Abstract
Apoptosis is observed during a spectrum of conditions including exogenous virus infection and endogenous cellular turnover. Adult female Aedes albopictus mosquitoes challenged with increasing titres of Sindbis virus (SINV) via intrathoracic inoculation demonstrated that the injection dosage did not result in significantly different levels of virus growth or mosquito survival at day 10 post-infection. Tissues probed for apoptosis using an in situ TUNEL assay revealed SINV-associated apoptotic cells scattered throughout the proximal and distal regions of the salivary gland (SG) lateral lobes but which were not detected in the median lobe or the midgut and hindgut. Apoptosis was also identified in SG duct cells in both infected and uninfected mosquitoes, suggesting routine tissue homeostasis. SINV-associated apoptosis sequestered to the SG lateral lobes indicates a differential epithelial cell response to an arbovirus and provides insight into mosquito defence mechanisms against pathogens and SG infection barriers, hurdles to transmission of arboviruses of public health concern.
Sindbis virus (SINV) is a member of the genus Alphavirus belonging to the family Togaviridae (Fields et al., 2001). This arthropod-borne virus (arbovirus) infects a variety of mosquito species and transmission in nature relies primarily upon a horizontal cycle between invertebrate and vertebrate hosts (Chamberlin, 1980). Female mosquitoes possess bilateral tri-lobed salivary glands (SGs) that synthesize and release a large repertoire of secretory proteins (Almeras et al., 2010; Arcà et al., 2007). Mosquito reproduction and arbovirus transmission depend on SG function during blood feeding. When imbibing a bloodmeal, the female ‘suck and spit’ behaviour secretes a plethora of products via saliva into the vertebrate host (Clements, 1996a, b; Ribeiro, 1992). Saliva is integral to virus transmission, and systemic infection of mosquitoes with some arboviruses has been shown to modify feeding behaviours (Platt et al., 1997; Qualls et al., 2012).
Apoptosis, a form of programmed cell death (PCD), is described as cellular suicide whereby the cell directly contributes to the death process (Wyllie, 1997). This process is fundamental to organogenesis during animal development and physiological cellular renewal in healthy adult tissues (Jacobson et al., 1997), involved in oncogenesis (Kerr et al., 1994), well-documented in Drosophila (McCall & Peterson, 2004) and provides an immunological response to virus infection (Girard et al., 2007). Apoptotic response to SINV infection has been observed in vertebrate cells (Levine et al., 1993; Lewis et al., 1996) and in mosquitoes following infection with alphaviruses and flaviviruses (Bowers et al., 1995, 2003; Girard et al., 2005; Mims et al., 1966; Vaidyanathan & Scott, 2006; Wang et al., 2012; Weaver et al., 1992, 1988).
SINV antigens detected in the SGs of adult female Aedes albopictus mosquitoes (Bowers et al., 1995) colocalized with a cytopathic effect (CPE) in the proximal and distal regions of the lateral lobes, effectively sparing median lobe insult (Bowers et al., 2003). Arbovirus-associated CPE in an organ directly essential to life and reproduction, while incidentally essential to virus transmission, led us to hypothesize the involvement of apoptosis as a mechanism of cytopathology. Insight into SINV-associated PCD in a mosquito host will further our understanding of the SG response to pathogens such as infection and escape barriers (Kramer et al., 1981), essential links in the chain of transmission of arboviruses of public health concern (Kuno & Chang, 2005; Vo & Bowers, 2006).
Colonized A. albopictus (Lake Charles Strain) mosquitoes were maintained at 25.5±0.5 °C, 70–80 % relative humidity and a 16 : 8 (light/dark) photoperiod with approximately 300 mosquitoes per bucket cage (Gerberg, 1970). Baby hamster kidney (BHK-21/13F) cells were grown in minimal essential medium (MEM) at 37 °C (Renz & Brown, 1976); the heat-resistant variant of SINV (SVHR; Burge & Pfefferkorn, 1966) was used for all virus assays (Bowers et al., 1995).
