Abstract
Oncolytic virus (OV) therapy is an emerging anti-cancer approach that utilizes viruses to preferentially infect and kill cancer cells, while not harming healthy cells. Vesicular stomatitis virus (VSV) is a prototypic non-segmented, negative-strand RNA virus with inherent OV qualities. Antiviral responses induced by type I interferon pathways are believed to be impaired in most cancer cells, making them more susceptible to VSV than normal cells. Several other factors make VSV a promising OV candidate for clinical use, including its well-studied biology, a small, easily manipulated genome, relative independence of a receptor or cell cycle, cytoplasmic replication without risk of host-cell transformation, and lack of pre-existing immunity in humans. Moreover, various VSV-based recombinant viruses have been engineered via reverse genetics to improve oncoselectivity, safety, oncotoxicity and stimulation of tumour-specific immunity. Alternative delivery methods are also being studied to minimize premature immune clearance of VSV. OV treatment as a monotherapy is being explored, although many studies have employed VSV in combination with radiotherapy, chemotherapy or other OVs. Preclinical studies with various cancers have demonstrated that VSV is a promising OV; as a result, a human clinical trial using VSV is currently in progress.
Introduction
Oncolytic virus (OV) therapy is an emerging anti-cancer approach that utilizes viruses to preferentially infect and kill cancer cells, while not harming healthy cells. Since 1893, scientists have observed tumour regression after virus infection (Kelly & Russell, 2007). However, research in the field has occurred mostly in the last 15 years, when almost every major group of animal viruses has been tested for OV potential, and impressive preclinical successes have been reported (Hammill et al., 2010). As a result, the adenovirus H101 was approved for clinical use in China in 2006 (Garber, 2006), and three other OVs based on vaccinia virus, herpes simplex virus and reovirus are currently in late-phase clinical trials and could soon be approved in the USA (Hammill et al., 2010).
This review focuses on vesicular stomatitis virus (VSV) as a promising OV, beginning with the inherent qualities of wild-type (WT) VSV. As with any OV, VSV has limitations that must be overcome for future clinical success. Compared with other OVs, VSV is advantageous due to a combination of several factors, including its well-studied biology, relative independence of a receptor or cell cycle, ability to infect a wide range of laboratory cell lines and to produce very high virus yields, cytoplasmic replication without risk of host-cell transformation, a small, easily manipulated genome, and lack of pre-existing immunity in humans. In the last 10 years, a great number of recombinant VSVs (rVSVs) have been generated via reverse genetics, with the goal of generating more potent OVs that work synergistically with host immunity and/or other therapies to reduce or eliminate tumour burden. To facilitate the comparison of these recombinant viruses, we have organized them in Table 1 based on their modifications and a problem that they were designed to address. Similarly, this review will summarize previous studies based on their attempts to improve oncoselectivity, safety and oncotoxicity; to minimize premature immune clearance; and/or to induce or stimulate tumour-specific immunity. As the most effective therapies are likely to involve combinational regimens of VSV and chemotherapy, radiotherapy or even other OVs, these approaches are also discussed.
Table 1. VSV recombinants used as oncolytic agents against cancer.
| Recombinant VSV | Virus description | Reference(s) | Designed to improve: | ||||
| Oncoselectivity | Safety | Oncotoxicity | VSV survival | Tumour immunity | |||
| WT and miscellaneous | |||||||
| WT VSV (‘Rose lab’) | The parental rWT VSV for most VSV-based OVs. The L gene and the N-terminal 49 residues of the N gene are derived from the Mudd-Summers strain, the rest is from the San Juan strain (both Indiana serotype) | Lawson et al. (1995) | |||||
| VSV-WT-XN2 (or XN1) | Derivative of rWT VSV (‘Rose lab’). Generated using pVSV-XN2 (or pVSV-XN1), a full-length VSV plasmid containing unique XhoI and NheI sites flanked by VSV transcription start and stop signals between G and L genes. pVSV-XN2 (or pVSV-XN1) is commonly used to generate recombinant VSVs encoding an extra gene | Schnell et al. (1996) | |||||
| WT VSV (‘Wertz lab’) | Alternative rWT VSV. The N, P, M and L genes originate from the San Juan strain; G gene from the Orsay strain (both Indiana serotype). Rarely used in OV studies | Whelan et al. (1995) | |||||
| VSV-WT-GFP, -RFP, -Luc, -LacZ | WT VSV encoding reporter genes (between G and L) to track virus infection. Based on pVSV-XN2. Toxicity similar to VSV-WT | Fernandez et al. (2002), Wu et al. (2008) | |||||
| VSV-G/GFP | GFP sequence fused to VSV G gene is inserted between the WT G and L genes (in addition to WT G). Toxicity similar to that of VSV-WT | Dalton & Rose (2001) | |||||
| VSV-rp30 | Derivative of VSV-G/GFP. Generated by positive selection on glioblastoma cells and contains two silent mutations and two missense mutations, one in P and one in L. ‘rp30’ indicates 30 repeated passages | Wollmann et al. (2005) | X | X | X | ||
| VSV-p1-GFP, VSV-p1-RFP | VSV expressing GFP or red fluorescent protein (RFP or dsRed) reporter gene at position 1. Attenuated because all VSV genes are moved downward, to positions 2–6. Safe and still effective as an OV | Wollmann et al. (2010) | X | X | |||
| VSV-dG-GFP (or RFP) (replication-defective) | Similar to VSV-p1-GFP or VSV-p1-RFP described above, but with the G gene deleted. Cannot generate a second round of infection. Poor ability to kill tumour cells | Wollmann et al. (2010) | X | X | |||
| VSV-ΔP, -ΔL, -ΔG (semi-replication-competent) | Each virus cannot replicate alone because of one VSV gene deleted, but when viruses co-infect, they show good replication, safety and oncolysis (especially the combination of VSVΔG/VSVΔL). VSVΔP and VSVΔL contain dsRed in place of the corresponding viral gene. VSVΔG contains GFP gene in place of G | Muik et al. (2012) | X | ||||
| M mutants | |||||||
| VSV-M51R | The M51R mutation was introduced into M | Kopecky et al. (2001) | X | X | |||
| VSV-ΔM51, VSV-ΔM51-GFP, - RFP, -FLuc, -Luc, - LacZ | The ΔM51 mutation was introduced into M. In addition, some recombinants encode a reporter gene between the G and L | Stojdl et al. (2003), Power & Bell (2007), Wu et al. (2008) | X | X | |||
| VSV-*Mmut | VSV with a single mutation or combination of mutations at the following M positions: M33A, M51R, V221F and S226R | Hoffmann et al. (2010) | X | X | |||
| VSV-M6PY >A4-R34E and other M mutants | The M51R mutation was introduced into the M gene, and, in addition, the mutations in the PSAP motif (residues 37–40) of M | Irie et al. (2007) | X | X | |||
| VSV-M(mut) | VSV M residues 52–54 are mutated from DTY to AAA. M(mut) cannot block nuclear mRNA export | Heiber & Barber (2011) | X | X | |||
| G mutants | |||||||
| VSV-G5, -G5R, -G6, -G6R | VSV-expressing mutant G with amino acid substitutions at various positions (between residues 100 and 471). Triggers type I IFN secretion as the M51R, but inhibits cellular transcription and host protein translation like WT | Janelle et al. (2011) | X | X | |||
| VSV-CT1 | The cytoplasmic tail of the G protein was truncated from 29 to 1 aa. Decreased neuropathology, but marginal oncolytic efficacy | Ozduman et al. (2009), Wollmann et al. (2010) | X | X | |||
| VSV-CT9-M51 | The cytoplasmic tail of VSV-G was reduced from 29 to 9 aa, also has ΔM51 mutation. Attenuated neurotoxicity and good OV abilities | Ozduman et al. (2009), Wollmann et al. (2010) | X | X | |||
| Foreign glycoproteins | |||||||
| VSV-DV/F(L289A) (same as rVSV-F) | VSV expressing the NDV fusion protein gene between G and L. The L289A mutation in this protein allows it to induce syncytia alone (without NDV HN protein) | Ebert et al. (2004) | X | X | X | ||
| VSV-S-GP | VSV with the native G gene deleted and replaced with a modified glycoprotein protein (GP) from Sindbis virus (SV). Also expressing mouse GM-CSF and GFP (between SV GP and VSV L). The modified GP protein recognizes the Her2 receptor, which is overexpressed on many breast cancer cells | Bergman et al. (2007) | X | X | X | ||
| VSV-ΔG-SV5-F | VSV G gene is replaced with the fusogenic simian parainfluenza virus 5 fusion protein (SV5-F) gene | Chang et al. (2010) | X | X | |||
| VSV-FAST, VSV-(ΔM51)-FAST | VSV or VSV-MΔ51 expressing the p14 FAST protein of reptilian reovirus (between VSV G and L) | Brown et al. (2009) | X | X | X | ||
| VSV-LCMV-GP (replication-defective) | VSV lacking the G gene was pseudotyped with the non-neurotropic glycoprotein of LMCV | Muik et al. (2011) | X | ||||
| VSV-H/F, -αEGFR, -αFR,-αPSMA (replication-defective) | VSV lacking the G gene was pseudotyped with the MV F and H displaying single-chain antibodies (scFv) specific for epidermal growth factor receptor, folate receptor, or prostate membrane-specific antigen. Retargeted VSV to cells that expressed the targeted receptor | Ayala-Breton et al. (2012) | X | X | X | X | |
| microRNA targets | |||||||
| VSV-let-7wt | The let-7 microRNA targets are inserted into the 3′-UTR of VSV M | Edge et al. (2008) | X | X | |||
| VSV-124, -125, -128, -134 (M or L mRNA) | VSV recombinants with neuron-specific microRNA (miR-124, 125, 128 or 134) targets inserted in the 3′-UTR of VSV M or L mRNA | Kelly et al. (2010) | X | X | |||
| Cancer suppressors | |||||||
| VSV-mp53, VSV-M(mut)-mp53 | VSV [WT or M(mut)] expressing the murine p53 gene. M(mut) has residues 52–54 of the M protein changed from DTY to AAA | Heiber & Barber (2011) | X | X | X | X | |
| Suicide genes | |||||||
| VSV-C : U | VSV expressing E. coli CD/UPRT, catalysing the modification of 5-fluorocytosine into chemotherapeutic 5-FU | Porosnicu et al. (2003) | X | X | |||
| VSV-C | VSV-MΔ51 expressing CD/UPRT | Leveille et al. (2011b) | X | X | X | ||
| VSV-(MΔ51)-NIS | VSV-MΔ51 expressing the human NIS gene (for ‘radiovirotherapy’ with 131I) | Goel et al. (2007) | X | X | X | ||
| VSV-TK | VSV expressing TK; can improve oncolysis if used with non-toxic prodrug ganciclovir | Fernandez et al. (2002) | X | X | |||
| Immunomodulation | |||||||
| VSV-mIFNβ, -hIFNβ, VSV-rIFNβ | VSV expressing the murine (m), human (h) or rat (r) IFN-β gene | Obuchi et al. (2003), Jenks et al. (2010) | X | X | X | X | |
| VSV-IL4 | VSV expressing IL-4 | Fernandez et al. (2002) | X | X | |||
| VSV-IL12 | VSV expressing IL-12 | Shin et al. (2007a) | X | X | |||
| VSV-IL23 | VSV expressing IL-23. Significantly attenuated in the CNS, but effective OV | Miller et al. (2010) | X | X | X | X | |
| VSV-IL28 | VSV expressing IL-28, a member of the type III IFN (IFN-λ) family | Wongthida et al. (2010) | X | X | X | X | |
| VSV-opt.hIL-15 | VSV-MΔ51 expressing a highly secreted version of human IL-15 | Stephenson et al. (2012) | X | X | X | X | |
| VSV-CD40L | VSV expressing CD40L, a member of the tumour necrosis factor (TNF) family of cell-surface molecules | Galivo et al. (2010) | X | X | |||
| VSV-Flt3L | VSV-MΔ51 expressing the soluble form of the human Flt3L, a growth factor activating DCs | Leveille et al. (2011a) | X | X | X | X | |
| VSV/hDCT | VSV-MΔ51 expressing hDCT | Boudreau et al. (2009) | X | X | X | X | |
| VSV-hgp100 | VSV expressing hgp100, an altered self-TAA against which tolerance is well-established in C57BL/6 mice | Wongthida et al. (2011b) | X | X | |||
| VSV-ova | VSV expressing chicken ovalbumin (for B16ova cancer model) | Diaz et al. (2007) | X | X | |||
| VSV-gG | VSV expressing EHV-1 glycoprotein G, a broad-spectrum viral chemokine-binding protein | Altomonte et al. (2008b) | X | X | |||
| VSV-UL141 | VSV expressing a secreted form of the human cytomegalovirus UL141 protein, known to inhibit the function of NK cells by blocking the ligand of NK cell-activating receptors | Altomonte et al. (2009) | X | X | |||
| VSV-(Δ51)-M3 | VSV-MΔ51 expressing the murine gammaherpesvirus-68 chemokine-binding protein M3 | Wu et al. (2008) | X | X | X | X | |
VSV biology
In this section we will review VSV biology with respect to its OV potential. VSV is a prototypic non-segmented, negative-sense RNA virus (order Mononegavirales, family Rhabdoviridae) and one of the best-studied animal viruses. Two major WT VSV serotypes, Indiana and New Jersey (VSVIN and VSVNJ), are endemic to much of Central and South America and parts of the USA (Lyles & Rupprecht, 2007). Natural hosts include horses, cattle, pigs and a range of other mammals and their insect vectors. Among livestock, WT VSV outbreaks occur seasonally and most infections are non-lethal, manifesting as fever and blister-like lesions of the oral cavity, feet and teats (Drolet et al., 2009; Hansen et al., 1985). In general, pre-existing immunity to VSV in human populations is very low, and VSV infection in humans is generally asymptomatic and limited to agricultural and laboratory workers (Lyles & Rupprecht, 2007). Only one case of WT VSVIN-mediated encephalitis in humans has been reported (Quiroz et al., 1988).
