Abstract
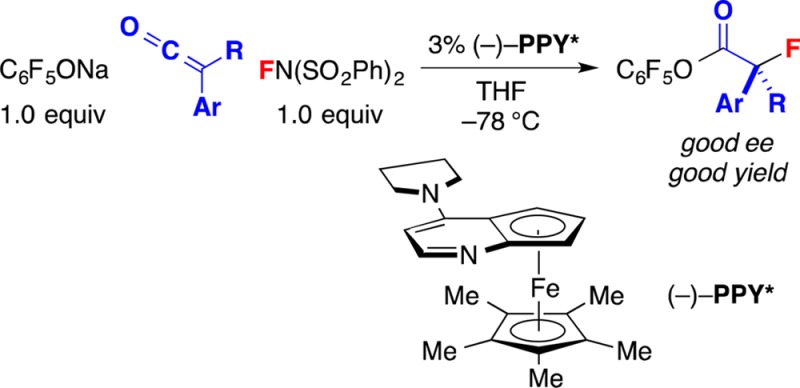
The catalytic asymmetric synthesis of alkyl fluorides, particularly α-fluorocarbonyl compounds, has been the focus of substantial effort in recent years. While significant progress has been described in the formation of enantioenriched secondary alkyl fluorides, advances in the generation of tertiary alkyl fluorides have been more limited. Here, we describe a method for the catalytic asymmetric coupling of aryl alkyl ketenes with commercially available N-fluorodibenzenesulfonimide (NFSI) and C6F5ONa to furnish tertiary α-fluoroesters. Mechanistic studies are consistent with the hypothesis that the addition of an external nucleophile (C6F5ONa) is critical for turnover, releasing the catalyst (PPY*) from an N-acylated intermediate. The available data can be explained by a reaction pathway wherein the enantioselectivity is determined in the turnover-limiting transfer of fluorine from NFSI to a chiral enolate derived from the addition of PPY* to the ketene. The structure and the reactivity of the product of this proposed elementary step, an α-fluoro-N-acylpyridinium salt, have been examined.
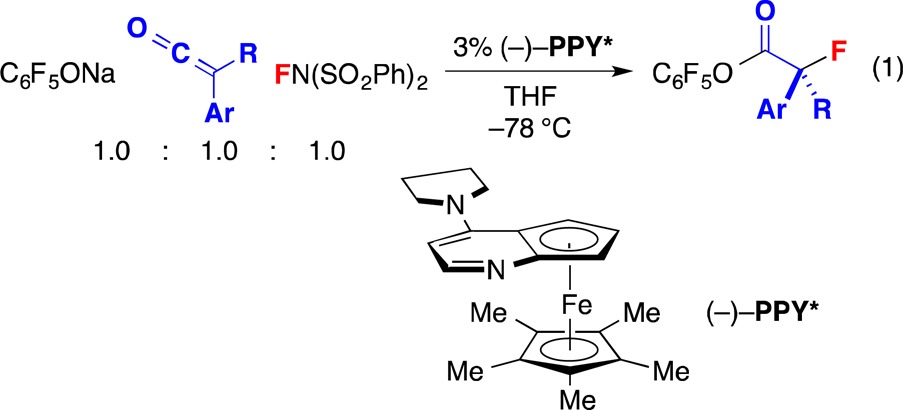
Because of properties such as the high electronegativity and the small size of fluorine, as well as the relative stability of the C–F bond, the incorporation of fluorine into organic molecules, including stereoselective processes, has become a widely used method in medicinal chemistry for altering the bioactivity of drug candidates.1 The catalytic enantioselective α-fluorination of carbonyl compounds has been a subject of particular interest,2 and an array of versatile methods have been described for the generation of such secondary alkyl fluorides.3,4 In contrast, except in the case of doubly activated molecules,2,5 there has been limited progress in the development of effective processes that furnish tertiary α-fluorocarbonyl compounds,6−8 and we are not aware of any methods that directly afford esters,9 with the exception of kinetic resolutions.10 Here, we establish that a planar-chiral nucleophilic catalyst (PPY*) can achieve the coupling of a ketene,11 an electrophilic fluorine source,12 and an alkoxide, thereby providing such tertiary alkyl fluorides with high enantioselectivity (eq 1). We also present mechanistic studies that help to illuminate the reaction pathway.