For investigation of mosquito survival and virus growth, adult females were cold-anaesthetized and injected via the intrathoracic route of inoculation (Rosen & Gubler, 1974). For survival analysis, experimental females were inoculated with 25 500 p.f.u. SVHR and mock-infected females were injected with an equal volume of MEM and monitored for 30 days post-infection (p.i.). To investigate the mosquito response to increasing titres of virus challenge, SINV was serially diluted to create tenfold inoculum titres between 255 000 and 255 p.f.u. per insect. Injected mosquitoes were incubated at standard insectary conditions and sampled at days 10 and 18 p.i. Each individual mosquito was triturated on a vortex for 60 s in a glass vial containing 300 µl amphotericin-B/PBS (2.5 µg ml−1) and 10 glass beads followed by quantification of the virus by plaque assay on BHK-21 cells at 28 °C (Bowers et al., 1995). Virus growth data were log-transformed to achieve normality and homoscedasticity. The effect of the level of inoculum on virus growth was tested using ANOVA, and the relationship between inoculum and percentage survival was tested using Pearson’s product–moment correlation.
To analyse SINV-associated apoptosis following high-titre inoculum (25 500 p.f.u. per insect), whole mount preparations of SGs, midguts and hindguts were dissected at days 5 and 7 p.i. Females were cold-anaesthetized on a chill plate at 4 °C, and organs were dissected in a 50/50 solution of insect haemolymph substitute (Seron et al., 2004) and PBS (pH 7.4) on a microscope slide. Tissue was incubated for 1 h in fresh 1 % paraformaldehyde/0.1 M sodium cacodylate buffer, and slides were washed three times in PBS rinses and stored at −20 °C. For TUNEL assays, slides were submerged in 70 % ice-cold ethanol for 3 h, rinsed three times (5 min each) in ice-cold PBS, and apoptosis was assayed using a APO-BrdU TUNEL Assay kit (Molecular Probes) using a modified protocol for fluorescence microscopy. Tissue was incubated in 50 µl DNA-labelling solution for 60 min, rocking gently at 24 °C, rinsed three times with buffer, followed by incubation in antibody solution for 30 min. Lastly, tissues were counterstained with propidium iodide, coverslipped in VectaShield (Vector Laboratories) and viewed on an Olympus BX60 epifluorescent microscope equipped with a U-MWU dual filter and KE/SE digital camera. Phase microscopy was used for orientation purposes and all images were captured using a SPOT/RT program.
A survival of 86 % was observed in A. albopictus at day 30 p.i. following injection with a high-titre inoculum. All infectious doses resulted in SINV replication and mosquito survival between 80 and 93 % (including medium and trauma) following 10 days incubation (Table 1). Virus replication ranged between 3.0×105 and 1.7×106 p.f.u. per whole body and SINV infection was persistent at day 18 p.i. The ANOVA results showed that different levels of inoculum did not result in significantly different levels of virus growth (F3,15 = 2.212, P = 0.129). There was also no relationship between inoculum dosage and percentage survival, as shown by the results of Pearson’s correlation (r = −0.277, P = 0.652).
Table 1. Virus growth and survival in A. albopictus at day 10 p.i. following injection with increasing titres of SINV.
| Inoculum SINV p.f.u. per insect | Virus growth (p.f.u. per insect) | Survival (%; n = 30) |
| 255 | 1.7±1.7×106, n = 3 | 93 |
| 2550 | 7.4±1.2×105, n = 3 | 80 |
| 2550* | 3.3±2.3×105, n = 4 | 93 |
| 25 500† | 1.4±1.4×106, n = 7 | 86 |
| 255 000 | 3.0±0.6×105, n = 3 | 85 |
| Medium-injected | 0 | 87 |
| Trauma‡ | 0 | 83 |
| Colony | 0 | 93 |
Virus growth and percentage survival at day 18 p.i.
High-titre SINV at day 10 p.i. and survival at day 30 p.i.; 86 % infected and 90 % uninfected (n = 50).
Intrathoracic inoculation without fluid instillation.