It is important to mention that VSV pathogenesis in natural hosts depends on the virus serotype. Thus, using a reverse-genetics approach, VSVIN and VSVNJ recombinants encoding a heterogeneous glycoprotein (G protein) were generated. The study demonstrated that the VSV G protein is a determinant of higher virulence of VSVNJ than of VSVIN in swine (Martinez et al., 2003).
VSV neurotoxicity has been studied extensively in different rodent systems. In principle, VSV can cause neurotoxicity in mice or rats when administered intracranially (Dal Canto et al., 1976), intranasally (Plakhov et al., 1995), intravascularly (Shinozaki et al., 2005) and intraperitoneally (Schellekens et al., 1984). Neurotoxicity following intranasal VSV infection (WT or non-attenuated VSV recombinants; Table 1) is very efficient and has been studied extensively. When administered intranasally, WT or non-attenuated VSV recombinants replicate rapidly in the nasal epithelium, spread to olfactory neurons, then move retrograde axonally to the brain, where they replicate and cause neuropathogenesis (Bi et al., 1995; Reiss et al., 1998; van den Pol et al., 2002). Following infection of the central nervous system (CNS), the onset of encephalitis was shown to be T-cell-independent as it is seen in athymic mice, and WT VSV neuropathology appears to be more related to the cytopathological nature of VSV infection rather than to T-cell-mediated mechanisms (Frei et al., 1989; Huneycutt et al., 1993). Both innate (nitric oxide produced by neurons and glial cells) and adaptive (expression of MHC molecules and T-cell infiltration) immunity are required for clearance of VSV from the CNS (Bi et al., 1995). Our own research demonstrated that WT VSV can infect microglia and astrocytes in vitro and in vivo, and suggests that infection of glial cells results in the production of inflammatory cytokines that may facilitate encephalitis (Chauhan et al., 2010; Furr et al., 2008, 2010). VSV-mediated encephalitis has also been observed in non-human primates (NHPs) and will be discussed in the ‘Improving VSV oncoselectivity and safety’ section, where we will describe attenuated VSV recombinants that lack neurotoxicity.
The 11 kb VSV genome encodes five genes encoding the nucleocapsid (N) protein, phosphoprotein (P), matrix (M) protein, G protein and large polymerase (L) (Fig. 1). At approximately 185×75 nm, the VSV virion is enveloped and bullet-shaped, and contains all five virus-encoded proteins (Ge et al., 2010; Lyles & Rupprecht, 2007), as well as a number of host proteins (Moerdyk-Schauwecker et al., 2011). The VSV G protein enables VSV to infect most, if not all, mammalian cell types. To date, no definitive cell-surface receptor has been identified for the VSV G protein, with binding attributed to negatively charged membrane lipids (Lyles & Rupprecht, 2007). Following attachment, VSV enters a cell through actin- and clathrin-dependent endocytosis (Cureton et al., 2010). Once internalized, endosomal acidification mediates G protein conformational changes that facilitate fusion of the viral envelope with the endosomal membrane, releasing the VSV ribonucleoprotein core into the cytoplasm (Stanifer et al., 2011).
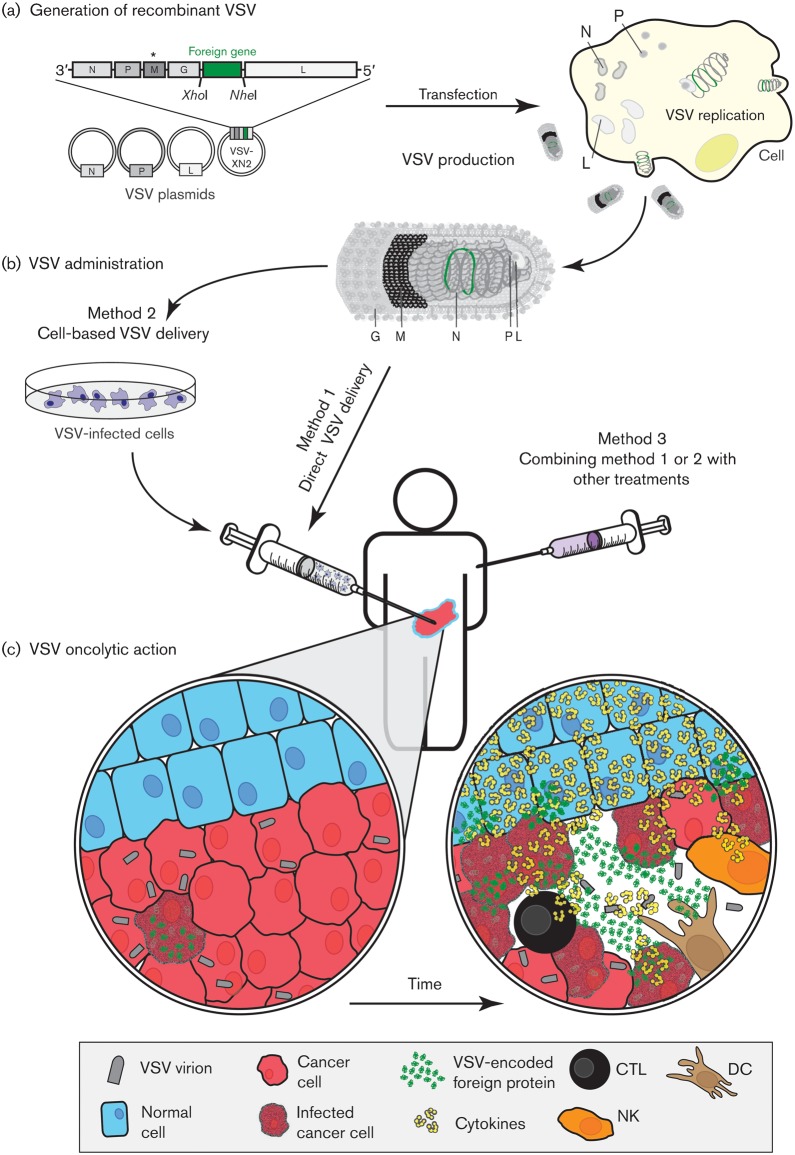
Fig. 1.
Scheme of VSV-based OV therapy. (a) Reverse genetics allows generation of a recombinant VSV encoding a foreign gene of interest between the VSV G and L genes. The asterisk above M indicates M protein mutation(s) resulting in VSV attenuation in normal cells. Plasmids encoding VSV replication machinery and the modified genome are co-transfected into a cell line, and complete virions are produced and amplified using good manufacturing practices. (b) For evaluation of oncolytic efficacy, VSV can be administered directly, via cell-based delivery, or in combination with other treatments (chemotherapy, radiotherapy or other OVs). (c) In infected cells, VSV recombinants may express a foreign gene that facilitates killing of the adjacent uninfected cancer cells (e.g. suicide-gene approach or immunostimulation). Innate antiviral responses and other mechanisms prevent cell death in normal cells. Ideally, stimulation of innate and adaptive immune cells by VSV and/or the foreign gene product should lead to tumour-specific immune responses, including memory responses that prevent cancer recurrence.
The VSV RNA genome is encapsidated tightly by the N protein, forming a nuclease-resistant nucleocapsid, which serves as a template for the viral RNA-dependent RNA polymerase. The VSV polymerase complex consists of the L and P proteins and is carried in virions from cell to cell (Lyles & Rupprecht, 2007). Initiation of transcription of downstream genes occurs as the polymerase pauses at each intergenic region before reinitiation, resulting in a quantitative gradient of mRNAs with N>M>P>G>L (Ball et al., 1999). Ongoing translation of these mRNAs is required for VSV genome replication. VSV is known for its fast replication, and assembly of enveloped progeny virions begins around the same time as secondary transcription, approximately 2–3 h post-infection (Lyles & Rupprecht, 2007). VSV infection culminates in cellular death through apoptosis (Gaddy & Lyles, 2005).
