Abstract
Starch phosphorylation mediated by the α-glucan, water dikinase (GWD) is crucial for transitory starch metabolism. The impact of the GWD action on transitory starch metabolism was analyzed in Arabidopsis mutants either lacking or revealing different reduced levels of GWD activity. In these mutants, glucose 6-phosphate (G6P) levels of the transitory leaf starch, the average leaf starch content, as well as alterations in the growth phenotype were determined under different light length conditions, including continuous light. Based on biochemical and growth phenotypical data, we found that the length of the light phase affects the phosphorylation state of the transitory starch and, by this, the average leaf starch content and the resulting growth of the plants. Additionally, we discuss data referring to an involvement of the GWD mediated glucan phosphorylation in starch synthesis, as, e.g., starch phosphorylation occurred even when a dark phase was omitted.
Keywords: Arabidopsis, GWD, glucan, starch, starch degradation, starch synthesis, water dikinase
The action of the starch-phosphorylating enzyme α-glucan, water dikinase (GWD) is well analyzed in the process of transitory starch degradation. GWD selectively phosphorylates the C6 position of glucosyl residues of amylopectin molecules.1 It has been shown that the action of GWD is an initial event of starch degradation.2-4 Glucan phosphorylation by GWD disrupts the crystalline structure of the starch granule surface and stimulates hydrolytic enzyme activities, e.g., AtBAM3.5
Mutations in the gene coding for GWD, SEX1, affect transitory starch turnover and have a deep impact on plant development. The GWD null mutant sex1–8 accumulates 5 times more starch than the Col-0 wild type.1 Moreover, mutant plants are massively compromised in growth.6,7
Recently, we have introduced sex1–8 mutants, which were partially complemented with wild type GWD. The expression of GWD in the lines gwd-c1 and gwd-c2 is lowered by 81% and 93%, respectively, compared with wild type.8 We found that the expression of GWD in gwd-c1 and gwd-c2 positively correlates with the starch bound glucose 6-phosphate (G6P) content.8 Interestingly, both mutants have an intermediate phenotype for parameters such as growth, average leaf starch content, and enzyme activities and protein levels of other starch-related enzymes. The protein levels of enzymes related to the starch phosphorylation/dephosphorylation cycle like PWD and SEX4 are considerably altered.4,8 In addition, the expression of SSIV is significantly increased, surely reflecting the observed increase of the starch granule number in case of reduced GWD activity.8-10 Thus, the system including wild type, sex1–8, as well as gwd-c1 and gwd-c2, is suitable for analysis of the impact of GWD activity to transitory starch turnover, as the resolution of the phenotypical alterations depending on residual GWD activity is increased.
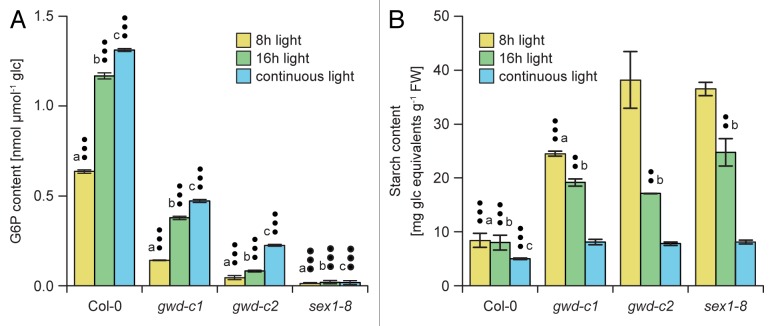
Interestingly, using this system, we were able to adjust the growth conditions such as length of the light phase in a way that the starch G6P content was similar for both wild type plants and mutants, although the activity of GWD varies in the different genotypes. As displayed in Figure 1A leaf starch of wild type grown in 8 h light /16 h dark and gwd-c1 grown under continuous light contained approximately the same amounts of esterified phosphate to the C6 position. Under these conditions, differences in the average leaf starch content (Fig. 1B) and growth phenotype (Fig. 2) were minor and both lines were more similar, indicating a positive correlation between these parameters and the residual GWD activities. Analyzing the effect of lacking GWD activity, we recently, presented data highlighting that GWD-mediated action contributes to the starch degrading, as well as the synthesizing pathway.
