Abstract
Lysine is an essential amino acid for human nutrition, which is generally low in cereal diets. Its biosynthesis via the aspartate-pathway and catabolism is controlled by complex feedback mechanisms. Recently, aspartate-derived amino acids were found to be elevated during pathogen infection in Arabidopsis and a lysine catabolite, pipecolic acid, was identified as critical regulator of systemic acquired resistance. Pipecolic acid is mobile in plants, functions as an intensifier of defense responses and mediates systemic acquired resistance establishment via signal amplification. The altered pathogen defense in several mutants with altered homeostasis of aspartate-derived amino acids, such as lysine, had already provided a genetic link with amino acid homeostasis. Furthermore, the modification of amino acid transport and distribution within tissues not only affected the plant growth performance, but also the plant-pathogen interaction. The ectopic overexpression of a gene encoding a high affinity importer with preference to basic amino acids, such as lysine, CATIONIC AMINO ACID TRANSPORTER1 (CAT1), improved the disease resistance to a hemibiotrophic bacterial pathogen in Arabidopsis via a constitutively activated salicylic acid pathway. The importance of Asp-derived amino acid homeostasis for plant systemic acquired resistance and on overall plant growth performance may be relevant to resistance and nutritional quality breeding. Whether nitrogen fertilization has an impact on crop pest control management via amino acid homeostasis is briefly discussed.
Keywords: Amino acid transporters, Lysine catabolism, Pathogen defense, Systemic acquired resistance, Plant immunity, Pipecolic acid, Salicylic acid, nitrogen, fertilization
The aspartate-derived amino acids (AAs), such as lysine (Lys), methionine (Met), threonine (Thr), or the branched amino acid isoleucine (Ile), are essential in human diets. Unfortunately, the content of these AAs is frequently low and thus limiting in cereal food and feed. Consequently, the enzymatic steps in Asp-derived AA biosynthesis and catabolism had been a focus of research, but the amino acid composition was difficult to improve genetically, because of the strong feedback regulation at various levels (e.g., the feedback-inhibition of aspartate kinase by Lys, the entrance enzyme into the Asp-pathway) and growth defects in many mutants.1-3
Low levels of Asp-derived amino acids and their impact on plant growth
When extracted from the leaves, the soluble AAs derived from the Asp-branch comprise in most plant species only a minor fraction (≤ 1%) of all soluble AAs. The total leaf AA concentrations are under diurnal control and in barley, their total amount doubles in the light, but Lys, Met, the aromatic AA tyrosine (Tyr), and the branched chain AAs Ile, valine (Val), and leucine (Leu), were almost constant at the total leaf and cytosolic level, similar as in other plants.4 These AAs are unable to sustain plant growth, which is easily visualized in young seedlings of Arabidopsis with primary root length assays under very low nitrogen (N), when each proteinogenic amino acid or ammonium nitrate (control) are supplied at 50 µM as the sole N source (Fig. 1A, B). The presence of each amino acid did not affect the germination, but basic-, hydroxyl-, sulfur-containing, and aromatic amino acids severely blocked primary root growth, with the most severe inhibition