Abstract
Mitogen-activated protein kinases and their targets have been in the limelight of plant stress research. Signaling pathways mediating the responses to multiple stresses deserve particular attention. In a recent study, we reported AZI1, a member of the lipid transfer protein family, to play a role in MPK3-mediated responses to salt stress in Arabidopsis thaliana. MPK3 controls AZI1 at the transcriptional and posttranslational level. The AZI1 protein has several properties that make it very attractive for genetic engineering. A model of multi-level control of AZI1 by MPK3 is proposed, and strategies toward optimizing AZI1 protein properties are briefly discussed.
Keywords: AZI1, Arabidopsis, Mitogen-activated protein kinase, WRKY, lipid transfer protein, multiple control, phosphorylation, salt stress, stress response
Abiotic and biotic stress largely impede plant development. Environmental challenges thus drastically limit agricultural productivity worldwide. Mitogen-activated protein kinase (MAPK) cascades are highly conserved signaling relays in eukaryotic organisms. In Arabidopsis, the MAPK MPK3 mediates numerous abiotic and biotic stress responses. Molecules directly targeted by MPK3 are attractive candidates for improving stress tolerance in plants.
We recently identified Arabidopsis lipid transfer protein AZI1 (azelaic acid induced 1) as a direct target of the stress-induced mitogen-acitvated protein kinase MPK3.1 Hitherto, evidence existed only for a cyto-nuclear distribution of MPK3. We found a subfraction of MPK3 to be associated with the plasma membrane. At distinct regions in the plasma membrane, MPK3 interacts with AZI1. Moreover, a cell-cell contact appears to be required for the interaction. Mutants affected in either gene are hypersensitive to salt stress. We found AZI1 overexpression to improve salt stress tolerance in transgenic plants. Importantly, this effect is clearly dependent on functional MPK3. Immunoblot studies on plants overexpressing AZI1 in the wild type or mpk3 mutant background point to a role of MPK3 as positive regulator of AZI1 protein stability. Accordingly, bioinformatics predictions compute a higher protein stability for phosphorylated AZI1 variants. Experimental evidence exists that MPK3 phosphorylates AZI1 at several residues (Pitzschke, unpublished). MPK3 also regulates AZI1 transcript abundance. Such multi-level control is also known from other MAPK substrates, including MYB44 and WRKY33.2,3
Transcriptional regulation of AZI1
MPK3 protein levels and activity increase upon diverse stress treatments within minutes.4 Most of these stress stimuli also trigger AZI1 gene expression (GENEVESTIGATOR). mpk3 mutants have lower levels of endogenous AZI1 transcript, as compared with wild type plants. Among the list of bona-fide and putative MPK3 substrates,5,6 transcription factors (TFs) are clearly over-represented. One straight-forward question arises from these observations: Is AZI1 gene expression controlled by a MPK3-activated transcription factor(s)? And if so, can candidates be predicted?
Transcription factors known to be directly activated by MPK3 phosphorylation include several members of the WRKY family (WRKY6, WRKY33, WRKY53, WRKY62) as well as SPEECHLESS, ERF,3,7 bZIP transcription factor VIP1,8 and MYB44.2 Various WRKY proteins, VIP1, and MYB44 have been implicated in the signaling of numerous and diverse stress responses.