Figure 1. Glucose 6-phosphate content of amylopectin and starch content of wild type and mutants (gwd-c1, gwd-c2, sex1–8), lacking GWD activity, grown in light-dark cycles or under continuous light. (A) Glucose 6-phosphate (G6P) content of 32 d old plants. Leaf material for starch isolation and measurement of G6P were harvested at the end of the light phase for 8 h light/ 16 h dark cycle (yellow bars), 16 h light/ 8 h dark cycle (green bars). Values displayed for continuous light are mean of several time points as indicated in Figure 3A (blue bars). (B) Average leaf starch content of the 4 genotypes. Results in (A) and (B) for plants grown in a light-dark cycle are mean values ± SD of 3 independent biological replicates. Dots and letters indicate significant differences of values of the 4 lines of the respective growth condition (a – 8 h light/ 16 h dark cycle; b – 16 h light/ 8 h dark cycle; c – continuous light) as determined by the Student t test (•• – P < 0.05; ••• – P < 0.01).
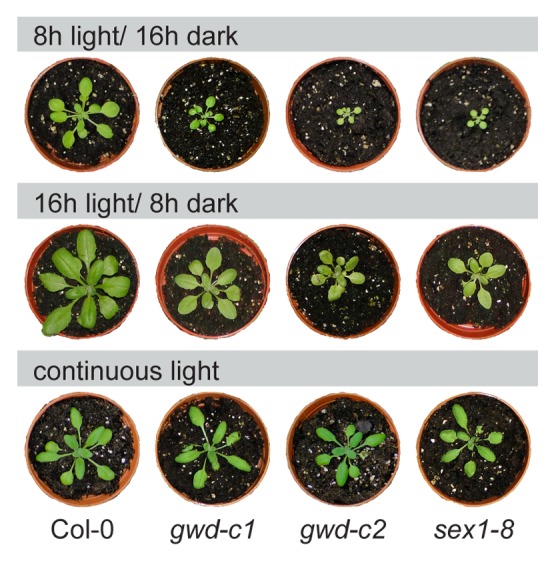
Figure 2. Growth phenotypes of wild type and GWD mutants (gwd-c1, gwd-c2, sex1–8) grown under different day length conditions. Plants were grown in an 8 h light/ 16 h dark cycle (upper panel), 16 h light/ 8 h dark cycle (middle panel) and in continuous light (lower panel) for 32 d.
Lacking GWD activity leads to alterations of the starch granule surface that is characterized by a higher frequency of free and accessible glucans and impedes the activity of starch degrading enzymes such as AtBAM3, isoamylase, and even GWD. These alterations also reduce the ability of recombinant soluble starch synthase I from Arabidopsis to transfer glucosyl moieties from ADP-glucose to native starch granules. However, as a result, over several photoperiods the starch morphology is altered.
When comparing the starch bound phosphate content in wild type and mutants grown either under 8 h light/ 16 h dark or 16 h light/ 8 h dark, it becomes obvious that the amount of phosphate incorporated during the light phase increased with a longer light phase while the average leaf starch content decreased. Thus, Arabidopsis plants accumulated more leaf starch when cultivated under short day conditions than under long day conditions, as seen for the wild type and even more pronounced in case of the mutants (Fig. 1B). In contrast, the starch bound phosphate levels were reduced under short day conditions. Thus, the higher starch bound phosphate content in wild type and mutants under long day could benefit a more effective degradation in the following (short) night as compared with short day conditions.
Another indication for a contribution of GWD activity to starch synthesis is the fact that glucan phosphorylation occured even when the dark phase was omitted (Fig. 1A). Moreover, starch phosphorylation was higher in wild type and mutants when plants were grown under continuous light compared with growth in a light-dark cycle (Fig. 1A). It remains possible that the incorporation of phosphate into starch under continuous light exceeds the level of glucan phosphorylation in the light-dark cycle as glucan dephosphorylation activity is thought to be restricted to the dark phase. Thus, the dephosphorylation of glucans would be prevented during continuous light conditions.
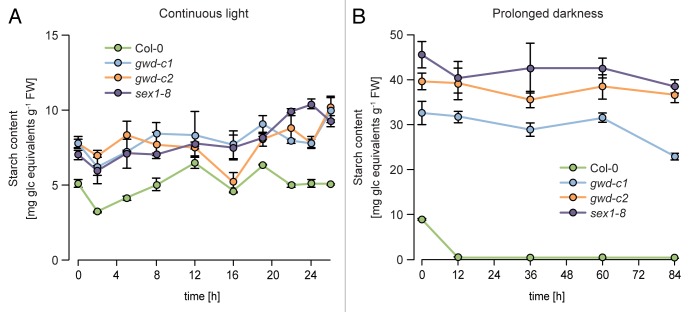
However, under continuous light, net starch degradation was almost omitted and, thus, the high phosphate level of the starch may not come into effect (Fig. 3A). Furthermore, the growth phenotypes of the mutants were more similar to wild type under continuous light compared with any light-dark cycle, indicating that the lack of GWD is decoupled from the growth phenotype under continuous light conditions.