 |
1 |
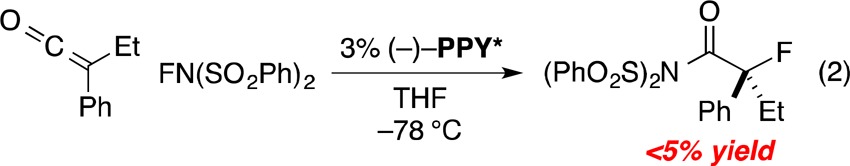
In an earlier study, we reported that PPY* can catalyze the asymmetric chlorination of ketenes by 2,2,6,6-tetrachlorocyclohexanone to produce tertiary α-chloroesters.13 When we attempted to extend this halogenation strategy to an analogous enantioselective fluorination of ketenes, building in part on the pioneering work of Lectka on the cinchona alkaloid/palladium/LiClO4-catalyzed synthesis of secondary alkyl fluorides from acid chlorides and N-fluorodibenzenesulfonimide (NFSI; commercially available),4 we obtained discouraging results (eq 2).
 |
2 |
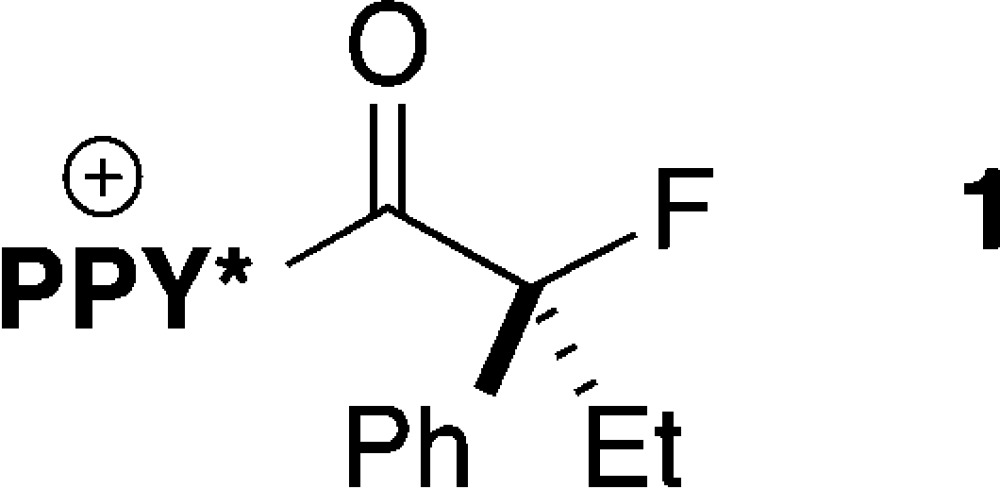
We postulated that our failure to achieve the desired catalytic asymmetric fluorination might be due to the stability of a potential N-acylpyridinium intermediate (e.g., 1) toward (SO2Ph)2N–.
 |
Consequently, we decided to determine if the addition to the reaction mixture of a stoichiometric quantity of a more reactive nucleophile might be beneficial. We were, of course, cognizant of the possibility that the nucleophilic additive might react directly with the ketene to generate an achiral enolate and racemic product; fortunately, however, we were able to identify a nucleophile that allowed us to achieve our objective (Table 1).
Table 1. Catalytic Asymmetric Synthesis of Tertiary Alkyl Fluorides: Effect of Added Nucleophilea.