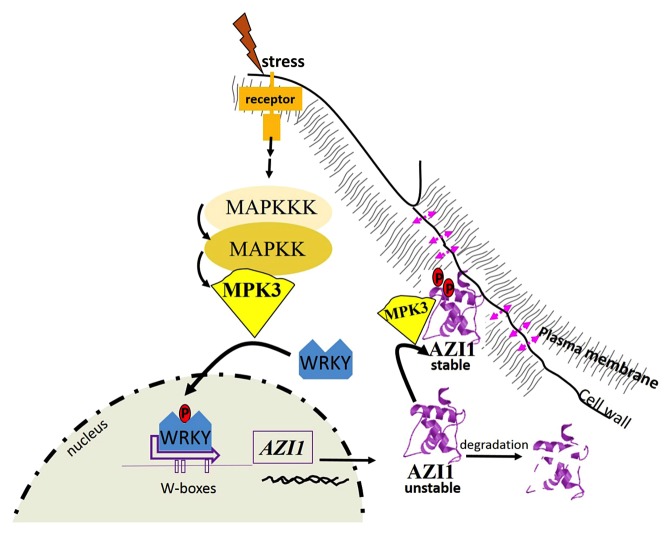
WRKY proteins recognize W-boxes (TTGAC). VIP1 binds to its cognate motive VRE (VIP1 response element; ACNGCT) in the promoters of multiple stress-responsive genes.8 MYB44 preferentially binds to MBSII (MYB binding site II; G(G/T)T(A/T)G(G/T)T). Screening of the AZI1 promoter sequence (1 kb upstream of transcription start) reveals several cis-elements potentially targeted by these TFs (Fig. 1). Particularly striking, a dense cluster is formed by 3 W-boxes (at position -276 to – 263). A (preliminary) model in which AZI1 gene expression is controlled by MPK3-activated transcription factors (WRKYs?) may therefore be proposed (Fig. 2).
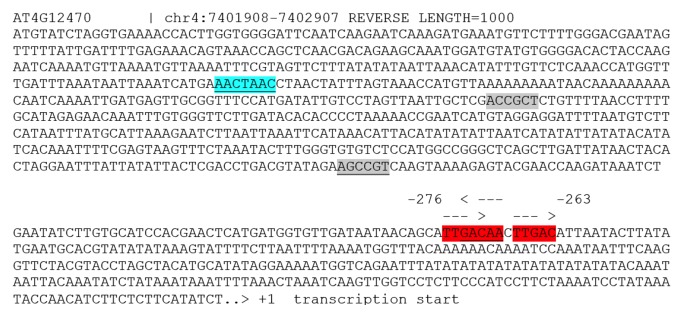
Figure 1.AZI1 1000 bp upstream regulatory region. Putative cis-regulatory elements potentially targeted by MPK3-activated transcription factors are highlighted. (reverse orientation underlined). MBSII (blue), VRE (gray), W-box (red).
Figure 2. Proposed model of AZI1 regulation in MPK3-mediated stress responses. Stress perception initiates MAPK cascade signaling. Stress-activated MPK3 phosphorylates WRKY transcription factor(s) (blue), which subsequently induce AZI1 gene expression through binding to W-boxes in the AZI1 Promoter. In mpk3 mutants, AZI1 protein is unstable. AZI1 and MPK3 interact at the plasma membrane; and a cell-cell-contact appears to be required for complex formation (pink arrows). Phosphorylation by MPK3 likely stabilizes the AZI1 protein.
Genetic engineering using phosphomimetic AZI1 variants
The AZI1 protein has various properties which render it an attractive target for biotechnological applications: 1) Its closest homolog, EARLI1, shows antimicrobial activity toward fungal pathogens and S. cerevisae.9 In addition, AZI1 and EARLI1 likely have bactericidal activities. Diverse and numerous attempts to express full-length recombinant proteins in E. coli failed, while high yields were obtained for deletion variants.1 2) AZI1 is required for systemic acquired resistance.10 Transgenic plants overexpressing AZI1 exhibit improved tolerance toward salt1 and freezing stress.11 What is more, AZI1 overexpression has positive effects also in non-plant eukaryotic systems: AZI1 and its closest homologs confer freezing and osmotic stress tolerance to yeast,11,12 an organism that naturally lacks LTPs but contains MAPKs.