In infected cells, VSV mRNAs are translated preferentially. It remains unclear what allows the translation of VSV mRNA but inhibits the translation of cellular mRNA, as viral and host mRNA are structurally similar (5′-capped and 3′-polyadenylated) (Lyles & Rupprecht, 2007). Previous studies suggest that this effect is independent of VSV mRNA cis-acting sequences. Instead, alterations of the translational machinery in VSV-infected cells, such as dephosphorylation of the cap-binding subunit eIF4E (Connor & Lyles, 2002), may favour newly produced mRNAs (Whitlow et al., 2006, 2008). A recent study suggests that the VSV WT M protein also plays a role in the preferential translation of VSV mRNAs, with M protein residue D125 being critical (Mire & Whitt, 2011). VSV secondary RNA synthesis was shown to occur predominantly in cytoplasmic inclusions and that viral mRNAs produced at the inclusions are transported away in a microtubule-dependent manner, which facilitates their translation (Heinrich et al., 2010). This mechanism may contribute to the preferential translation of viral mRNAs in VSV-infected cells. In addition, although replication of VSV is believed to be generally cell-cycle-independent, VSV infection of primary T-lymphocytes was shown to depend on the cell-cycle transition from the G0 to the G1 phase, characterized by robust ribogenesis and mRNA translation, illustrating the dependence of VSV on robust mRNA translation machinery (Oliere et al., 2008).
Cellular responses to VSV infection are multi-pronged and are initiated by pattern-recognition receptors. Toll-like receptor (TLR) 4, TLR3 and TLR7 induce expression of type I interferons (IFNs) after sensing VSV proteins or genome (Georgel et al., 2007; Rieder & Conzelmann, 2009). A novel TLR13 may also induce type I IFNs (Shi et al., 2011). Retinoic acid-inducible gene I (RIG-I)-like receptors are found in the cytoplasm of most cells. RIG-I stimulates mitochondrial antiviral signalling (MAVS/IPS-1/VISA/Cardif), which induces type I IFN production (Hornung et al., 2006). IFN molecules act in an autocrine or paracrine manner to upregulate expression of IFN-stimulated genes (ISGs) in the infected cell and in proximal uninfected cells. Several ISGs, including MxA (Staeheli & Pavlovic, 1991), PKR (Krishnamoorthy et al., 2008), PCBP1/PCBP2 (Dinh et al., 2011), PML (Chelbi-Alix et al., 1998), IFITM2/3 (Weidner et al., 2010) and tetherin (BST-2) (Sarojini et al., 2011), have been reported to inhibit VSV replication directly.
The ability of VSV to suppress cellular responses to infection is required for continued replication. The M protein can localize to the nuclear envelope, where it interacts with Rae1 and Nup98 to inhibit nucleocytoplasmic trafficking of cellular mRNAs and small nuclear RNAs (Petersen et al., 2000). VSV M mutants with defects in Rae1–Nup98 binding are unable to inhibit mRNA nuclear export (Faria et al., 2005). Interestingly, a study by Chakraborty et al. (2009) showed that interaction of the VSV M protein with the Rae1–Nup98 complex during mitosis causes spindle abnormalities and triggers cell death during metaphase. The M protein may also inhibit host gene expression via inhibition of cellular mRNA synthesis (Yuan et al., 2001). In a susceptible host, M protein inhibition of cellular gene expression allows VSV to complete its replication cycle and to produce high titres of progeny before the initially infected cell mounts any significant antiviral response. This is the major mechanism by which VSV evades the host IFN system (Rieder & Conzelmann, 2009).
It is important to note that recent advances in VSV biology are possible thanks to the methods established to recover infectious recombinant virus from cDNA, so-called reverse-genetics systems (Lawson et al., 1995; Whelan et al., 1995). Reverse genetics enables the use of genetic engineering of VSV to study all aspects of the virus life cycle and to create rVSVs better suited for vaccine and oncolytic applications (Finke & Conzelmann, 2005). In a typical reverse-genetics approach, mammalian cells expressing bacteriophage T7 are transfected with a plasmid mixture containing VSV N, P, L and full-length genomic plasmid containing the desired modifications (Fig. 1, Table 1). Most VSV-based OVs contain VSV sequences similar to the recombinant WT (rWT) VSVIN, designated rWT VSV (‘Rose lab’) in Table 1. This particular rWT VSV, generated in Jack Rose’s laboratory (Lawson et al., 1995), has the L gene and the N-terminal 49 residues of the N gene derived from the Mudd-Summers strain; the rest is from the San Juan strain (both strains are of the Indiana serotype). Although most VSV-based recombinants used in OV applications have VSV sequences similar to that of this rWT VSV, we refer readers to original papers for specific details of each ‘WT VSV’ used in those studies.
Improving VSV oncoselectivity and safety
The type I IFN-associated antiviral potential of a cell is the key determinant of VSV oncoselectivity. VSV cannot distinguish non-malignant ‘normal’ cells from cancer cells based on differential receptor expression or cell-cycle state. The relative independence of VSV on a receptor can be an advantage when targeting cancer cells. For instance, not all cancer cells express sufficient coxsackievirus and adenovirus receptor for efficient entry of adenovirus 5-based OVs (Pearson et al., 1999). Although normal cells can be infected by VSV, they sense virus infection and produce, secrete and respond to type I IFNs to impede virus replication by inducing an antiviral state in the cell. In many cancer cells, VSV oncoselectivity is based largely on defective or reduced type I IFN responses (Barber, 2004; Lichty et al., 2004; Stojdl et al., 2000, 2003). In many cancer cells, specific genes associated with type I IFN responses are downregulated or functionally inactive (Balachandran & Barber, 2004; Marozin et al., 2008, 2010; Moussavi et al., 2010; Zhang et al., 2010). In addition, IFN signalling can be inhibited by MEK/ERK signalling, a cascade often upregulated in cancer cells (Noser et al., 2007). Abrogation of IFN signalling in cancer cells can also be caused by epigenetic silencing of IFN-responsive transcription factors IRF7 or IRF5 (Li & Tainsky, 2011). In addition to defective IFN signalling, continuously proliferating cancer cells often have abnormal translation machinery that favours VSV replication (Oliere et al., 2008). Several cellular proteins, including PKR, eIF2β, eIF4E, AKT and NFAR1/2, have been shown to play a role in mRNA translation as a determinant of VSV oncoselectivity (Balachandran & Barber, 2007; Barber, 2005; Harashima et al., 2010).
However, some cancer cells do not have these defects and resist VSV infection like normal cells (Naik & Russell, 2009; Stojdl et al., 2000). This includes some mesotheliomas (Saloura et al., 2010), melanomas (Linge et al., 1995; Wong et al., 1997), lymphomas (Sun et al., 1998), bladder cancers (Matin et al., 2001), renal cancers (Pfeffer et al., 1996) and possibly others (Stojdl et al., 2003). Our own research showed that several human pancreatic ductal adenocarcinoma cancer cell lines resistant to VSV infection are sensitive to IFN-α treatment and capable of secreting IFN-β following VSV infection (Murphy et al., 2012). Defects in the IFN pathway are not surprising, considering that IFN responses generally create conditions unfavourable for tumour formation, as they are anti-proliferative, anti-angiogenic and pro-apoptotic (Wang et al., 2011).
Understanding the mechanisms of VSV oncoselectivity is important for creating new, safe OVs designed for selective replication in cancer cells. While high sensitivity to IFN would eventually stop VSV replication and dissemination in healthy tissues of an immunocompetent host, VSV replication in an immunocompromised cancer patient requires additional safety measures to minimize its potential neurovirulence.
As described in the ‘VSV biology’ section above, WT VSV can cause severe neurotoxicity in rodents, especially when administered intracranially or intranasally. Therefore, the development of any clinical application involving replication-competent VSV vectors requires understanding of potential VSV neuropathogenesis in humans and appropriate attenuation of VSV to remove it. Many relevant studies analysed WT (or rWT) VSV and VSV recombinants with regard to their potential as vaccine vectors, and these studies are relevant to the applications of VSV recombinants as oncolytic agents. In fact, many oncolytic VSV recombinants were originally developed as vaccine vectors, and will be discussed later in this section. Some approaches, such as the rearrangement of VSV resulting in its attenuation, have yet to be applied to OV therapy, but will probably be explored in the future (Flanagan et al., 2001).
In NHP infection models, which resemble human disease pathogenesis more closely, intranasal or intramuscular injection of WT (or rWT) VSV and VSV recombinants caused no clinically adverse signs (Egan et al., 2004; Johnson et al., 2007; Rose et al., 2001). However, intrathalamic administration can result in severe neurological disease (Johnson et al., 2007). In this study, when WT VSV, rWT VSV and two rVSV-HIV (human immunodeficiency virus) vectors were administered intranasally to NHPs, there was no evidence of VSV spread to CNS tissues. However, macaques inoculated intrathalamically with WT VSV developed severe neurological disease. Interestingly, rWT VSV was attenuated significantly compared with WT VSV, and all of the macaques in the rVSV-HIV vector groups showed no clinical signs of disease. The attenuation of rWT VSV (compared with WT VSV) was probably due to spontaneous mutations generated during the reverse-genetics process or due to sequence differences between WT VSV and rWT VSV (Table 1). The attenuation of rVSV-HIV (compared with rWT VSV) was probably due to the presence of the additional gene (HIV Gag) and the CT1 mutation (described later in this review). With regard to OV therapy, a recent study tested VSV-IFNβ on rhesus macaques via intrahepatic injection; no neurological signs were observed at any time point (Jenks et al., 2010). As a result, a phase I clinical trial using VSV-IFNβ is currently in progress to evaluate the safety of intratumoral administration of VSV-IFNβ to human patients with hepatocellular carcinoma (ClinicalTrials.gov, 2012, trial ID NCT01628640; http://clinicaltrials.gov/ct2/show/NCT01628640).
Currently, at least eight approaches have been shown to improve VSV oncoselectivity and neurotropism safety without compromising its oncolytic abilities: (i) mutating the VSV M protein; (ii) VSV-directed IFN-β expression; (iii) attenuation of VSV through disruption of normal gene order; (iv) mutating the VSV G protein; (v) introducing targets for microRNA from normal cells into the VSV genome; (vi) pseudotyping VSV; (vii) experimental adaptation of VSV to cancer cells; and (viii) using semi-replicative VSV.
Employing VSVs encoding a mutated M protein, which are unable to evade antiviral innate responses in normal cells, is possibly the most common approach to improve both oncoselectivity and safety of VSV (Table 1). Such VSV mutants retain their oncotoxicity in cancer cells defective in their antiviral responses. Most studies use VSV M recombinants containing a mutation or deletion of the methionine residue at position 51 of the M protein (Black et al., 1993; Coulon et al., 1990). Alternatively, an M mutant with residues 52–54 mutated from DTY to AAA has been used (Heiber & Barber, 2011). These mutations prevent the M protein from binding to the Rae1–Nup98 mRNA export complex and inhibiting cellular gene expression in normal cells, and thus provide enhanced safety, including no neurotoxicity in vivo. Even safer VSVs have been generated by additional M modifications within the PSAP region (residues 33–44) (Irie et al., 2007). It is important to note that inactivation of the ability of the M protein to inhibit cellular gene expression is a strategic advantage not only for safety reasons (e.g. normal cells can produce type I IFN and ISGs), but also when cellular gene expression is desirable (e.g. for tumour antigen presentation).
The oncoselectivity and safety of VSV are greatly improved in VSVs encoding mouse, rat or human IFN-β (which are species-specific), and one is being used in an ongoing clinical trial to evaluate VSV-IFNβ in human patients with hepatocellular carcinoma (ClinicalTrials.gov, 2012, trial ID NCT01628640; http://clinicaltrials.gov/ct2/show/NCT01628640). IFN-β stimulates innate immune responses in normal cells, but not in cancers with defective type I IFN signalling (Jenks et al., 2010; Obuchi et al., 2003; Saloura et al., 2010). In addition to enhanced oncoselectivity and safety, VSV-directed IFN-β expression also generates desirable immunostimulation of the tumour microenvironment (discussed later).