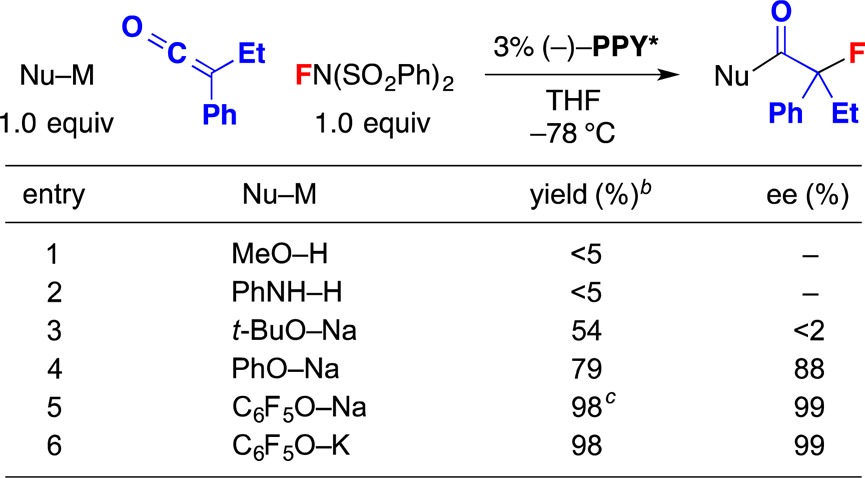
All data are the averages of two experiments.
Determined through GC analysis with the aid of an internal standard.
Yield of purified product.
Whereas the addition of MeOH or PhNH2 did not result in a significant amount of the desired tertiary alkyl fluoride (Table 1, entries 1 and 2), the use of alkoxides led to a substantial quantity (entries 3–5). In the case of sodium tert-butoxide, the product was racemic (entry 3); on the other hand, sodium phenoxide furnished good enantioselectivity (entry 4), and a less nucleophilic phenoxide, sodium pentafluorophenoxide, provided excellent yield and ee (entry 5).14
Our optimized method is effective for the catalytic enantioselective synthesis of tertiary α-fluoroesters from an array of aryl alkyl ketenes (Table 2).15 The alkyl group of the ketene can vary in size from Me to cyclopentyl (entries 1–5; lower ee is observed with larger alkyl groups), and the aromatic substituent can be electron-poor, electron-rich, para-substituted, or meta-substituted (entries 6–9), as well as naphthyl or heteroaryl (entries 10 and 11). On a gram scale, the fluorination illustrated in entry 2 of Table 2 proceeded in 99% ee and 90% yield.
Table 2. Catalytic Asymmetric Synthesis of Tertiary Alkyl Fluoridesa.
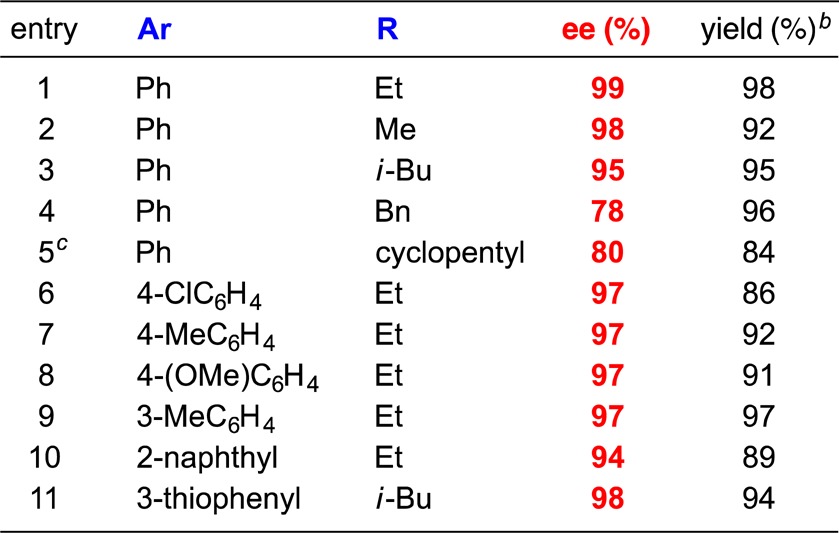
For the reaction conditions, see eq 1. All data are the averages of two experiments.