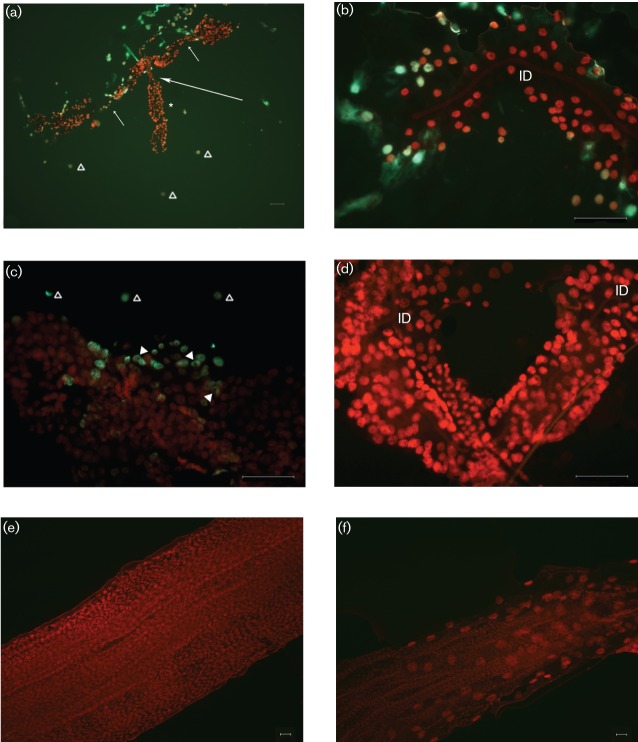
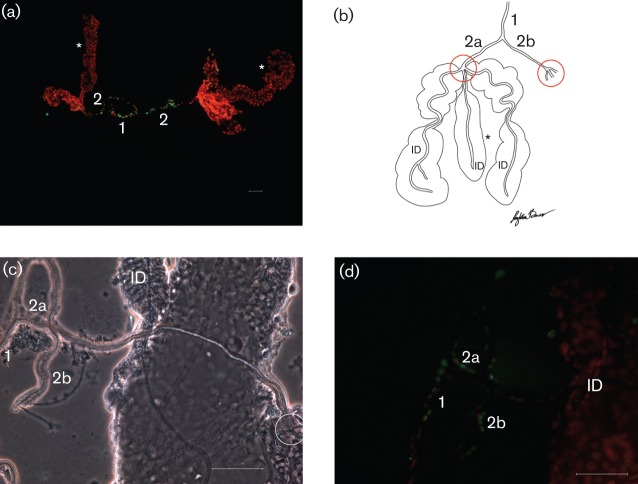
Apoptotic cells were observed in cells throughout the proximal and distal regions of the lateral lobes (Fig. 1a–c) at days 5 and 7 p.i., but starkly absent from the median lobe (Fig. 1a). Apoptosis was also detected in detached cells scattered around the SG preparations from SINV-infected mosquitoes (Fig. 1a, c; ▵) but not observed in mock-infected mosquitoes (Figs 1d and 2a). Lateral lobes of SINV-infected insects presented a decreased cell density (Fig. 1b, c) when compared with uninfected lateral lobes (Fig. 1d). Apoptosis was not detected at day 5 or 7 p.i. in peristaltic muscles associated with the midgut (Fig. 1e) or hindgut (Fig. 1f). Apoptosis was identified in SG gland duct cells in both virus-infected and naïve mosquitoes (Fig. 2a). Cells expressing apoptosis were observed in the epithelium integral to the common duct (Fig. 2a; 1) and the two main ducts (Fig. 2a; 2) but were not detected in the internal ducts (Figs 1b, d and 2d; ID) embedded in the parenchyma of all three lobes. The graphic drawing of duct structures (Fig. 2b) aids the orientation of the phase-contrast microscopy image (Fig. 2c) and enables anatomical localization of apoptosis (Fig. 2d). Using the triad (point where main ducts branch into three internal ducts; Fig. 2b, c; circled) as a reference, apoptosis was never observed distal to the triad in either infected (Fig. 1b; ID) or naïve (Figs 1d and 2d; ID) mosquitoes.
Fig. 1.