Figure 3. Alterations in leaf starch content of wild type and GWD mutants (gwd-c1, gwd-c2, sex1–8) grown in continuous light or transferred to a prolonged dark phase. (A) Wild type and mutants were cultivated in continuous light and samples were taken as indicated during the day. (B) Plants were grown in a strict 12 h light/ 12 h dark cycle for 5 weeks and at the end of the light phase subjected to a prolonged darkness. Data in (A) and (B) represent the mean ± SD of 3 independent biological replicates, each.
In another experiment, wild type and mutants were grown in a strict 12 h light/ 12 h dark cycle for 5 weeks and then subjected to continuous darkness. The average leaf starch content of the 4 genotypes is shown in Figure 3B for indicated time points of the prolonged dark phase. In contrast to wild type, the sex1–8 mutant displayed a starch excess phenotype even after 84 h of darkness, which is in accordance to a previous report.11 Also gwd-c1 and gwd-c2 revealed a starch excess phenotype in the beginning of the dark phase as expected.Interestingly, the residual GWD activity in the partially complemented mutants gwd-c1 and gwd-c2, which is less than in wild type, was insufficient to mobilize the leaf starch to wild type level even in a prolonged dark phase (Fig. 3B). This indicates again that starch phosphorylation by GWD in the light phase is in parts necessary for proper starch degradation in the following night. Overall, there are clear indications for the involvement of GWD in both starch synthesis and degradation. For a deeper understanding of the GWD functions in starch metabolism a separation of the enzyme action during the light and dark phase is necessary. This could be archived by inducible expression systems and time resolved analysis of the starch metabolism, as previously shown for trehalose-phosphate synthase.12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ritte G, Heydenreich M, Mahlow S, Haebel S, Kötting O, Steup M. Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett. 2006;580:4872–6. doi: 10.1016/j.febslet.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 2.Zeeman SC, Smith SM, Smith AM. The breakdown of starch in leaves. New Phytol. 2004;163:247–61. doi: 10.1111/j.1469-8137.2004.01101.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochem J. 2007;401:13–28. doi: 10.1042/BJ20061393. [DOI] [PubMed] [Google Scholar]
- 4.Fettke J, Fernie AR, Steup M. Transitory starch and its degradation in higher plant cells. In: Starch: Origins, Structure and Metabolism. Tetlow IJ, ed. London, UK: The Society for Experimental Biology, 2012. 309–72. [Google Scholar]
- 5.Edner C, Li J, Albrecht T, Mahlow S, Hejazi M, Hussain H, Kaplan F, Guy C, Smith SM, Steup M, et al. Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol. 2007;145:17–28. doi: 10.1104/pp.107.104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T-S, Kofler H, Häusler RE, Hille D, Flügge U-I, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell. 2001;13:1907–18. doi: 10.1105/tpc.13.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M. The starch-related R1 protein is an α -glucan, water dikinase. Proc Natl Acad Sci U S A. 2002;99:7166–71. doi: 10.1073/pnas.062053099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahlow S, Hejazi M, Kuhnert F, Garz A, Brust H, Baumann O, Fettke J. Phosphorylation of transitory starch by α-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules. New Phytol. 2014 doi: 10.1111/nph.12801. In press. [DOI] [PubMed] [Google Scholar]
- 9.Roldán I, Wattebled F, Mercedes Lucas M, Delvallé D, Planchot V, Jiménez S, Pérez R, Ball S, D’Hulst C, Mérida A. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- 10.Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell. 2009;21:2443–57. doi: 10.1105/tpc.109.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 2005;137:242–52. doi: 10.1104/pp.104.055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins MC, Hejazi M, Fettke J, Steup M, Feil R, Krause U, Arrivault S, Vosloh D, Figueroa CM, Ivakov A, et al. Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiol. 2013;163:1142–63. doi: 10.1104/pp.113.226787. [DOI] [PMC free article] [PubMed] [Google Scholar]