Yield of purified product (contains ≤5% of the non-fluorinated ester).
Catalyst loading: 10%.
Although our other chiral 4-(dimethylamino)pyridine (DMAP)-catalyzed reactions of ketenes have generally not been effective for dialkyl ketenes,16 in the case of this asymmetric fluorination process, we have observed a promising level of enantioselectivity (eq 3).
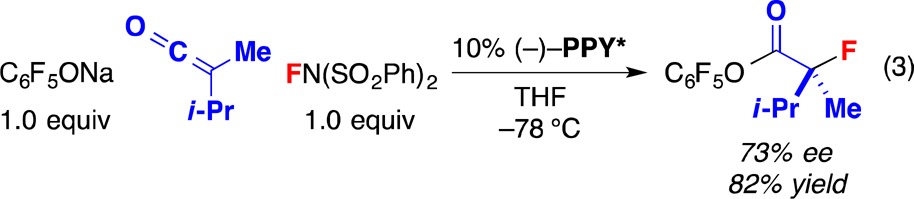 |
3 |
The enantioenriched α-fluoroesters that are generated in this catalytic asymmetric C–F bond-forming process can be transformed in good yield into a variety of other tertiary alkyl fluorides (Figure 1).
Figure 1.
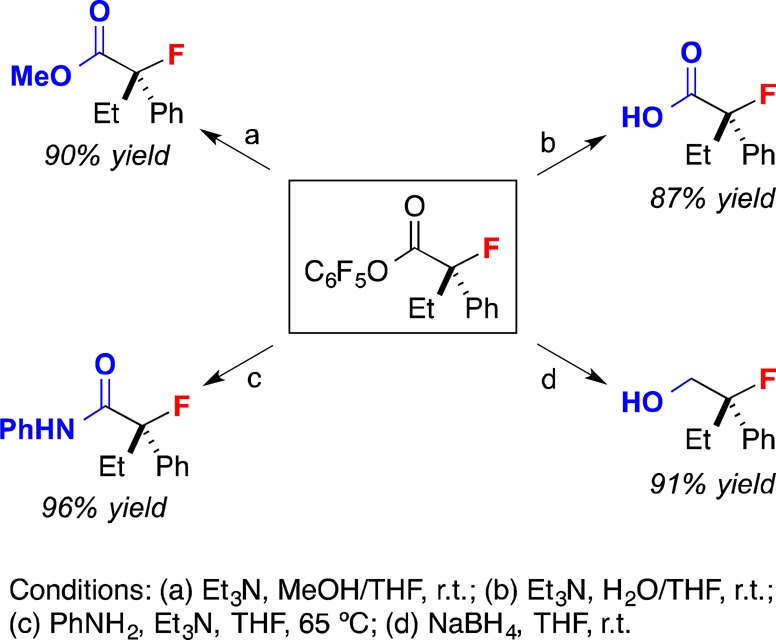
Transformations of an enantioenriched tertiary α-fluoroester.
Two of the possible pathways for the PPY*-catalyzed enantioselective fluorination of ketenes to generate enantioenriched tertiary α-fluoroesters are illustrated in Figure 2. In one mechanism, a PPY*-derived chiral enolate (2) is a key intermediate (top), and in the other a PPY*-derived chiral fluorinating agent (4) is critical (bottom).17 To gain insight into the operative pathway, we determined the rate law for the reaction of phenyl ethyl ketene, which is first order in PPY* and in NFSI, and zeroth order in the ketene and in C6F5ONa.18 The rate of product formation does vary with the identity of the ketene, suggesting that the ketene is involved in the rate-determining step.
Figure 2.