Theoretically, any significant attenuation of VSV can improve oncoselectivity and safety. This approach has been used to generate VSV-p1-GFP and similar recombinants with a foreign gene inserted in position 1 of the VSV genome (before the N gene). While a typical insertion of a foreign gene between the VSV G and L genes affects VSV replication only marginally (L polymerase mRNA can be downregulated without dramatic consequences for VSV fitness), insertions at position 1 negatively affect expression of all VSV genes. The resulting VSV-p1-GFP lacks neurotoxicity, but retains good oncolytic abilities in an intracranial human glioblastoma tumour xenograft mouse model (Wollmann et al., 2010).
WT VSV can also be attenuated by mutations in the G protein (Table 1). CT1 and CT9 mutants have the cytoplasmic tail of G truncated by removal of residues 1–29 and 9–29, respectively (Ozduman et al., 2009). The best oncolytic ability and safety was shown for VSV-CT9-M51, which combined the CT9 and M51 mutations in a mouse model of human neuroblastoma (Ozduman et al., 2009; Wollmann et al., 2010). Another study, examining four VSVs with point mutations in the G protein against a variety of cancer cell lines, showed that mutant VSV-G6R (E238G substitution in the G protein) is as efficient as WT VSV at cell killing and inhibition of cellular transcription and host protein translation. Surprisingly, VSV-G6R triggers type I IFN secretion as efficiently as a VSV M51 mutant (Janelle et al., 2011).
Altered expression of microRNAs in cancer cells can also be exploited to increase oncoselectivity and safety of VSV. A recombinant containing the highly conserved let-7 microRNA target sequence in the M mRNA 3′-UTR resulted in attenuation via lower M expression in normal cells that express high levels of let-7, but not in cancer cells that express low levels of let-7 in vitro and in vivo, and caused no neurotoxicity after intranasal virus infection of mice (Edge et al., 2008). Insertion of neuronal miR125 targets into VSV, particularly the L mRNA 3′-UTR, reduced neurotoxicity even when virus was injected intracranially into mice, while retaining oncolytic abilities (Kelly et al., 2010).
VSV neurotropism can also be inhibited through a pseudotyping approach (Table 1). Pseudotyped VSV-LCMV-GP virions, containing the non-neurotropic envelope glycoprotein of lymphocytic choriomeningitis virus (LCMV) instead of the VSV G protein, showed enhanced infectivity of malignant glioma cells while sparing primary human and rat neurons (Muik et al., 2011). Although only replication-defective viruses were used, this proof-of-principle study demonstrated that VSV-LCMV-GP has a better therapeutic window than VSV, especially for clinical applications targeting brain cancers.
While the approaches described above were designed to prevent VSV replication in normal cells, several studies designed VSVs specifically to target cancer cells. One approach used VSV-S-GP, where a modified glycoprotein from Sindbis virus replaced VSV G (Bergman et al., 2007; Gao et al., 2006). The modified glycoprotein was designed to specifically recognize the Her2 receptor, which is overexpressed on many breast cancer cells. This approach successfully targeted and eliminated Her2-expressing tumours in mice in vivo. In a separate study, replication-defective VSV was pseudotyped with measles virus (MV) fusion and haemagglutinin glycoproteins displaying single-chain antibodies to target and infect cells expressing epidermal growth factor receptor, folate receptor or prostate membrane-specific antigen (Ayala-Breton et al., 2012) in human tumour xenografts in mice.
VSV oncoselectivity can be increased via adaptation to cancer cells using serial passages. This approach successfully adapted VSV-S-GP (described above) to a murine mammary tumour cell line expressing the Her2 receptor (Gao et al., 2006). In a separate study, VSV-rp30 was generated by passaging VSV-G/GFP (Table 1) 30 times on glioblastoma cells (Wollmann et al., 2005). VSV-rp30 contains two silent mutations and two missense mutations, one in P and one in L.
Whilst oncolytic virotherapy is based predominantly on replication-competent viruses, some studies have examined replication-defective viruses, which do not produce infectious progeny, similar to those employed in most standard gene-therapy studies (Galivo et al., 2010). While it is unlikely that replication-defective recombinants could be as effective as replicative VSVs (unless used mainly to deliver anti-cancer genes, induce adaptive immunity, etc.), so-called semi-replicative viruses have been generated and tested. Two trans-complementing recombinants, VSV*ΔG and VSVΔL-dsRed, lack the VSV G and L genes, respectively, and are non-replicative alone (Muik et al., 2012). However, co-infection of a cell with the two recombinants results in production and spread of non-replicative progeny. The VSVΔG/VSVΔL-dsRed combination was as potent as WT VSV in vitro and induced long-term glioblastoma tumour regression in mice in vivo without neurotoxicity.
There are several options for treating VSV-resistant cancer cells. Pre-screening cells against an array of VSVs or other OVs could identify the best OV for treating a particular tumour. OV therapy can also be combined with chemical inhibitors to overcome VSV resistance. For example, the mammalian target of rapamycin stimulates type I IFN production via phosphorylation of its effectors. Using rapamycin, the inhibitor of this protein, in combination with VSVΔM51 increased survival of immunocompetent rats with malignant gliomas (Alain et al., 2010). Histone deacetylase inhibitors influence epigenetic changes within cells, ultimately altering gene expression and affecting antiviral responses. Indeed, these inhibitors reversibly compromise host antiviral responses in multiple cancer cell lines and allow enhanced spread of VSV that correlates with inhibited IFN responses and VSV-mediated oncolysis in cancer cells (Nguyên et al., 2008).
The resistance of cancer cells to VSV can also be overcome using a combination of VSV with other OVs, e.g. the double-deleted vaccinia virus (VV). The deletions restrict VV to cells that overexpress transcription factor E2F and have activated epithelial growth factor receptor pathways, a common cancer cell signature. Expression of the VV-encoded B18R protein antagonizes the innate cellular antiviral response to allow more robust VSVΔM51 replication in colon cancer xenografts in mice (Le Boeuf et al., 2010). Furthermore, a recent analysis showed that previous infection of cervical carcinoma cancer cells with human papillomavirus (HPV) improved VSV infection and killing, compared with cervical carcinomas not infected with HPV (Le Boeuf et al., 2012). HPV can inhibit IFN signalling, possibly creating a more hospitable environment for VSV.
Increased oncotoxicity
The ultimate goal of any successful OV therapy is the selective killing of cancer cells. There are at least seven approaches that have been shown to improve the oncolytic abilities (‘oncotoxicity’) of VSV independent of the immune system (as discussed in the last section): (i) combination of VSV with chemical agents; (ii) viral expression of tumour-suppressor genes; (iii) viral expression of ‘suicide genes’; (iv) syncytium induction; (v) radiovirotherapy; (vi) combining VSV with tumour embolization; and (vii) combining VSV with anti-angiogenic agents.
VSV kills infected cells by inducing apoptosis via the mitochondrial (intrinsic) or death receptor (extrinsic) pathway, or both (Cary et al., 2011; Gaddy & Lyles, 2005, 2007; Sharif-Askari et al., 2007). The mechanisms of induction can be cell-type-specific, and many cancer cells inhibit apoptosis to allow prolonged proliferation (Hamacher et al., 2008; Hanahan & Weinberg, 2011). WT VSV induces apoptosis primarily via the intrinsic pathway, while recombinants with an M51 mutation induce apoptosis primarily via the extrinsic pathway, although this is not absolute (Cary et al., 2011; Gaddy & Lyles, 2005). Understanding the interplay between VSV and cellular apoptotic mechanisms may be critical for developing and selecting OV treatment. Overexpression of the anti-apoptotic B-cell lymphoma 2 (BCL-2) protein was shown to impair VSV-mediated oncolysis, while this resistance was reversed when VSV was combined with obatoclax, a small-molecule BCL-2 inhibitor (Samuel et al., 2010). Another BCL-2 inhibitor, EM20-25, rendered apoptosis-resistant cancer cells susceptible to VSV-induced apoptosis (Tumilasci et al., 2008). In another study, infection by WT VSV (but not VSV with the M51 mutation) increased degradation of an anti-apoptotic myeloid cell leukaemia 1 protein (Mcl-1) that contributes to chemotherapy resistance (Schache et al., 2009). This VSV-mediated Mcl-1 degradation sensitized apoptosis-resistant cancer cells to doxorubicin, an approved chemotherapeutic. This combined ‘chemovirotherapy’ had an enhanced therapeutic effect compared with each treatment alone in mice (Schache et al., 2009).
The oncotoxicity of VSV can be enhanced by the expression of functional tumour-suppressor genes in cancer cells, e.g. VSV-M(mut)-mp53, which encodes p53 in addition to the mutated M protein and induces potent anti-tumour responses in mice (Heiber & Barber, 2011). This study showed that VSV-M(mut)-mp53 retained the selective ability to lyse cancer cells and also directed expression of high levels of functional p53. Importantly, VSV-M(mut)-mp53 showed improved safety when attenuated in vivo due to the activation of innate immune genes (such as type I IFNs) by p53, and induced enhanced adaptive tumour-specific immune responses.
One limitation of a standard OV therapy is that oncotoxicity is normally limited to virus-infected cancer cells. To address this issue, several approaches aim to increase the bystander effect and to kill uninfected cancer cells. One approach uses VSV expressing so-called ‘suicide genes’. These genes catalyse conversion of a non-toxic prodrug into a toxic form that, in addition to its toxicity in infected cancer cells, can diffuse to neighbouring uninfected cancer cells through gap junctions. Administration of VSV expressing the herpesvirus thymidine kinase (TK) protein to mice in combination with the prodrug ganciclovir exerted a great oncolytic effect through TK/ganciclovir-mediated apoptosis, enhanced the bystander effect and induced tumour-specific immune responses in breast or melanoma tumours in mice (Fernandez et al., 2002). Cytosine deaminase (CD) catalyses the conversion of 5-fluorocytosine (5-FC) to the commonly used chemotherapeutic drug 5-fluorouracil (5-FU), while uracil phosphoribosyltransferase (UPRT) converts 5-FU into the active 5-fluoro-UMP form. Intratumoral inoculation with VSV expressing these two proteins (VSV-C : U) followed by 5-FC administration improved tumour regression significantly compared with VSV or 5-FU alone, and activated tumour-specific immune responses against lymphoma or mammary carcinoma in mice (Porosnicu et al., 2003). This approach was further optimized by combining the M51 mutation with CD/UPRT expression (VSV-C) (Leveille et al., 2011b).
One critical limitation of OVs is their relatively poor penetration and spread within tumour masses. Several studies have attempted to address this problem through the generation of VSVs that spread by forming giant, multinuclear cells called syncytia. While VSV is generally not fusogenic, several fusogenic recombinants have been generated and showed promising results in different cancer models. VSV-NDV/F(L289A) (later designated rVSV-F) encodes the Newcastle disease virus (NDV) fusion protein gene with an L289A mutation to allow syncytium formation in the absence of the NDV haemagglutinin–neuraminidase (HN) protein (Altomonte et al., 2008b; Ebert et al., 2004; Shin et al., 2007b). VSV-ΔG-SV5-F expresses the simian parainfluenza virus F protein (Chang et al., 2010) and VSV/FAST virus expresses the p14 FAST protein of reptilian reovirus (Brown et al., 2009). Adding fusogenic genes to the VSV genome should be done with caution as VSV/FAST with WT M showed dramatically increased neuropathology in mice, although VSV M51 expressing p14 remained attenuated (Brown et al., 2009).