The gathered knowledge on AZI1 and its regulation can now be employed for targeted engineering of an AZI1 variant with optimised properties to improve stress tolerance in plants. As strongly suggested in our recent study, phosphorylation positively controls both protein stability and the stress tolerance-enhancing effects of AZI1. It is currently unknown whether AZI1 is recognized as MAPK substrate in organisms other than Arabidopsis. For these reasons, a phosphomimetic AZI1 variant seems the most promising candidate for genetic engineering. Data from our ongoing research suggest AZI1 to be phosphorylated at several residues. Furthermore, bioinformatic analyses compute a gradual increase in AZI1 protein stability upon successive replacement of the 5 putative MAPK-targeted sites by phosphomimetic amino acids (Asp or Glu). Therefore, multiple exchanges in the “phosphorylation island” (comprising Ser33, Ser41, Ser59, Thr66, Thr70) to Asp or Glu residues likely yield an AZI1 variant with the desired characteristics (Fig. 3).
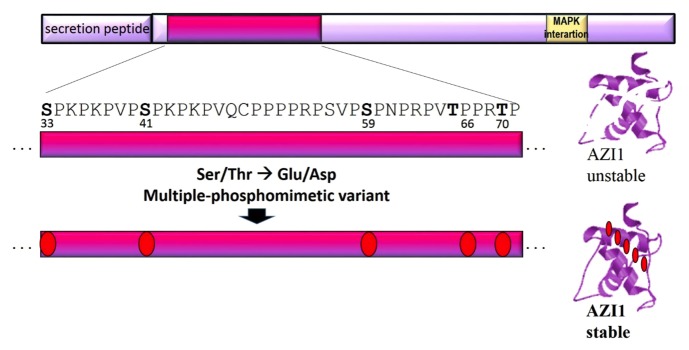
Figure 3. Optimised AZI1 variant proposed for genetic engineering. Top: schematic image of AZI1 primary protein sequence. The region spanning the 5 putative MAPK phosphorylation sites is highlighted and enlarged. Replacing all or a subset of these residues by phosphomimetic amino acids is expected to improve AZI1 protein stability and to make AZI1 protein function independent of MAPK phosphorylation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Funding by the Austrian Research Foundation (FWF), projects P21851 (H.P, S.D.) and V-167-B09 (A.P.) is gratefully acknowledged.
References
- 1.Pitzschke A, Datta S, Persak H. Salt Stress in Arabidopsis: Lipid Transfer Protein AZI1 and Its Control by Mitogen-Activated Protein Kinase MPK3. Mol Plant. 2013 doi: 10.1093/mp/sst157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persak H, Pitzschke A. Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signalling. PLoS One. 2013;8:e57547. doi: 10.1371/journal.pone.0057547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23:1639–53. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Šamajová O, Plíhal O, Al-Yousif M, Hirt H, Šamaj J. Improvement of stress tolerance in plants by genetic manipulation of mitogen-activated protein kinases. Biotechnol Adv. 2013;31:118–28. doi: 10.1016/j.biotechadv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, Dinesh-Kumar SP. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoehenwarter W, Thomas M, Nukarinen E, Egelhofer V, Röhrig H, Weckwerth W, Conrath U, Beckers GJ. Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol Cell Proteomics. 2013;12:369–80. doi: 10.1074/mcp.M112.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasson E, Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010;15:106–13. doi: 10.1016/j.tplants.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Pitzschke A, Djamei A, Teige M, Hirt H. VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc Natl Acad Sci U S A. 2009;106:18414–9. doi: 10.1073/pnas.0905599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Zhang C, Xu D, Schläppi M, Xu ZQ. Expression of recombinant EARLI1, a hybrid proline-rich protein of Arabidopsis, in Escherichia coli and its inhibition effect to the growth of fungal pathogens and Saccharomyces cerevisiae. Gene. 2012;506:50–61. doi: 10.1016/j.gene.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 10.Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZY, Zhang X, Schläppi M, Xu ZQ. Cold-inducible expression of AZI1 and its function in improvement of freezing tolerance of Arabidopsis thaliana and Saccharomyces cerevisiae. J Plant Physiol. 2011;168:1576–87. doi: 10.1016/j.jplph.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Schläppi M. Cold responsive EARLI1 type HyPRPs improve freezing survival of yeast cells and form higher order complexes in plants. Planta. 2007;227:233–43. doi: 10.1007/s00425-007-0611-2. [DOI] [PubMed] [Google Scholar]