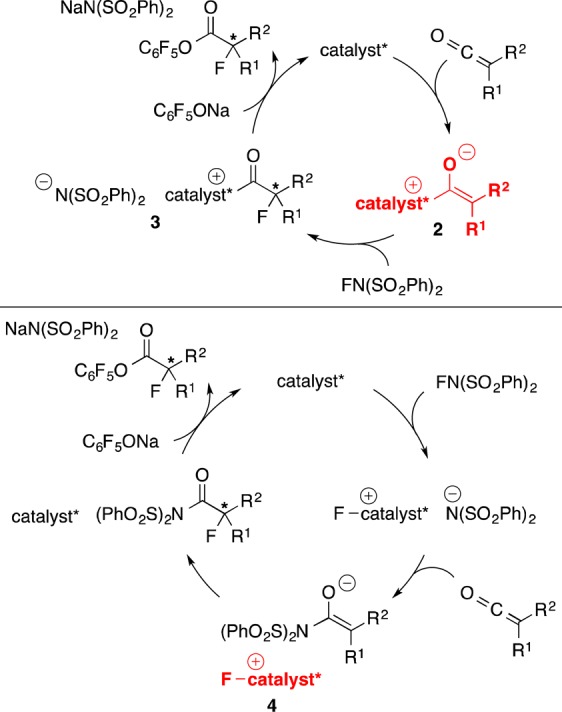
An outline of two of the possible mechanisms for the PPY*-catalyzed enantioselective α-fluorination of ketenes: a “chiral enolate” pathway (top) and a “chiral fluorinating agent” pathway (bottom).
These observations are consistent with a “chiral enolate” pathway (top of Figure 2; but not with a “chiral fluorinating agent” pathway (bottom of Figure 2)19) wherein nucleophilic addition of PPY* to the ketene affords enolate 2, this enolate is the resting state of the catalytic cycle,20 and, in the turnover-limiting step, enolate 2 is fluorinated by NFSI to provide enantioenriched N-acylpyridinium salt 3. Reaction with the phenoxide then furnishes the tertiary α-fluoroester and regenerates the catalyst, PPY*.
In view of our earlier inability to achieve PPY*-catalyzed asymmetric fluorination of ketenes with NFSI in the absence of an added nucleophile (eq 2), we hypothesized that N-acylpyridinium salt 3 (Figure 2) might be isolable. Indeed, reaction of PPY*, phenyl benzyl ketene, and NFSI (1:1:1) provided α-fluorinated acylpyridinium salt 5 (eq 4).21,22 This ion pair is stable at room temperature under nitrogen for at least 6 months.
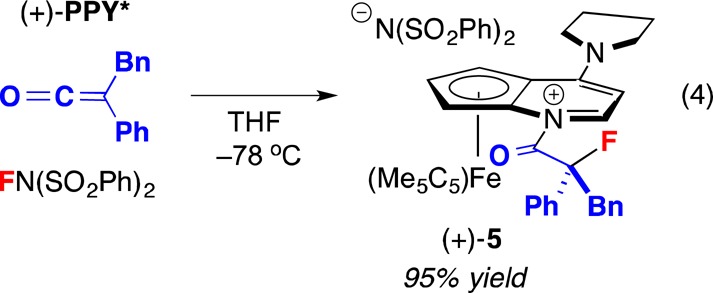 |
4 |
Although we were not able to obtain X-ray-quality crystals of the sulfonimide salt of the N-acylpyridinium ion, anion exchange of N(SO2Ph)2 for a carborane through treatment with CsCB11H12 furnished a suitable crystal (Figure 3).23 The pyrrolidino group, the pyridine ring, and the carbonyl group are approximately coplanar, consistent with extended conjugation of the π system, which decreases the electrophilicity of the carbonyl group. With regard to the orientation around the N–Ccarbonyl bond, the smaller group (carbonyl oxygen) is positioned on the side of the fused cyclopentadienyl ring, presumably to minimize unfavorable steric interactions. The stereochemistry of the fluorine-bearing carbon of the N-acylpyridinium ion is the same as for the major enantiomer of the α-fluoroester that is generated by (+)-PPY* under our standard fluorination conditions.
Figure 3.