One of the most elegant approaches to increase VSV oncotoxicity is based on a combination of viro- and radiotherapy (‘radiovirotherapy’). VSV-MΔ51-NIS expresses a human sodium iodide symporter (NIS) protein that mediates high-concentration iodide uptake and, in mice, has a synergistic effect with iodine. VSV-NIS in combination with iodine-123 allows sensitive monitoring of infection, while iodine-131 is used for treatment of radiosensitive tumours (Goel et al., 2007; Naik et al., 2012).
VSV-based OV therapy of liver cancers can be improved significantly when combined with tumour embolization (‘viroembolization’), the blocking of arterial blood flow in the liver, thereby prolonging exposure of tumour cells to the therapeutic agent. When VSV was administered to rats in combination with degradable starch microspheres, an embolic agent currently used clinically for liver tumours, massive tumour necrosis and substantially prolonged survival were observed in test animals compared with monotherapy with either VSV or the embolic agent alone (Altomonte et al., 2008a).
Finally, VSV oncotoxicity can be improved by targeting tumour vasculature. A recent study has shown the ability of VSV to target tumour vasculature and angiogenesis when administered subcutaneously to mice with colon adenocarcinoma (Breitbach et al., 2011). The use of anti-angiogenic vascular endothelial growth factor 165 inhibitor combined with VSV led to increased tumour regression and improved virus titre and dissemination, even within tumours that previously supported poor VSV replication (Kottke et al., 2010).
Preventing premature clearance of VSV
Safe virotherapy ultimately requires clearance of the OV from the body. Unfortunately, those same mechanisms can eliminate the OVs prematurely, before they complete their task. Prior to initiation of infection, circulating antibodies (Abs), non-specific host proteins or complement proteins can neutralize virus particles. Virus sequestration to certain organs can also result in ineffective OV therapy. Several approaches have been developed to protect VSV-based OVs from premature clearance, including: (i) physical delivery methods hiding/masking virus from Abs, other host components or immune cells; (ii) VSVs expressing genes favouring VSV survival; and (iii) combination of VSV with chemicals favouring VSV survival.
Various cell-based methods to deliver OVs to tumours via carrier cells have been reviewed by Nakashima et al. (2010) and Power & Bell (2008). With regard to VSV, murine OT-I CD8+ T-cells, specific for an epitope of the ovalbumin antigen (Ag), were infected ex vivo with VSV and delivered to established B16-OVA melanoma tumours in the lungs of immunocompetent mice (Kottke et al., 2008a; Qiao et al., 2008a). These virus ‘Trojan horses’ demonstrated significantly improved therapy compared with VSV or T-cells alone. Importantly, this therapy was effective even in mice with pre-existing Abs against VSV, indicating that therapy with virus-loaded T-cells may be useful even in patients with pre-existing immunity to VSV (Kottke et al., 2008a). A new approach called aptamer-facilitated virus immunoshielding (AptaVISH) uses aptamer technology to mask OVs from their respective neutralizing Abs, and is currently in development for several OVs, including VSV (Labib et al., 2012).
While these studies physically hide or mask VSV from the immune system, other approaches have attempted to modulate the immune system environment to favour virus survival. For instance, VSV-gG expresses the equine herpesvirus (EHV)-1 glycoprotein G, a broad-spectrum chemokine-binding protein. Addition of EHV-1 G increases the oncolytic potency of VSV due to substantial suppression of host antiviral inflammatory responses in rats (Altomonte et al., 2008b). Similarly, VSV-M51-M3 expresses the murine gammaherpesvirus-68 M3 protein, which binds a broad range of chemokines and reduces the inflammatory response and NK and neutrophil accumulation in lesions in rats (Wu et al., 2008). Furthermore, recombinant VSV-UL141 expressing UL141, which downregulates NK cell-activating ligand CD155, inhibited NK-cell recruitment in rats (Altomonte et al., 2009).
Additional studies investigated the prevention of Ab-mediated VSV neutralization by combined administration of VSV and cyclophosphamide (CPA). CPA enhances delivery of OVs through reductions in levels of neutralizing Abs, suppression of innate immune effectors (Ikeda et al., 2000; Qiao et al., 2008b), depletion of number of Tregs (Kottke et al., 2008b) and activation of immune cells (Ghiringhelli et al., 2007). While a single dose of CPA has been shown to be insufficient to control primary anti-VSV immune responses in animal models, a clinically approved multi-dose CPA regimen suppressed antiviral Ab responses against VSV, even in mice with pre-existing Abs against VSV (Peng et al., 2012). However, a recent study surprisingly showed that the combination of CPA and VSV was less effective than CPA alone, despite increased intratumoral VSV titres (Willmon et al., 2011). This study suggests that CPA-mediated oncotherapy is dependent upon both CD4 T-cell and NK-cell activation, which are suppressed upon VSV infection, and serves as a warning of unforeseen consequences of experimental therapies involving immune modulation.
Inducing tumour-specific immunity
Fully effective OV therapy may require the activation of tumour-specific adaptive immune responses (Melcher et al., 2011). Although all VSVs have immunostimulatory abilities, in this section we focus on VSV-based OVs designed specifically to induce tumour-specific immune responses.
A number of tested VSVs encode immunostimulatory host genes (Table 1), including interleukin (IL)-4 (Fernandez et al., 2002), IL-12 (Shin et al., 2007a), IL-15 (Stephenson et al., 2012), IL-23 (Miller et al., 2009, 2010), type III IFN-λ (also called IL-28) (Wongthida et al., 2010), Fms-like tyrosine kinase 3 ligand (Flt3L) (Leveille et al., 2011a) and CD40L (Galivo et al., 2010) (Table 1). Interestingly, a study utilizing VSV-CD40L with or without VSV G indicated that therapeutic success may not depend on progressive rounds of VSV replication, as non-replicative VSV-CD40L-ΔG was equally as effective as fully replication-competent VSV-CD40L in mice. This result illustrates that tumour-specific immune responses could play a dominant role, at least in the employed experimental system (Galivo et al., 2010). Some interleukins provide not only immunostimulation but also improved safety. For example, the incorporation of IL-23 into the VSV genome stimulated NK and CD4 cells and enhanced nitric oxide production in the CNS, aiding viral clearance from neurons (Miller et al., 2009, 2010).
While these approaches stimulated the immune system and often resulted in tumour-specific memory responses, several studies investigated whether VSV can be designed specifically to facilitate the presentation of tumour-associated antigen (TAA) to immune cells. In a proof-of-principle study, the VSV-OVA virus was generated to express the chicken ovalbumin (ova) gene (Diaz et al., 2007). Injection of VSV-OVA into established B16-OVA tumours increased the number of ova-specific T-cells significantly compared with VSV-GFP (Wongthida et al., 2011b). VSV expressing an altered version of the murine self-TAA gp100 was able to stimulate gp100-specific T-cells despite pre-existing immune tolerance. Although tumour reduction was not improved significantly compared with VSV-GFP, combining VSV-hgp100 infection with adoptive transfer of naïve gp100-specific T-cells improved efficacy greatly, suggesting the potential of this treatment strategy (Wongthida et al., 2011b).
Dendritic cells (DCs) have the ability to activate Ag-specific T-cells and NK cells. While DCs do not support robust VSV replication, they can be infected ex vivo, then used to mount a specific anti-tumour response. DCs infected with VSV encoding human melanoma-associated Ag dopachrome tautomerase (hDCT) endogenously expressed by B16-OVA cells, or luciferase tagged with the immunodominant class-I epitope SIINFEKL, were able to mature and produce pro-inflammatory cytokines (Boudreau et al., 2009). When mice with metastatic tumours received DC-VSV/hDCT, tumour growth was controlled by both NK and CD8+ T-cells (Boudreau et al., 2009). In an even more sophisticated approach, a combination of an adenovirus and VSV both expressing hDCT were used sequentially. The adenovirus pre-immunization of in vivo murine DCT tumours did not prevent intratumoral VSV infection. Furthermore, this treatment resulted in reduced VSV replication in normal cells and a shift in immune activation from viral Ags to TAAs (Bridle et al., 2010).
These approaches may be useful if a specific TAA is stably expressed, but, in most cancer types, TAA expression is variable, transient and often unknown for individual tumours. In a new approach, a VSV-cDNA library was used to identify TAAs capable of inducing enhanced tumour-specific immunity (Pulido et al., 2012). The screen identified three viruses encoding putative TAAs, and their therapeutic effect against B16 murine melanoma tumours was reconstituted in vivo when these viruses were used together.
A fine balance between antiviral and anti-cancer responses is probably needed for effective OV therapy using VSV. For example, TLR signalling through myeloid differentiation primary response gene 88 (MyD88) activates specific antiviral immune responses that inhibit virus replication within the tumour, but also induces critical anti-cancer responses; a recent study has shown that VSV anti-tumour therapy in the B16-OVA mouse model depends on antiviral signalling through MyD88 (Wongthida et al., 2011a). Finally, while the majority of studies have demonstrated the desirable immunomodulation of the tumour microenvironment following VSV infection to favour tumour rejection, the opposite situation can also occur. A recent study demonstrated that VSV infection can negatively affect surface expression of immunostimulatory NKG2D-ligand, allowing viruses to escape immune recognition by NK cells, but negatively affecting anti-tumour immune responses (Jensen et al., 2011).
Concluding remarks/future directions
While significant advances have been made in the use of VSV as an OV, room for improvement still remains, along with many challenges to be addressed. The creation of recombinants has improved the OV qualities of VSV, but it is important to be mindful of biosafety, especially with recombinants designed to evade host antiviral responses. Future recombinants will need to address the visualization of VSV infection and spread in vivo. Imaging of VSV-GFP or VSV-luciferase is widely used in rodent models, but requires levels of expression that may not be attainable in human clinical studies. Whilst VSV-NIS in combination with iodine-123 allows sensitive monitoring of infection, it is mostly applicable to studies involving radioisotopes (Goel et al., 2007; Naik et al., 2012).
To prevent cancer recurrence, successful OV should not only penetrate tumours, but also kill cancer stem cells. Whilst studied extensively in other viruses, few have looked at this issue with regard to VSV (Cripe et al., 2009; Friedman et al., 2012). To provide the best virus for clinical use, the ‘perfect’ therapeutic oncolytic VSV should cause no neurotoxicity, retain WT-like oncolytic ability, be easily adaptable to target specific cancer types, and induce immune memory. Development of good manufacturing practices is necessary to ensure that the VSVs are grown in the most appropriate cell line, free of contaminants and optimized for clinical use (Ausubel et al., 2011; Diallo et al., 2012). A recent study using VSV-IFNβ demonstrated successfully that systemic delivery in immunocompetent mice destroyed disseminated myeloma (Naik et al., 2012). Currently, a phase I clinical trial using VSV-IFNβ is in progress (ClinicalTrials.gov, 2012, trial ID NCT01628640; http://clinicaltrials.gov/ct2/show/NCT01628640). The study aims to evaluate the safety of intratumoral administration of VSV-IFNβ to human patients with hepatocellular carcinoma refractory or intolerant to sorafenib, a standard agent for systemic chemotherapy. Expected to finish in 2013, the outcome will help to determine future maximum tolerated dose, assess tumour-response rate and look at immune responses, and will pave the way for future research and clinical trials.