Epifluorescent micrographs of whole-mount preparations of SGs, midgut and hindgut from adult female A. albopictus mosquitoes, following injection with high-titre SINV (25 500 p.f.u. per insect). Tissues were stained using the TUNEL assay to detect apoptotic cells (green) and counterstained with propidium iodide (red). (a) Apoptosis was associated with the proximal and distal regions of two lateral lobes at day 5 p.i. (short arrows; neck region separating proximal and distal lateral lobes). Apoptosis was not detected in median lobes (*, long arrow; neck region of median lobe). Gross pathology (distension and cellular disruption) was observed in lateral lobes but not in the median lobe. Apoptotic-positive cells scattered around the SGs (▵). (b) Distal region of lateral lobe at day 5 p.i. Note apoptotic-positive cells integral to the lobe epithelium. Note the overall low cell density compared with the uninfected lobe (d) and absence of apoptosis in the internal duct (ID). (c) Distal region of lateral lobe at day 7 p.i. Note apoptotic-positive cells integral to lobe (arrowheads; indicate cytoplasmic staining) and apoptotic-positive cells scattered around SGs (▵). (d) Distal region of lateral lobe from an uninfected mosquito at day 5 p.i. Note overall high cell density compared with infected tissue (b, c). Apoptosis was not detected in the SG parenchyma, internal ducts (ID) or in cells scattered around the SGs (compare with a and c). Midgut (e) and hindgut (f) from infected mosquitoes (day 5 p.i.). Note the absence of apoptosis (green) in both gut regions. Bars, 50 µm (a–d); 10 µm (e, f).
Fig. 2.
Whole-mount SG preparations from uninfected A. albopictus mosquitoes. SG common duct (1) and main ducts (2; 2a, 2b). Triad structure (circled) indicates the junction where the main ducts (2) branch into the internal ducts (ID). (a) Epifluorescent image stained with the TUNEL assay; SGs with ducts intact. Note the presence of apoptosis (green) associated with the common duct (1) and main ducts (2). *, Median lobes. (b) Graphic drawing for orientation. (c) Phase-contrast image for orientation. (d) Apoptotic cells (green) outline the common (1) and main (2a, 2b) ducts, but are not observed in the internal ducts (ID). Bars, 10 µm (a); 50 µm (c, d).
Mosquito survival of ≥80 % at days 10 and 30 p.i. was observed following all inoculations, indicating tolerance of virus and injection procedures. While the virus grew to a range of titres, this was not significantly different between inoculum titres and probably represents slight differences in mosquito sizes. Intrathoracic inoculation was essential to ensure exposure of the SGs to SINV (Bowers et al., 1995), a necessary prerequisite to understanding the mechanism of CPE in secondary target organs. Analysis of apoptosis was conducted using high-titre inoculum because (i) there was an acceptable mosquito survival and (ii) to ensure an effective m.o.i. of SGs (Fields et al., 2001).
Both SINV-associated apoptosis, a PCD mechanism for the elimination of damaged cells, and apoptosis integral to cellular homeostasis were detected in the SGs of adult female A. albopictus mosquitoes. Hence, SG tissue is capable of an apoptotic virus defence response while maintaining routine cellular turnover. Detection of SINV-associated PCD in cytopathic regions of the lateral lobes but not the median lobe confirms and expands upon previously documented colocalization of SINV antigens and CPE (Bowers et al., 2003). Detection of apoptosis in some lateral lobe cells (Fig. 1b, c) but not in all cells, suggests that virus-associated apoptosis is cell-specific within this organ. Varying degrees of apoptosis have been identified in three lines of A. albopictus cultured cells following exposure to SINV (Karpf & Brown, 1998), probably reflecting different tissue types of origin (Singh, 1967). We found SG tissue from infected mosquitoes to be friable, tearing easily during resection, and a gross decrease in cell density observed in lateral lobes (Fig. 1b, c) that was not noted in uninfected tissue (Fig. 1d). Girard et al. (2007) revealed a CPE and an accelerated SG decline in Culex species following infection with the flavivirus West Nile virus, describing a CPE similar to normal ageing of SG tissue but occurring earlier in infected mosquitoes. Research looking at gene silencing demonstrated that recombinant SINV (pro-apoptotic genes) resulted in decreased virus replication in mosquito cell culture (Wang, et al., 2008), indicating that virus-associated apoptosis resulted in a decline in virus production. Induction of apoptosis in SGs may be a host-induced evolutionary strategy for virus sequestration to minimize systemic viraemia and/or transmission (Krakauer & Payne, 1997). Our observations of detached apoptotic cells scattered around infected SGs could quite possibly be infected haemocytes, a tropism critical to the systemic spread of arboviruses to secondary target tissues (Parikh et al., 2009). It can be speculated that an early pre-emptive host cell death would minimize virus numbers, resulting in decreased virus release and an effective increase in the SG escape barrier.