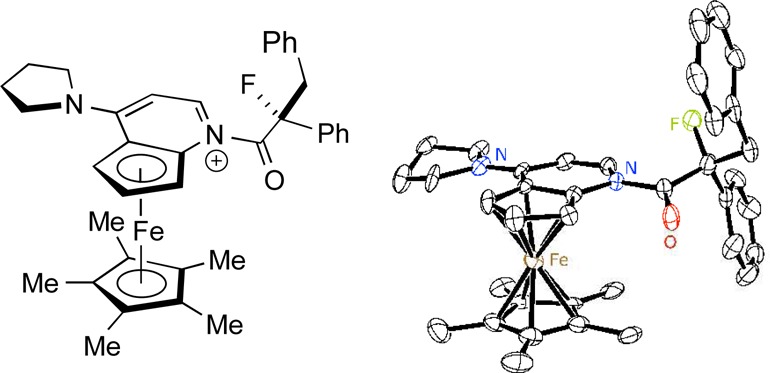
ORTEP diagram of N-acylated (+)-PPY*. Thermal ellipsoids are illustrated at the 35% probability level. For clarity, the carborane counteranion, the hydrogen atoms, the solvent, and the second molecule in the asymmetric unit are omitted, and only the major component of the disorder in the Cp* group is shown.
We have confirmed that N-acylpyridinium salt 5 is a chemically and kinetically competent potential intermediate in the proposed catalytic cycle that leads to the enantioenriched tertiary α-fluoroester (top of Figure 2). Thus, upon treatment of this salt with C6F5ONa in THF at −78 °C, the enantioenriched ester is formed with retention of stereochemistry (eq 5).
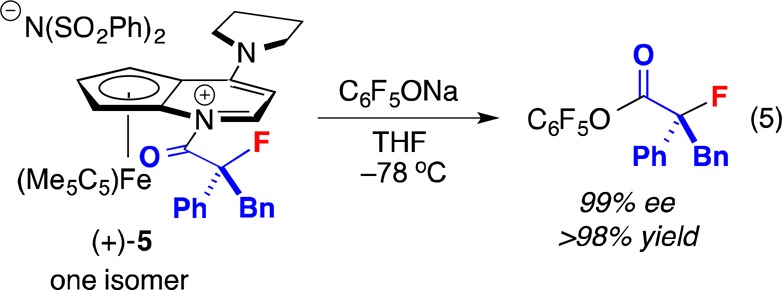 |
5 |
It is interesting to note that this catalyst-regeneration issue from an N-acylated catalyst intermediate is not encountered in “related” processes such as the PPY*-catalyzed α-chlorination of ketenes13 or the cinchona alkaloid-catalyzed α-fluorination of acid chlorides (via monosubstituted ketenes) to afford secondary α-fluoroesters.4
In conclusion, we have described a method for the catalytic enantioselective coupling of aryl alkyl ketenes (and a dialkyl ketene) with NFSI and C6F5ONa to produce tertiary α-fluoroesters, a family of target molecules that have rarely been directly accessed via asymmetric catalysis. The addition of C6F5ONa was critical to the success of this effort, as it enables regeneration of the catalyst (PPY*) from a relatively stable N-acylated intermediate, which we have synthesized and investigated independently. Our mechanistic studies are consistent with a catalytic cycle wherein PPY* adds to the ketene and generates a chiral enolate which, in the stereochemistry- and rate-determining step, reacts with NFSI to furnish the enantioenriched N-acylpyridinium salt.
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences R01-GM57034) and the Deutsche Forschungsgemeinschaft (fellowship to S.N.). We thank Trixia M. Buscagan, Dr. Nathan D. Schley, Dr. Michael K. Takase (X-ray Crystallography Facility; a Bruker KAPPA APEX II X-ray diffractometer was purchased via NSF CRIF:MU award CHE-0639094 to the California Institute of Technology), Dr. David VanderVelde (NMR Facility), and Dr. Scott C. Virgil (Caltech Center for Catalysis and Chemical Synthesis, supported by the Gordon and Betty Moore Foundation) for assistance.