Acknowledgements
The authors are grateful to Megan Moerdyk-Schauwecker, Didier Dréau and Ian Marriott for critical review of the manuscript. The authors apologize to those whose work has not been discussed due to space limitations. V. Z. G. is funded by the National Cancer Institute, National Institutes of Health (Bethesda, MD, USA), grant no. 1R15CA167517-01.
References
- Alain T., Lun X., Martineau Y., Sean P., Pulendran B., Petroulakis E., Zemp F. J., Lemay C. G., Roy D. & other authors (2010). Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci U S A 107, 1576–1581 10.1073/pnas.0912344107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J., Braren R., Schulz S., Marozin S., Rummeny E. J., Schmid R. M., Ebert O. (2008a). Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology 48, 1864–1873 10.1002/hep.22546 [DOI] [PubMed] [Google Scholar]
- Altomonte J., Wu L., Chen L., Meseck M., Ebert O., García-Sastre A., Fallon J., Woo S. L. (2008b). Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol Ther 16, 146–153 10.1038/sj.mt.6300343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomonte J., Wu L., Meseck M., Chen L., Ebert O., Garcia-Sastre A., Fallon J., Mandeli J., Woo S. L. (2009). Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther 16, 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel L. J., Meseck M., Derecho I., Lopez P., Knoblauch C., McMahon R., Anderson J., Dunphy N., Quezada V. & other authors (2011). Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum Gene Ther 22, 489–497 10.1089/hum.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Breton C., Barber G. N., Russell S. J., Peng K. W. (2012). Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum Gene Ther 23, 484–491 10.1089/hum.2011.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S., Barber G. N. (2004). Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5, 51–65 10.1016/S1535-6108(03)00330-1 [DOI] [PubMed] [Google Scholar]
- Balachandran S., Barber G. N. (2007). PKR in innate immunity, cancer, and viral oncolysis. Methods Mol Biol 383, 277–301 [DOI] [PubMed] [Google Scholar]
- Ball L. A., Pringle C. R., Flanagan B., Perepelitsa V. P., Wertz G. W. (1999). Phenotypic consequences of rearranging the P, M, and G genes of vesicular stomatitis virus. J Virol 73, 4705–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber G. N. (2004). Vesicular stomatitis virus as an oncolytic vector. Viral Immunol 17, 516–527 10.1089/vim.2004.17.516 [DOI] [PubMed] [Google Scholar]
- Barber G. N. (2005). VSV-tumor selective replication and protein translation. Oncogene 24, 7710–7719 10.1038/sj.onc.1209042 [DOI] [PubMed] [Google Scholar]
- Bergman I., Griffin J. A., Gao Y., Whitaker-Dowling P. (2007). Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int J Cancer 121, 425–430 10.1002/ijc.22680 [DOI] [PubMed] [Google Scholar]
- Bi Z., Barna M., Komatsu T., Reiss C. S. (1995). Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol 69, 6466–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B. L., Rhodes R. B., McKenzie M., Lyles D. S. (1993). The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J Virol 67, 4814–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau J. E., Bridle B. W., Stephenson K. B., Jenkins K. M., Brunellière J., Bramson J. L., Lichty B. D., Wan Y. (2009). Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Mol Ther 17, 1465–1472 10.1038/mt.2009.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach C. J., De Silva N. S., Falls T. J., Aladl U., Evgin L., Paterson J., Sun Y. Y., Roy D. G., Rintoul J. L. & other authors (2011). Targeting tumor vasculature with an oncolytic virus. Mol Ther 19, 886–894 10.1038/mt.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridle B. W., Stephenson K. B., Boudreau J. E., Koshy S., Kazdhan N., Pullenayegum E., Brunellière J., Bramson J. L., Lichty B. D., Wan Y. (2010). Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther 18, 1430–1439 10.1038/mt.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. W., Stephenson K. B., Hanson S., Kucharczyk M., Duncan R., Bell J. C., Lichty B. D. (2009). The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol 83, 552–561 10.1128/JVI.01921-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary Z. D., Willingham M. C., Lyles D. S. (2011). Oncolytic vesicular stomatitis virus induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J Virol 85, 5708–5717 10.1128/JVI.02393-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Seemann J., Mishra R. K., Wei J. H., Weil L., Nussenzveig D. R., Heiber J., Barber G. N., Dasso M., Fontoura B. M. (2009). Vesicular stomatitis virus inhibits mitotic progression and triggers cell death. EMBO Rep 10, 1154–1160 10.1038/embor.2009.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G., Xu S., Watanabe M., Jayakar H. R., Whitt M. A., Gingrich J. R. (2010). Enhanced oncolytic activity of vesicular stomatitis virus encoding SV5-F protein against prostate cancer. J Urol 183, 1611–1618 10.1016/j.juro.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Chauhan V. S., Furr S. R., Sterka D. G., Jr, Nelson D. A., Moerdyk-Schauwecker M., Marriott I., Grdzelishvili V. Z. (2010). Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology 400, 187–196 10.1016/j.virol.2010.01.025 [DOI] [PubMed] [Google Scholar]
- Chelbi-Alix M. K., Quignon F., Pelicano L., Koken M. H., de Thé H. (1998). Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J Virol 72, 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. H., Lyles D. S. (2002). Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J Virol 76, 10177–10187 10.1128/JVI.76.20.10177-10187.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon P., Deutsch V., Lafay F., Martinet-Edelist C., Wyers F., Herman R. C., Flamand A. (1990). Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J Gen Virol 71, 991–996 10.1099/0022-1317-71-4-991 [DOI] [PubMed] [Google Scholar]
- Cripe T. P., Wang P. Y., Marcato P., Mahller Y. Y., Lee P. W. (2009). Targeting cancer-initiating cells with oncolytic viruses. Mol Ther 17, 1677–1682 10.1038/mt.2009.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton D. K., Massol R. H., Whelan S. P., Kirchhausen T. (2010). The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog 6, e1001127. 10.1371/journal.ppat.1001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. (1976). Status spongiosus resulting from intracerebral infection of mice with temperature-sensitive mutants of vesicular stomatitis virus. Br J Exp Pathol 57, 321–330 [PMC free article] [PubMed] [Google Scholar]
- Dalton K. P., Rose J. K. (2001). Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology 279, 414–421 10.1006/viro.2000.0736 [DOI] [PubMed] [Google Scholar]
- Diallo J. S., Vähä-Koskela M., Le Boeuf F., Bell J. (2012). Propagation, purification, and in vivo testing of oncolytic vesicular stomatitis virus strains. Methods Mol Biol 797, 127–140 10.1007/978-1-61779-340-0_10 [DOI] [PubMed] [Google Scholar]
- Diaz R. M., Galivo F., Kottke T., Wongthida P., Qiao J., Thompson J., Valdes M., Barber G., Vile R. G. (2007). Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res 67, 2840–2848 10.1158/0008-5472.CAN-06-3974 [DOI] [PubMed] [Google Scholar]
- Dinh P. X., Beura L. K., Panda D., Das A., Pattnaik A. K. (2011). Antagonistic effects of cellular poly(C) binding proteins on vesicular stomatitis virus gene expression. J Virol 85, 9459–9471 10.1128/JVI.05179-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet B. S., Stuart M. A., Derner J. D. (2009). Infection of Melanoplus sanguinipes grasshoppers following ingestion of rangeland plant species harboring vesicular stomatitis virus. Appl Environ Microbiol 75, 3029–3033 10.1128/AEM.02368-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert O., Shinozaki K., Kournioti C., Park M. S., García-Sastre A., Woo S. L. (2004). Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res 64, 3265–3270 10.1158/0008-5472.CAN-03-3753 [DOI] [PubMed] [Google Scholar]
- Edge R. E., Falls T. J., Brown C. W., Lichty B. D., Atkins H., Bell J. C. (2008). A let-7 microRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther 16, 1437–1443 10.1038/mt.2008.130 [DOI] [PubMed] [Google Scholar]
- Egan M. A., Chong S. Y., Rose N. F., Megati S., Lopez K. J., Schadeck E. B., Johnson J. E., Masood A., Piacente P. & other authors (2004). Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses 20, 989–1004 10.1089/aid.2004.20.989 [DOI] [PubMed] [Google Scholar]
- Faria P. A., Chakraborty P., Levay A., Barber G. N., Ezelle H. J., Enninga J., Arana C., van Deursen J., Fontoura B. M. (2005). VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol Cell 17, 93–102 10.1016/j.molcel.2004.11.023 [DOI] [PubMed] [Google Scholar]
- Fernandez M., Porosnicu M., Markovic D., Barber G. N. (2002). Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol 76, 895–904 10.1128/JVI.76.2.895-904.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke S., Conzelmann K. K. (2005). Recombinant rhabdoviruses: vectors for vaccine development and gene therapy. Curr Top Microbiol Immunol 292, 165–200 10.1007/3-540-27485-5_8 [DOI] [PubMed] [Google Scholar]
- Flanagan E. B., Zamparo J. M., Ball L. A., Rodriguez L. L., Wertz G. W. (2001). Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J Virol 75, 6107–6114 10.1128/JVI.75.13.6107-6114.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K., Malipiero U. V., Leist T. P., Zinkernagel R. M., Schwab M. E., Fontana A. (1989). On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol 19, 689–694 10.1002/eji.1830190418 [DOI] [PubMed] [Google Scholar]
- Friedman G. K., Cassady K. A., Beierle E. A., Markert J. M., Gillespie G. Y. (2012). Targeting pediatric cancer stem cells with oncolytic virotherapy. Pediatr Res 71, 500–510 10.1038/pr.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr S. R., Chauhan V. S., Sterka D., Jr, Grdzelishvili V. Z., Marriott I. (2008). Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol 14, 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr S. R., Moerdyk-Schauwecker M., Grdzelishvili V. Z., Marriott I. (2010). RIG-I mediates nonsegmented negative-sense RNA virus-induced inflammatory immune responses of primary human astrocytes. Glia 58, 1620–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy D. F., Lyles D. S. (2005). Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol 79, 4170–4179 10.1128/JVI.79.7.4170-4179.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy D. F., Lyles D. S. (2007). Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol 81, 2792–2804 10.1128/JVI.01760-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F., Diaz R. M., Thanarajasingam U., Jevremovic D., Wongthida P., Thompson J., Kottke T., Barber G. N., Melcher A., Vile R. G. (2010). Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum Gene Ther 21, 439–450 10.1089/hum.2009.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Whitaker-Dowling P., Watkins S. C., Griffin J. A., Bergman I. (2006). Rapid adaptation of a recombinant vesicular stomatitis virus to a targeted cell line. J Virol 80, 8603–8612 10.1128/JVI.00142-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. (2006). China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst 98, 298–300 10.1093/jnci/djj111 [DOI] [PubMed] [Google Scholar]