The absence of SINV antigen, CPE and apoptosis in the median lobe indicate that while lateral lobe cells are virus susceptible, median lobe cells may be virus refractory (Bowers et al., 1995, 2003). Barreau et al. (1999) observed that various mAbs bound differentially to the lateral and median lobes of Aedes aegypti, documenting region-specific biochemical differences on SG surfaces. The absence of SINV receptors on the median lobe would prevent attachment, precluding both virus entry and replication, explaining the absence of CPEs and apoptosis. Spatial mapping of gene expression of 30 endogenous transcripts in the SGs of A. aegypti revealed unique localization patterns (Juhn et al., 2011). These researchers identified five genes exclusive to the median lobe and seven genes co-existing in the median and lateral lobes. If, in fact, the median lobe is spared CPEs following SINV infection, potential gene products of these 12 genes could be retained.
While mosquito gut peristaltic muscles are permissive to SINV infection (Vo et al., 2010), we did not detect apoptosis in midgut or hindgut musculature (Bowers, et al., 1995). Using gene silencing to manipulate apoptosis, SINV caused midgut alterations and increased insect mortality, suggesting that apoptosis can influence arbovirus replication in mosquitoes and may be necessary for dissemination (Wang et al., 2012). Our study used parenteral inoculation of SINV; hence, the process of dissemination was not addressed. The lack of apoptosis in muscle tissue further supports a cell/tissue-specific PCD response to SINV, highlighting that apoptosis is not a pantropic tissue defence to virus.
Apoptosis is essential for organogenesis during insect development (Cooper et al., 2007); however, this mechanism appears infrequently in adults. While apoptosis was detected in the epithelial cells lining the common and main ducts, its absence beyond the triad delineates a structure–function junction in the saliva duct continuum. We suggest that distinct cellular differences exist between the lining of the common and main ducts (apoptotic positive) from the internal ducts (apoptotic negative). Apoptosis in the SG ducts may reflect routine cellular turnover during tissue homeostasis and perhaps the wear-and-tear of salivation deems constitutive regeneration of mitotic epithelia, affecting external ducts.
Mosquitoes are vectors of numerous arboviruses and this study offers insight into the complexities of virus–host interactions at the cell/tissue/organ levels. Here we document a lobe-specific apoptotic response to SINV in SGs. This suggests that mosquito cells can respond differentially to SINV in different locations within the organism (midgut, hindgut or SGs) as well as within the same organ (SG lobes). Localization of SINV-associated apoptosis to infected lateral lobes, but not infected gut muscles suggests that SINV-associated apoptosis may be tissue-type specific; glandular epithelia (apoptotic positive) versus muscle (apoptotic negative). Girard et al. (2007) also documented that salivary secretions from West Nile virus-infected Culex species decreased over time; from 83 % at 14 days p.i. to 39 % at days 25–28 p.i. Arbovirus-associated apoptosis in the SGs may also influence virus concentration in saliva, production of SG secretions, changes in host feeding behaviour such as prolonged probing or feeding times (Platt et al., 1997; Qualls et al., 2012) and hopefully shed light on SG infection and escape barriers; hurdles to and links in the chain of transmission of arboviruses (Vo & Bowers, 2006) of public health concern.
Acknowledgements
We thank Leighton T. Bowers, Florida State University, for his original graphic drawing of the mosquito salivary gland. We also thank David Wilson, Center for Instruction and Research Technology, University of North Florida, for his expertise and magic in computer graphics and image presentation. The research was supported by the National Institute of Allergy and Infectious Diseases via grant number R15AI060654. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the NIH.