Supporting Information Available
Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For example, see:; a Fluorine in Pharmaceutical and Medicinal Chemistry; Gouverneur V., Müller K., Eds.; Imperial College Press: London, 2012. [Google Scholar]; b Fluorine in Medicinal Chemistry and Chemical Biology; Ojima I., Ed.; John Wiley & Sons: Chichester, 2009. [Google Scholar]
- For reviews that include catalytic asymmetric fluorination, see:; a Liang T.; Neumann C. N.; Ritter T. Angew. Chem., Int. Ed. 2013, 52, 8214. [DOI] [PubMed] [Google Scholar]; b Gouverneur V.; Lozano O. In Science of Synthesis; De Vries J. G., Molander G. A., Evans P. A., Eds.; Georg Thieme: Stuttgart, 2011; Vol. 3, p 851. [Google Scholar]; c Valero G.; Companyó X.; Rios R. Chem.—Eur. J. 2011, 17, 2018. [DOI] [PubMed] [Google Scholar]; d Cahard D.; Xu X.; Couve-Bonnaire S.; Pannecoucke X. Chem. Soc. Rev. 2010, 39, 558. [DOI] [PubMed] [Google Scholar]; e Lectard S.; Hamashima Y.; Sodeoka M. Adv. Synth. Catal. 2010, 352, 2708. [Google Scholar]
- For pioneering examples of catalytic, asymmetric α-fluorinations of aldehydes, see:; a Marigo M.; Fielenbach D.; Braunton A.; Kjærsgaard A.; Jørgensen K. A. Angew. Chem., Int. Ed. 2005, 44, 3703. [DOI] [PubMed] [Google Scholar]; b Steiner D. D.; Mase N.; Barbas C. F. III. Angew. Chem., Int. Ed. 2005, 44, 3706. [DOI] [PubMed] [Google Scholar]; c Beeson T. D.; MacMillan D. W. C. J. Am. Chem. Soc. 2005, 127, 8826. [DOI] [PubMed] [Google Scholar]
- a Paull D. H.; Scerba M. T.; Alden-Danforth E.; Widger L. R.; Lectka T. J. Am. Chem. Soc. 2008, 130, 17260. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Erb J.; Paull D. H.; Dudding T.; Belding L.; Lectka T. J. Am. Chem. Soc. 2011, 133, 7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a pioneering example, see:Hintermann L.; Togni A. Angew. Chem., Int. Ed. 2000, 39, 4359. [DOI] [PubMed] [Google Scholar]
- For examples of oxindoles, see:; a Shibata N.; Kohno J.; Takai K.; Ishimaru T.; Nakamura S.; Toru T.; Kanemasa S. Angew. Chem., Int. Ed. 2005, 44, 4204. [DOI] [PubMed] [Google Scholar]; b Hamashima Y.; Suzuki T.; Takano H.; Shimura Y.; Sodeoka M. J. Am. Chem. Soc. 2005, 127, 10164. [DOI] [PubMed] [Google Scholar]; c Ishimaru T.; Shibata N.; Horikawa T.; Yasuda N.; Nakamura S.; Toru T.; Shiro M. Angew. Chem., Int. Ed. 2008, 47, 4157. [DOI] [PubMed] [Google Scholar]; d Wu L.; Falivene L.; Drinkel E.; Grant S.; Linden A.; Cavallo L.; Dorta R. Angew. Chem., Int. Ed. 2012, 51, 2870. [DOI] [PubMed] [Google Scholar]
- For examples of ketones, see:; a Bélanger É.; Cantin K.; Messe O.; Tremblay M.; Paquin J.-F. J. Am. Chem. Soc. 2007, 129, 1034. [DOI] [PubMed] [Google Scholar]; b Phipps R. J.; Toste F. D. J. Am. Chem. Soc. 2013, 135, 1268. [DOI] [PubMed] [Google Scholar]; c Yang X.; Phipps R. J.; Toste F. D. J. Am. Chem. Soc. 2014, 136, 5225. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liang Y.; Fu G. C. J. Am. Chem. Soc. 2014, 136, 5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a few examples of the catalytic enantioselective synthesis of other families of tertiary alkyl fluorides, see:; a Phipps R. J.; Hiramatsu K.; Toste F. D. J. Am. Chem. Soc. 2012, 134, 8376. [DOI] [PubMed] [Google Scholar]; b Shunatona H. P.; Früh N.; Wang Y.-M.; Rauniyar V.; Toste F. D. Angew. Chem., Int. Ed. 2013, 52, 7724. [DOI] [PubMed] [Google Scholar]; c Wu J.; Wang Y.-M.; Drljevic A.; Rauniyar V.; Phipps R. J.; Toste F. D. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For an example of a bioactive tertiary α-fluoroester, see:Yasuhara A.; Nakamura M.; Sakagami K.; Shimazaki T.; Yoshikawa R.; Chaki S.; Ohta H.; Nakazato A. Bioorg. Med. Chem. 2006, 14, 4193. [DOI] [PubMed] [Google Scholar]