- Ge P., Tsao J., Schein S., Green T. J., Luo M., Zhou Z. H. (2010). Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 327, 689–693 10.1126/science.1181766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P., Jiang Z., Kunz S., Janssen E., Mols J., Hoebe K., Bahram S., Oldstone M. B., Beutler B. (2007). Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362, 304–313 10.1016/j.virol.2006.12.032 [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F., Menard C., Puig P. E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. (2007). Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56, 641–648 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Carlson S. K., Classic K. L., Greiner S., Naik S., Power A. T., Bell J. C., Russell S. J. (2007). Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Δ51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood 110, 2342–2350 10.1182/blood-2007-01-065573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher R., Schmid R. M., Saur D., Schneider G. (2008). Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer 7, 64. 10.1186/1476-4598-7-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammill A. M., Conner J., Cripe T. P. (2010). Oncolytic virotherapy reaches adolescence. Pediatr Blood Cancer 55, 1253–1263 10.1002/pbc.22724 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hansen D. E., Thurmond M. C., Thorburn M. (1985). Factors associated with the spread of clinical vesicular stomatitis in California dairy cattle. Am J Vet Res 46, 789–795 [PubMed] [Google Scholar]
- Harashima A., Guettouche T., Barber G. N. (2010). Phosphorylation of the NFAR proteins by the dsRNA-dependent protein kinase PKR constitutes a novel mechanism of translational regulation and cellular defense. Genes Dev 24, 2640–2653 10.1101/gad.1965010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiber J. F., Barber G. N. (2011). Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent. J Virol 85, 10440–10450 10.1128/JVI.05408-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. S., Cureton D. K., Rahmeh A. A., Whelan S. P. (2010). Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathog 6, e1000958. 10.1371/journal.ppat.1000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Wu Y. J., Gerber M., Berger-Rentsch M., Heimrich B., Schwemmle M., Zimmer G. (2010). Fusion-active glycoprotein G mediates the cytotoxicity of vesicular stomatitis virus M mutants lacking host shut-off activity. J Gen Virol 91, 2782–2793 10.1099/vir.0.023978-0 [DOI] [PubMed] [Google Scholar]
- Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K. K. & other authors (2006). 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 10.1126/science.1132505 [DOI] [PubMed] [Google Scholar]
- Huneycutt B. S., Bi Z., Aoki C. J., Reiss C. S. (1993). Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol 67, 6698–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Wakimoto H., Ichikawa T., Jhung S., Hochberg F. H., Louis D. N., Chiocca E. A. (2000). Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol 74, 4765–4775 10.1128/JVI.74.10.4765-4775.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T., Carnero E., Okumura A., García-Sastre A., Harty R. N. (2007). Modifications of the PSAP region of the matrix protein lead to attenuation of vesicular stomatitis virus in vitro and in vivo. J Gen Virol 88, 2559–2567 10.1099/vir.0.83096-0 [DOI] [PubMed] [Google Scholar]
- Janelle V., Brassard F., Lapierre P., Lamarre A., Poliquin L. (2011). Mutations in the glycoprotein of vesicular stomatitis virus affect cytopathogenicity: potential for oncolytic virotherapy. J Virol 85, 6513–6520 10.1128/JVI.02484-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks N., Myers R., Greiner S. M., Thompson J., Mader E. K., Greenslade A., Griesmann G. E., Federspiel M. J., Rakela J. & other authors (2010). Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-β in rodents and nonhuman primates. Hum Gene Ther 21, 451–462 10.1089/hum.2009.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H., Andresen L., Nielsen J., Christensen J. P., Skov S. (2011). Vesicular stomatitis virus infection promotes immune evasion by preventing NKG2D-ligand surface expression. PLoS ONE 6, e23023. 10.1371/journal.pone.0023023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Nasar F., Coleman J. W., Price R. E., Javadian A., Draper K., Lee M., Reilly P. A., Clarke D. K. & other authors (2007). Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360, 36–49 10.1016/j.virol.2006.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E., Russell S. J. (2007). History of oncolytic viruses: genesis to genetic engineering. Mol Ther 15, 651–659 [DOI] [PubMed] [Google Scholar]
- Kelly E. J., Nace R., Barber G. N., Russell S. J. (2010). Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol 84, 1550–1562 10.1128/JVI.01788-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky S. A., Willingham M. C., Lyles D. S. (2001). Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol 75, 12169–12181 10.1128/JVI.75.24.12169-12181.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T., Diaz R. M., Kaluza K., Pulido J., Galivo F., Wongthida P., Thompson J., Willmon C., Barber G. N. & other authors (2008a). Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol Ther 16, 1910–1918 10.1038/mt.2008.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T., Galivo F., Wongthida P., Diaz R. M., Thompson J., Jevremovic D., Barber G. N., Hall G., Chester J. & other authors (2008b). Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol Ther 16, 1217–1226 10.1038/mt.2008.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T., Hall G., Pulido J., Diaz R. M., Thompson J., Chong H., Selby P., Coffey M., Pandha H. & other authors (2010). Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest 120, 1551–1560 10.1172/JCI41431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy J., Mounir Z., Raven J. F., Koromilas A. E. (2008). The eIF2α kinases inhibit vesicular stomatitis virus replication independently of eIF2alpha phosphorylation. Cell Cycle 7, 2346–2351 [DOI] [PubMed] [Google Scholar]
- Labib M., Zamay A. S., Muharemagic D., Chechik A., Bell J. C., Berezovski M. V. (2012). Electrochemical sensing of aptamer-facilitated virus immunoshielding. Anal Chem 84, 1677–1686 10.1021/ac202978r [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Stillman E. A., Whitt M. A., Rose J. K. (1995). Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A 92, 4477–4481 10.1073/pnas.92.10.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F., Diallo J. S., McCart J. A., Thorne S., Falls T., Stanford M., Kanji F., Auer R., Brown C. W. & other authors (2010). Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther 18, 888–895 10.1038/mt.2010.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F., Niknejad N., Wang J., Auer R., Weberpals J. I., Bell J. C., Dimitroulakos J. (2012). Sensitivity of cervical carcinoma cells to vesicular stomatitis virus-induced oncolysis: potential role of human papilloma virus infection. Int J Cancer 131, E204–E215 10.1002/ijc.27404 [DOI] [PubMed] [Google Scholar]
- Leveille S., Goulet M. L., Lichty B. D., Hiscott J. (2011a). Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation. J Virol 85, 12160–12169 10.1128/JVI.05703-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille S., Samuel S., Goulet M. L., Hiscott J. (2011b). Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther 18, 435–443 10.1038/cgt.2011.14 [DOI] [PubMed] [Google Scholar]
- Li Q., Tainsky M. A. (2011). Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS ONE 6, e28683. 10.1371/journal.pone.0028683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty B. D., Power A. T., Stojdl D. F., Bell J. C. (2004). Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med 10, 210–216 10.1016/j.molmed.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Linge C., Gewert D., Rossmann C., Bishop J. A., Crowe J. S. (1995). Interferon system defects in human malignant melanoma. Cancer Res 55, 4099–4104 [PubMed] [Google Scholar]
- Lyles D. S., Rupprecht C. E. (2007). Rhabdoviridae. In Fields Virology, 5th edn, pp. 1363–1408 Edited by Knipe D. M., Howley P. M. Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Marozin S., Altomonte J., Stadler F., Thasler W. E., Schmid R. M., Ebert O. (2008). Inhibition of the IFN-β response in hepatocellular carcinoma by alternative spliced isoform of IFN regulatory factor-3. Mol Ther 16, 1789–1797 10.1038/mt.2008.201 [DOI] [PubMed] [Google Scholar]
- Marozin S., De Toni E. N., Rizzani A., Altomonte J., Junger A., Schneider G., Thasler W. E., Kato N., Schmid R. M., Ebert O. (2010). Cell cycle progression or translation control is not essential for vesicular stomatitis virus oncolysis of hepatocellular carcinoma. PLoS ONE 5, e10988. 10.1371/journal.pone.0010988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I., Rodriguez L. L., Jimenez C., Pauszek S. J., Wertz G. W. (2003). Vesicular stomatitis virus glycoprotein is a determinant of pathogenesis in swine, a natural host. J Virol 77, 8039–8047 10.1128/JVI.77.14.8039-8047.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin S. F., Rackley R. R., Sadhukhan P. C., Kim M. S., Novick A. C., Bandyopadhyay S. K. (2001). Impaired α-interferon signaling in transitional cell carcinoma: lack of p48 expression in 5637 cells. Cancer Res 61, 2261–2266 [PubMed] [Google Scholar]
- Melcher A., Parato K., Rooney C. M., Bell J. C. (2011). Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 19, 1008–1016 10.1038/mt.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Bidula S. M., Jensen T. M., Reiss C. S. (2009). Cytokine-modified VSV is attenuated for neural pathology, but is both highly immunogenic and oncolytic. Int J Infereron Cytokine Mediator Res 1, 15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Bidula S. M., Jensen T. M., Reiss C. S. (2010). Vesicular stomatitis virus modified with single chain IL-23 exhibits oncolytic activity against tumor cells in vitro and in vivo. Int J Infereron Cytokine Mediator Res 2010, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire C. E., Whitt M. A. (2011). The protease-sensitive loop of the vesicular stomatitis virus matrix protein is involved in virus assembly and protein translation. Virology 416, 16–25 10.1016/j.virol.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Moerdyk-Schauwecker M., Destephanis D., Hastie E., Grdzelishvili V. Z. (2011). Detecting protein–protein interactions in vesicular stomatitis virus using a cytoplasmic yeast two hybrid system. J Virol Methods 173, 203–212 10.1016/j.jviromet.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi M., Fazli L., Tearle H., Guo Y., Cox M., Bell J., Ong C., Jia W., Rennie P. S. (2010). Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res 70, 1367–1376 10.1158/0008-5472.CAN-09-2377 [DOI] [PubMed] [Google Scholar]
- Muik A., Kneiske I., Werbizki M., Wilflingseder D., Giroglou T., Ebert O., Kraft A., Dietrich U., Zimmer G. & other authors (2011). Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol 85, 5679–5684 10.1128/JVI.02511-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A., Dold C., Geiß Y., Volk A., Werbizki M., Dietrich U., von Laer D. (2012). Semireplication-competent vesicular stomatitis virus as a novel platform for oncolytic virotherapy. J Mol Med (Berl) 90, 959–970 10.1007/s00109-012-0863-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. M., Besmer D. M., Moerdyk-Schauwecker M., Moestl N., Ornelles D. A., Mukherjee P., Grdzelishvili V. Z. (2012). Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J Virol 86, 3073–3087 10.1128/JVI.05640-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Russell S. J. (2009). Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther 9, 1163–1176 10.1517/14712590903170653 [DOI] [PubMed] [Google Scholar]
- Naik S., Nace R., Federspiel M. J., Barber G. N., Peng K. W., Russell S. J. (2012). Curative one-shot systemic virotherapy in murine myeloma. Leukemia 26, 1870–1878 10.1038/leu.2012.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Kaur B., Chiocca E. A. (2010). Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev 21, 119–126 10.1016/j.cytogfr.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyên T. L., Abdelbary H., Arguello M., Breitbach C., Leveille S., Diallo J. S., Yasmeen A., Bismar T. A., Kirn D. & other authors (2008). Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A 105, 14981–14986 10.1073/pnas.0803988105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noser J. A., Mael A. A., Sakuma R., Ohmine S., Marcato P., Lee P. W., Ikeda Y. (2007). The RAS/Raf1/MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol Ther 15, 1531–1536 10.1038/sj.mt.6300193 [DOI] [PubMed] [Google Scholar]