References
- Almeras L. A., Fontaine A., Belghazi M., Bourdon S., Boucomont-Chapeaublanc E., Orlandi-Pradines E., Baragatti M., Corre-Catelin N., Reiter P. & other authors (2010). Salivary gland protein repertoire from Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis 10, 391–402 10.1089/vbz.2009.0042 [DOI] [PubMed] [Google Scholar]
- Arcà B., Lombardo F., Francischetti I. M., Pham V. M., Mestres-Simon M., Andersen J. F., Ribeiro J. M. (2007). An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol 37, 107–127 10.1016/j.ibmb.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Barreau C., Conrad J., Fischer E., Lujan H. D., Vernick K. D. (1999). Identification of surface molecules on salivary glands of the mosquito, Aedes aegypti, by a panel of monoclonal antibodies. Insect Biochem Mol Biol 29, 515–526 10.1016/S0965-1748(99)00025-9 [DOI] [PubMed] [Google Scholar]
- Bowers D. F., Abell B. A., Brown D. T. (1995). Replication and tissue tropism of the alphavirus Sindbis in the mosquito Aedes albopictus. Virology 212, 1–12 10.1006/viro.1995.1447 [DOI] [PubMed] [Google Scholar]
- Bowers D. F., Coleman C. G., Brown D. T. (2003). Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae). J Med Entomol 40, 698–705 10.1603/0022-2585-40.5.698 [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. (1966). Complementation between temperature-sensitive mutants of Sindbis virus. Virology 30, 214–223 10.1016/0042-6822(66)90097-3 [DOI] [PubMed] [Google Scholar]
- Chamberlin R. W. (1980). Epidemiology of arthropod-borne Togaviruses: the role of arthropods as hosts and vectors and of vertebrate hosts in natural transmission cycles. In Togaviridae and Flaviridae, pp. 175–227. Edited by Schleisinger S., Schleisinger J. J. New York: Plenum Press [Google Scholar]
- Clements A. N. (1996a). Adult food and feeding mechanisms. In The Biology of Mosquitoes, pp. 222 London: Chapman and Hall [Google Scholar]
- Clements A. N. (1996b). Adult salivary glands and their secretions. In The Biology of Mosquitoes, pp. 251–262 London: Chapman and Hall [Google Scholar]
- Cooper D. M., Pio F., Thi E. P., Theilmann D., Lowenberger C. (2007). Characterization of Aedes Dredd: a novel initiator caspase from the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 37, 559–569 10.1016/j.ibmb.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Fields B. N., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Strausm B., Snipe D. M. (2001). Fields Virology, 4th edn New York: Lippincott Williams & Wilkins [Google Scholar]
- Gerberg E. J. (1970). Manual for Mosquito Rearing and Experimental Techniques. California: American Mosquito Control Assocociation, Inc [Google Scholar]
- Girard Y. A., Popov V., Wen J., Han V., Higgs S. (2005). Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae). J Med Entomol 42, 429–444 10.1603/0022-2585(2005)042[0429:USOWNV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Girard Y. A., Schneider B. S., McGee C. E., Wen J., Han V. C., Popov V., Mason P. W., Higgs S. (2007). Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am J Trop Med Hyg 76, 118–128 [PubMed] [Google Scholar]
- Jacobson M. D., Weil M., Raff M. C. (1997). Programmed cell death in animal development. Cell 88, 347–354 10.1016/S0092-8674(00)81873-5 [DOI] [PubMed] [Google Scholar]
- Juhn J., Naeem-Ullah U., Maciel Guedes B. A., Majid A., Coleman J., Paolucci Pimenta P. F., Akram W., James A. A., Marinotti O. (2011). Spatial mapping of gene expression in the salivary glands of the dengue vector mosquito, Aedes aegypti. Parasit Vectors 4, 1–13 10.1186/1756-3305-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf A. R., Brown D. T. (1998). Comparison of Sindbis virus-induced pathology in mosquito and vertebrate cell cultures. Virology 240, 193–201 10.1006/viro.1997.8914 [DOI] [PubMed] [Google Scholar]
- Kerr J. F. R., Winterford C. M., Harmon B. V. (1994). Apoptosis. Its significance in cancer and cancer therapy. Cancer 73, 2013–2026 [DOI] [PubMed] [Google Scholar]
