growth in the face of altered PMEI expression.6 Brassinosteroid signaling is upregulated as a result of PMEI5 overexpression,7 and brassinosteroids have repressive effects on organ boundary regulating genes of the shoot meristem.17 This type of mechanism may underlie the lack of organ separation observed in the present study for the PMEI5 OE lines.
Stem epidermal cells are normally covered with cutin and other waxes; the cuticle is established while the cells grow.18 Synthesis and deposition of cuticular waxes is rapidly increased to thicken the cuticle when bolting sets in.18 This requires the transport of the fatty acids that will be integrated into the cuticle through the expanding cell wall. Altered pectin methylesterification may have affected lipid transport through changes in the porosity of the pectin matrix. In addition, the proportion of negatively charged C6 carboxyl groups would be reduced. This would lead to reduced Ca2+-bridges, as well as to a lowered density of negative charges associated with the galacturonic acid residues of the pectins, perhaps leading to a deficiency of anchor points for interaction with the lipid transport or cutin synthesis machinery. The discontinuous cuticle that we have identified by the patchy staining pattern has also been observed in other mutants that display organ fusions.9 A normal cuticle may be paramount to facilitating organ separation;9 the structurally defective cuticle that characterized the PMEI5 OE plants of the present study may well have led to the abnormal fusions between the stem and lateral branches, and to a reduced elasticity of the stems. Organ fusion defects are very common to mutants with defective cuticles.19
Materials and Methods
Plant materials
Arabidopsis wild-type plants (Col-0) (WT) and plants overexpressing the PMEI5 gene under control of the CaMV (35S) promotor7 were grown in soil in pots maintained in a growth chamber at 22°C with a 16 h photoperiod (long days).
Cell wall composition analysis and quantification of methylesters
Dried stems from mature Arabidopsis plants were ground in a ball mill (Retsch, www.retsch.de) to a fine powder and cell wall material was prepared as described.7 The pellet was resuspended in water and heated to 60°C for 15 min under constant agitation to extract the pectins. This procedure was repeated twice and the pectin-rich supernatants were pooled (hot water fraction). The remaining pellet was treated as described.7 The content of neutral monosaccharides was determined by GC-MS analysis of the respective alditol acetates, as previously described.20 Methylester quantification on stem sections of PMEI OE and WT plants was performed as described.7
Immunofluorescence analyses
Stem sectioning of WT and PMEI OE plants, sample preparation, immunostaining, and microscopy was performed as described.7
Evaluation of Elasticity
Measurements of WT and PME OE stems were performed on a Microtest 200 (Gatan, UK) with a load cell range of 2N. The pre-dried stems were cut into segments (5 cm) including 2 internodes each, and attached to the tester frame with clamps on both ends. The test length between the clamps was 3 cm. A strain rate of 0.5 mm/min was applied. The experiment was stopped when the samples detached at the clamps or in the case of the PMEI OE stems, they broke off at the nodes. The linear part of the stress/strain curves was used to calculate the elasticity modules (WT: between 0.5 and 2 MPa, PMEI OE: between 0.05 and 0.2 MPa) as previously described.21 The diameter of the PMEI OE stems was approximately twice that of the WT stems. The exponential phase occurred later in the PMEI OE stems, as they were straightened first.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Christiane Nawrath and Dr. Rochus Franke for their helpful discussions regarding cuticle integrity and thank Dr. Markus Günl for his help with the GC-MS analysis of alditol acetates. We are also grateful for the facilities provided by The Centre of Plant Integrative Biology, University of Nottingham.
References
- 1.Wolf S, Mouille G, Pelloux J. Homogalacturonan methyl-esterification and plant development. Mol Plant. 2009;2:851–60. doi: 10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- 2.Wakabayashi K, Hoson T, Huber DJ. Methyl de-esterification as a major factor regulating the extent of pectin depolymerization during fruit ripening: a comparison of the action of avocado (Persea americana) and tomato (Lycopersicon esculentum) polygalacturonases. J Plant Physiol. 2003;160:667–73. doi: 10.1078/0176-1617-00951. [DOI] [PubMed] [Google Scholar]
- 3.Hongo S, Sato K, Yokoyama R, Nishitani K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell. 2012;24:2624–34. doi: 10.1105/tpc.112.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall H, Ellis B. Transcriptional programming during cell wall maturation in the expanding Arabidopsis stem. BMC Plant Biol. 2013;13:14. doi: 10.1186/1471-2229-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz EA, Haughn GW. LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell. 1991;3:771–81. doi: 10.1105/tpc.3.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf S, Mravec J, Greiner S, Mouille G, Höfte H. Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol. 2012;22:1732–7. doi: 10.1016/j.cub.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B, Haughn G, Kermode AR. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013;161:305–16. doi: 10.1104/pp.112.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lolle SJ, Hsu W, Pruitt RE. Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics. 1998;149:607–19. doi: 10.1093/genetics/149.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Tanaka H, Chinoku M, Wanatabe M, Machida Y. A new method for rapid visualisation of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004;37:139–46. doi: 10.1046/j.1365-313X.2003.01946.x. [DOI] [PubMed] [Google Scholar]
- 10.Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–21. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- 11.Willats WG, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res. 2000;327:309–20. doi: 10.1016/S0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- 12.Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. Arabidopsis - a powerful model system for plant cell wall research. Plant J. 2010;61:1107–21. doi: 10.1111/j.1365-313X.2010.04161.x. [DOI] [PubMed] [Google Scholar]
- 13.Verhertbruggen Y, Marcus SE, Chen J, Knox JP. Cell wall pectic arabinans influence the mechanical properties of Arabidopsis thaliana inflorescence stems and their response to mechanical stress. Plant Cell Physiol. 2013;54:1278–88. doi: 10.1093/pcp/pct074. [DOI] [PubMed] [Google Scholar]
- 14.Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol. 2011;21:1720–6. doi: 10.1016/j.cub.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Kierzkowski D, Nakayama N, Routier-Kierzkowska AL, Weber A, Bayer E, Schorderet M, Reinhardt D, Kuhlemeier C, Smith RS. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science. 2012;335:1096–9. doi: 10.1126/science.1213100. [DOI] [PubMed] [Google Scholar]
- 16.Peaucelle A, Louvet R, Johansen JN, Salsac F, Morin H, Fournet F, Belcram K, Gillet F, Höfte H, Laufs P, et al. The transcription factor BELLRINGER modulates phyllotaxis by regulating the expression of a pectin methylesterase in Arabidopsis. Development. 2011;138:4733–41. doi: 10.1242/dev.072496. [DOI] [PubMed] [Google Scholar]
- 17.Gendron JM, Liu J-S, Fan M, Bai M-Y, Wenkel S, Springer PS, Barton MK, Wang Z-Y. Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis. Proc Natl Acad Sci U S A. 2012;109:21152–7. doi: 10.1073/pnas.1210799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 2005;139:1649–65. doi: 10.1104/pp.105.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krolikowski KA, Victor JL, Wagler TN, Lolle SJ, Pruitt RE. Isolation and characterization of the Arabidopsis organ fusion gene HOTHEAD. Plant J. 2003;35:501–11. doi: 10.1046/j.1365-313X.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 20.Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. J Vis Exp. 2010 doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kompella MK, Lambros J. Micromechanical characterization of cellulose fibers. Polym Test. 2002;21:523–30. doi: 10.1016/S0142-9418(01)00119-2. [DOI] [Google Scholar]