- Obuchi M., Fernandez M., Barber G. N. (2003). Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 77, 8843–8856 10.1128/JVI.77.16.8843-8856.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliere S., Arguello M., Mesplede T., Tumilasci V., Nakhaei P., Stojdl D., Sonenberg N., Bell J., Hiscott J. (2008). Vesicular stomatitis virus oncolysis of T lymphocytes requires cell cycle entry and translation initiation. J Virol 82, 5735–5749 10.1128/JVI.02601-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozduman K., Wollmann G., Ahmadi S. A., van den Pol A. N. (2009). Peripheral immunization blocks lethal actions of vesicular stomatitis virus within the brain. J Virol 83, 11540–11549 10.1128/JVI.02558-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A. S., Koch P. E., Atkinson N., Xiong M., Finberg R. W., Roth J. A., Fang B. (1999). Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res 5, 4208–4213 [PubMed] [Google Scholar]
- Peng K. W., Myers R., Greenslade A., Mader E., Greiner S., Federspiel M. J., Dispenzieri A., Russell S. J. (2012). Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther (in press). 10.1038/gt.2012.31 10.1038/gt.2012.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. M., Her L. S., Varvel V., Lund E., Dahlberg J. E. (2000). The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol 20, 8590–8601 10.1128/MCB.20.22.8590-8601.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang C., Constantinescu S. N., Croze E., Blatt L. M., Albino A. P., Nanus D. M. (1996). Human renal cancers resistant to IFN’s antiproliferative action exhibit sensitivity to IFN’s gene-inducing and antiviral actions. J Urol 156, 1867–1871 10.1016/S0022-5347(01)65555-1 [DOI] [PubMed] [Google Scholar]
- Plakhov I. V., Arlund E. E., Aoki C., Reiss C. S. (1995). The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology 209, 257–262 10.1006/viro.1995.1252 [DOI] [PubMed] [Google Scholar]
- Porosnicu M., Mian A., Barber G. N. (2003). The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res 63, 8366–8376 [PubMed] [Google Scholar]
- Power A. T., Bell J. C. (2007). Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther 15, 660–665 [DOI] [PubMed] [Google Scholar]
- Power A. T., Bell J. C. (2008). Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther 15, 772–779 10.1038/gt.2008.40 [DOI] [PubMed] [Google Scholar]
- Pulido J., Kottke T., Thompson J., Galivo F., Wongthida P., Diaz R. M., Rommelfanger D., Ilett E., Pease L. & other authors (2012). Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol 30, 337–343 10.1038/nbt.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Wang H., Kottke T., Diaz R. M., Willmon C., Hudacek A., Thompson J., Parato K., Bell J. & other authors (2008a). Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther 15, 604–616 10.1038/sj.gt.3303098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Wang H., Kottke T., White C., Twigger K., Diaz R. M., Thompson J., Selby P., de Bono J. & other authors (2008b). Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res 14, 259–269 10.1158/1078-0432.CCR-07-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz E., Moreno N., Peralta P. H., Tesh R. B. (1988). A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am J Trop Med Hyg 39, 312–314 [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Plakhov I. V., Komatsu T. (1998). Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci 855, 751–761 10.1111/j.1749-6632.1998.tb10655.x [DOI] [PubMed] [Google Scholar]
- Rieder M., Conzelmann K. K. (2009). Rhabdovirus evasion of the interferon system. J Interferon Cytokine Res 29, 499–510 10.1089/jir.2009.0068 [DOI] [PubMed] [Google Scholar]
- Rose N. F., Marx P. A., Luckay A., Nixon D. F., Moretto W. J., Donahoe S. M., Montefiori D., Roberts A., Buonocore L., Rose J. K. (2001). An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106, 539–549 10.1016/S0092-8674(01)00482-2 [DOI] [PubMed] [Google Scholar]
- Saloura V., Wang L. C., Fridlender Z. G., Sun J., Cheng G., Kapoor V., Sterman D. H., Harty R. N., Okumura A. & other authors (2010). Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum Gene Ther 21, 51–64 10.1089/hum.2009.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S., Tumilasci V. F., Oliere S., Nguyên T. L., Shamy A., Bell J., Hiscott J. (2010). VSV oncolysis in combination with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance in chronic lymphocytic leukemia. Mol Ther 18, 2094–2103 10.1038/mt.2010.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S., Theofanis T., Reiss C. S. (2011). Interferon-induced tetherin restricts vesicular stomatitis virus release in neurons. DNA Cell Biol 30, 965–974 10.1089/dna.2011.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schache P., Gürlevik E., Strüver N., Woller N., Malek N., Zender L., Manns M., Wirth T., Kühnel F., Kubicka S. (2009). VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther 16, 849–861 10.1038/gt.2009.39 [DOI] [PubMed] [Google Scholar]
- Schellekens H., Smiers-de Vreede E., de Reus A., Dijkema R. (1984). Antiviral activity of interferon in rats and the effect of immune suppression. J Gen Virol 65, 391–396 10.1099/0022-1317-65-2-391 [DOI] [PubMed] [Google Scholar]
- Schnell M. J., Buonocore L., Kretzschmar E., Johnson E., Rose J. K. (1996). Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci U S A 93, 11359–11365 10.1073/pnas.93.21.11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Askari E., Nakhaei P., Oliere S., Tumilasci V., Hernandez E., Wilkinson P., Lin R., Bell J., Hiscott J. (2007). Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology 365, 20–33 10.1016/j.virol.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Shi Z., Cai Z., Sanchez A., Zhang T., Wen S., Wang J., Yang J., Fu S., Zhang D. (2011). A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem 286, 4517–4524 10.1074/jbc.M110.159590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E. J., Wanna G. B., Choi B., Aguila D., III, Ebert O., Genden E. M., Woo S. L. (2007a). Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope 117, 210–214 10.1097/01.mlg.0000246194.66295.d8 [DOI] [PubMed] [Google Scholar]
- Shin E. J., Chang J. I., Choi B., Wanna G., Ebert O., Genden E. M., Woo S. L. (2007b). Fusogenic vesicular stomatitis virus for the treatment of head and neck squamous carcinomas. Otolaryngol Head Neck Surg 136, 811–817 10.1016/j.otohns.2006.11.046 [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ebert O., Suriawinata A., Thung S. N., Woo S. L. (2005). Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J Virol 79, 13705–13713 10.1128/JVI.79.21.13705-13713.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Pavlovic J. (1991). Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol 65, 4498–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer M. L., Cureton D. K., Whelan S. P. (2011). A recombinant vesicular stomatitis virus bearing a lethal mutation in the glycoprotein gene uncovers a second site suppressor that restores fusion. J Virol 85, 8105–8115 10.1128/JVI.00735-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson K. B., Barra N. G., Davies E., Ashkar A. A., Lichty B. D. (2012). Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther 19, 238–246 10.1038/cgt.2011.81 [DOI] [PubMed] [Google Scholar]
- Stojdl D. F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J. C. (2000). Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 6, 821–825 10.1038/77558 [DOI] [PubMed] [Google Scholar]
- Stojdl D. F., Lichty B. D., tenOever B. R., Paterson J. M., Power A. T., Knowles S., Marius R., Reynard J., Poliquin L. & other authors (2003). VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4, 263–275 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- Sun W. H., Pabon C., Alsayed Y., Huang P. P., Jandeska S., Uddin S., Platanias L. C., Rosen S. T. (1998). Interferon-α resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91, 570–576 [PubMed] [Google Scholar]
- Tumilasci V. F., Olière S., Nguyên T. L., Shamy A., Bell J., Hiscott J. (2008). Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol 82, 8487–8499 10.1128/JVI.00851-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Dalton K. P., Rose J. K. (2002). Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol 76, 1309–1327 10.1128/JVI.76.3.1309-1327.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. X., Rahbar R., Fish E. N. (2011). Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res 31, 545–552 10.1089/jir.2010.0158 [DOI] [PubMed] [Google Scholar]
- Weidner J. M., Jiang D., Pan X. B., Chang J., Block T. M., Guo J. T. (2010). Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol 84, 12646–12657 10.1128/JVI.01328-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S. P., Ball L. A., Barr J. N., Wertz G. T. (1995). Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A 92, 8388–8392 10.1073/pnas.92.18.8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow Z. W., Connor J. H., Lyles D. S. (2006). Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome. J Virol 80, 11733–11742 10.1128/JVI.00971-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow Z. W., Connor J. H., Lyles D. S. (2008). New mRNAs are preferentially translated during vesicular stomatitis virus infection. J Virol 82, 2286–2294 10.1128/JVI.01761-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon C., Diaz R. M., Wongthida P., Galivo F., Kottke T., Thompson J., Albelda S., Harrington K., Melcher A., Vile R. (2011). Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intratumoral oncolytic virus and cyclophosphamide. Mol Ther 19, 140–149 10.1038/mt.2010.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G., Tattersall P., van den Pol A. N. (2005). Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J Virol 79, 6005–6022 10.1128/JVI.79.10.6005-6022.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G., Rogulin V., Simon I., Rose J. K., van den Pol A. N. (2010). Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol 84, 1563–1573 10.1128/JVI.02040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. H., Krauer K. G., Hatzinisiriou I., Estcourt M. J., Hersey P., Tam N. D., Edmondson S., Devenish R. J., Ralph S. J. (1997). Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3γ. J Biol Chem 272, 28779–28785 10.1074/jbc.272.45.28779 [DOI] [PubMed] [Google Scholar]
- Wongthida P., Diaz R. M., Galivo F., Kottke T., Thompson J., Pulido J., Pavelko K., Pease L., Melcher A., Vile R. (2010). Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res 70, 4539–4549 10.1158/0008-5472.CAN-09-4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P., Diaz R. M., Galivo F., Kottke T., Thompson J., Melcher A., Vile R. (2011a). VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling. Mol Ther 19, 150–158 10.1038/mt.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P., Diaz R. M., Pulido C., Rommelfanger D., Galivo F., Kaluza K., Kottke T., Thompson J., Melcher A., Vile R. (2011b). Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum Gene Ther 22, 1343–1353 10.1089/hum.2010.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Huang T. G., Meseck M., Altomonte J., Ebert O., Shinozaki K., García-Sastre A., Fallon J., Mandeli J., Woo S. L. (2008). rVSV(MΔ51)-M3 is an effective and safe oncolytic virus for cancer therapy. Hum Gene Ther 19, 635–647 10.1089/hum.2007.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Puckett S., Lyles D. S. (2001). Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J Virol 75, 4453–4458 10.1128/JVI.75.9.4453-4458.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. X., Matsui Y., Hadaschik B. A., Lee C., Jia W., Bell J. C., Fazli L., So A. I., Rennie P. S. (2010). Down-regulation of type I interferon receptor sensitizes bladder cancer cells to vesicular stomatitis virus-induced cell death. Int J Cancer 127, 830–838 [DOI] [PubMed] [Google Scholar]