- Krakauer D. C., Payne R. J. H. (1997). The evolution of virus-induced apoptosis. Proc Biol Sci 264, 1757–1762 10.1098/rspb.1997.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L. D., Hardy J. L., Presser S. B., Houk E. J. (1981). Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg 30, 190–197 [DOI] [PubMed] [Google Scholar]
- Kuno G., Chang G.-J. (2005). Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev 18, 608–637 10.1128/CMR.18.4.608-637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Huang Q., Isaacs J. T., Reed J. C., Griffin D. E., Hardwick J. M. (1993). Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361, 739–742 10.1038/361739a0 [DOI] [PubMed] [Google Scholar]
- Lewis J., Wesselingh S. L., Griffin D. E., Hardwick J. M. (1996). Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol 70, 1828–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall K., Peterson J. S. (2004). Detection of apoptosis in Drosophila. In Apoptosis Methods and Protocols, pp. 191–206 Edited by Brady H. J. M. Totowa, NJ: Humana Press; 10.1385/1-59259-812-9:191 [DOI] [PubMed] [Google Scholar]
- Mims C. A., Day M. F., Marshall I. D. (1966). Cytopathic effect of Semliki Forest virus in the mosquito Aedes aegypti. Am J Trop Med Hyg 15, 775–784 [DOI] [PubMed] [Google Scholar]
- Parikh G. R., Oliver J. D., Bartholomay L. C. (2009). A haemocyte tropism for an arbovirus. J Gen Virol 90, 292–296 10.1099/vir.0.005116-0 [DOI] [PubMed] [Google Scholar]
- Platt K. B., Linthicum K. J., Myint K. S. A., Innis B. L., Lerdthusnee K., Vaughn D. W. (1997). Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg 57, 119–125 [DOI] [PubMed] [Google Scholar]
- Qualls W. A., Day J. F., Xue R.-D., Bowers D. F. (2012). Sindbis virus infection alters blood feeding responses and DEET repellency in Aedes aegypti (Diptera: Culicidae). J Med Entomol 49, 418–423 10.1603/ME11102 [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. (1976). Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (mosquito) cells. J Virol 19, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M. C. (1992). Characterization of a vasodilator from the salivary glands of the yellow fever mosquito Aedes aegypti. J Exp Biol 165, 61–71 [DOI] [PubMed] [Google Scholar]
- Rosen L., Gubler D. (1974). The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg 23, 1153–1160 [DOI] [PubMed] [Google Scholar]
- Seron T. J., Hill J., Linser P. J. (2004). A GPI-linked carbonic anhydrase expressed in the larval mosquito midgut. J Exp Biol 207, 4559–4572 10.1242/jeb.01287 [DOI] [PubMed] [Google Scholar]
- Singh K. R. P. (1967). Cell cultures derived from larvae of Aedes albopictus (Skuse) and Aedes aegypti. Curr Sci 36, 506–508 [Google Scholar]
- Vaidyanathan R., Scott T. W. (2006). Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 11, 1643–1651 10.1007/s10495-006-8783-y [DOI] [PubMed] [Google Scholar]
- Vo M., Bowers D. F. (2006). The arbovirus lifecycle: links in a chain. Tech Bull Fl Mosq Contr Assoc 7, 31–34 [Google Scholar]
- Vo M., Linser P. J., Bowers D. F. (2010). Organ-associated muscles in Aedes albopictus (Diptera: Culicidae) respond differentially to Sindbis virus. J Med Entomol 47, 215–225 10.1603/ME09041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Blair C. D., Olson K. E., Clem R. J. (2008). Effects of inducing or inhibiting apoptosis on Sindbis virus replication in mosquito cells. J Gen Virol 89, 2651–2661 10.1099/vir.0.2008/005314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Gort T., Boyle D. L., Clem R. J. (2012). Effects of manipulating apoptosis on Sindbis virus infection of Aedes aegypti mosquitoes. J Virol 86, 6546–6554 10.1128/JVI.00125-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Lorenz L. H., Lerdthusnee K., Romoser W. S. (1988). Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J Virol 62, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Lorenz L. H., Scott T. W. (1992). Pathologic changes in the midgut of Culex tarsalis following infection with Western equine encephalomyelitis virus. Am J Trop Med Hyg 47, 691–701 [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. (1997). Apoptosis: an overview. Br Med Bull 53, 451–465 10.1093/oxfordjournals.bmb.a011623 [DOI] [PubMed] [Google Scholar]