- For examples, see:; a Schlosser M.; Michel D.; Guo Z.-w.; Sih C. J. Tetrahedron 1996, 52, 8257. [Google Scholar]; b Tengeiji A.; Shiina I. Molecules 2012, 17, 7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews of catalytic asymmetric reactions of ketenes, see:Chen S.; Salo E. C.; Kerrigan N. J. In Science of Synthesis: Asymmetric Organocatalysis; List B., Maruoka K., Eds.; Georg Thieme: Stuttgart, 2012; Vol. 1, p 455. [Google Scholar]
- For a review of electrophilic N–F fluorinating agents, see:Baudoux J.; Cahard D. Org. React. 2007, 69, 347. [Google Scholar]
- Lee E. C.; McCauley K. M.; Fu G. C. Angew. Chem., Int. Ed. 2007, 46, 977. [DOI] [PubMed] [Google Scholar]
- When C6F5OH was employed in place of C6F5ONa, essentially none of the desired product was observed.
- Notes:; a Under our optimized condition, solutions of the ketene and the nucleophile are added dropwise via a syringe pump to a solution of the catalyst and NFSI; Selectfluor reagent is not a suitable substitute for NFSI; the ee of the product is constant during the course of the reaction; an o-tolyl-substituted and a tert-butyl-substituted ketene were not effective reaction partners; b Both enantiomers of PPY* are available from Strem Chemicals
- For the exception, see:Wilson J. E.; Fu G. C. Angew. Chem., Int. Ed. 2004, 43, 6358. [DOI] [PubMed] [Google Scholar]
- We have hypothesized that planar-chiral DMAP derivatives can serve both as enantioselective nucleophilic catalysts (e.g.,; Fu G. C.Acc. Chem. Res. 2004, 37, 542) [DOI] [PubMed] [Google Scholar]; and, in protonated form, as enantioselective Brønsted-acid catalysts (e.g.,; Dai X.; Nakai T.; Romero J. A. C.; Fu G. C.. Angew. Chem., Int. Ed. 2007, 46, 4367).The catalytic cycle for the Brønsted-acid mode of reactivity parallels the chiral fluorinating agent pathway (substitution of F-catalyst* with H-catalyst* at the bottom of Figure 2) [DOI] [PubMed] [Google Scholar]
- PPY* and t-BuONa react with phenyl ethyl ketene in THF-d8 at −78 °C, whereas C6F5ONa does not.
- In THF-d8 at −78 °C, no reaction is observed when PPY* is mixed with NFSI.
- We have not yet been able to identify the resting state of the catalyst under our standard fluorination conditions.
- The yield was determined by 1H NMR spectroscopy with the aid of an internal standard.
- We were also able to prepare the corresponding N-acylpyridinium salt derived from phenyl ethyl ketene, but it was more difficult to purify than salt 4.
- Previously, we have structurally characterized an N-acylpyridinium salt generated through the reaction of a planar-chiral DMAP derivative with an acid chloride:Tao B.; Ruble J. C.; Hoic D. A.; Fu G. C. J. Am. Chem. Soc. 1999, 121, 5091. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