by Lys. This amino acid therefore can even shape root morphology: in plants overexpressing or lacking the Lys-transporting amino acid transporter CATIONIC AMINO ACID TRANSPORTER1 (CAT1), the lateral root density was increased by Lys and affected in the mutants.5 The inhibitory effects of Asp-derived AAs are largely consistent with the results from Arabidopsis seedlings at higher basal N supply,6,7 and these are at least partially physiologically explained by the complex feedback inhibition that these AAs impose on the primary biosynthesis of essential amino acids.8
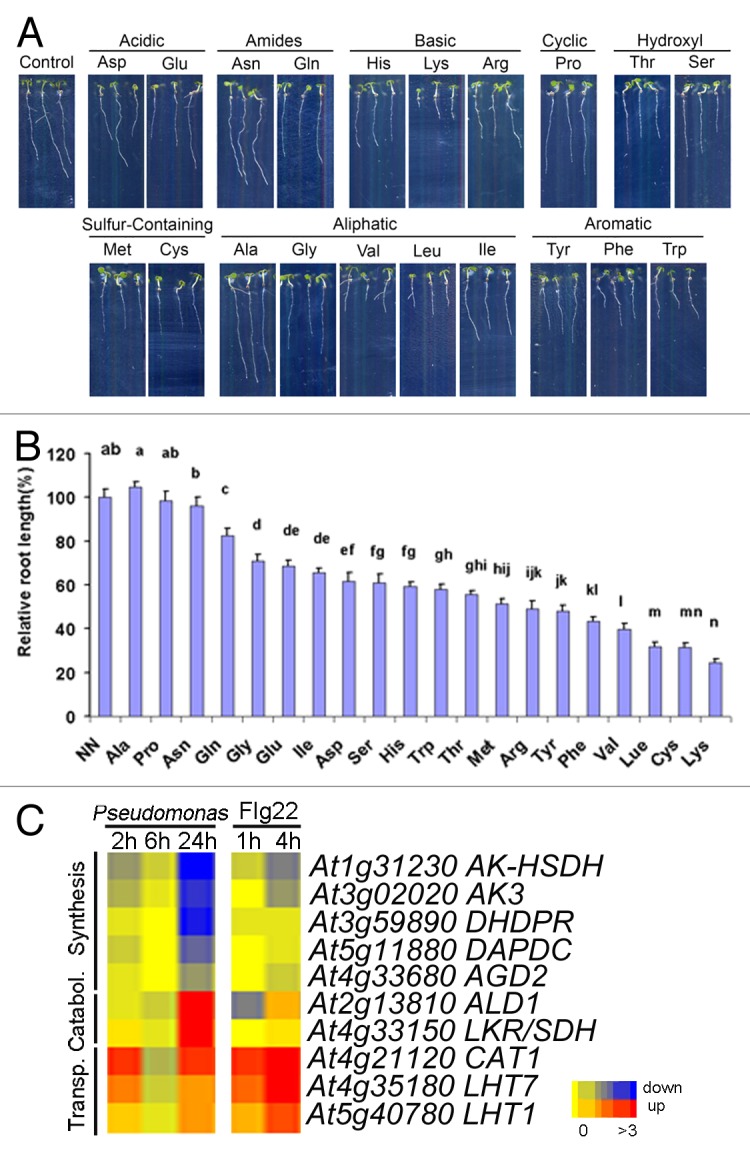
Figure 1. (A, B) Inhibitory effects of proteinogenic amino acids on primary root growth. Seedling growth on the vertical plates containing diluted Hoagland media with amino acids (or ammonium nitrate in the control) at 50 µM as the only nitrogen source (n = 9). (C) Transcriptional regulation of selected genes involved in the biosynthesis of Lys, degradation of Lys, and transport of amino acids upon P. syringae inoculation after 2, 6, 24 h, and only after Flg22 elicitor inoculation (1 and 4 h). AK-HSDH: Aspartate Kinase/Homoserine Dehydrogenase1, AK3: Aspartate Kinase3, DHDPR: Dihydrodipicolinate Reductase, DAPDC: Diaminopimelate Decarboxylase, AGD2: Aberrant Growth And Death2 (Diaminopimelate Aminotransferase), ALD1: AGD2-like Defense Response Protein 1; LKR/SDH: Lysine-Ketoglutarate Reductase/Saccharopine Dehydrogenase, CAT1: Cationic Amino Acid Transporter1, LHT1: Lysine Histidine Transporter1, LHT7: Lysine Histidine Transporter7. The color code for upregulation (red), downregulation (blue), and no change (yellow) is given as log values and is shown in the legend at the bottom.
Nearly a decade ago, it was found that a knock-down mutant in ABERRANT GROWTH AND DEATH2 (AGD2), a gene encoding a diaminopimelate aminotransferase involved in Lys biosynthesis, resulted in elevated disease resistance via an elevated salicylic acid (SA) pathway in Arabidopsis. By contrast, the loss of a gene involved in Lys catabolism, AGD2-LIKE DEFENSE RESPONSE PROTEIN1 (ALD1), reduced salicylic acid (SA), and lowered the plant resistance to Pseudomonas syringae.9-11 The miss-regulation of the amino acid homeostasis in the mutants came with a growth trade-off, as typically does the constitutive activation of systemic acquired resistance (SAR) in plants. There are further examples that enzyme mutants in the Asp-branch of AAs affect disease susceptibility12 and more recently, genetic studies on the improved resistance against an obligate biotrophic oomycete identified a loss-of-inhibition allele of ASPARTATE KINASE2 (AK2) and a loss-of-function allele of DIHYDRODIPICOLINATE SYNTHASE2 (DHDPS2). These mutations were associated with impaired growth and increased levels of the end products of the Asp-pathway: Met, Thr, Ile (and Lys in ak2). This was expected, since AK2 functions at the common entry into the Asp-pathway, while DHDPS2 is at the branching point, where Lys biosynthesis separates from the synthesis of the other AAs. These mutations, however, did not cause the constitutive activation of defense reactions, they accumulated less salicylic acid, had reduced expression of the systemic acquired resistance marker PATHOGENESIS-RELATED GENE1 (PR-1), and remained susceptible to another fungus.13
Interestingly, a recent study showed that the amino acid concentrations in Arabidopsis leaves increased upon bacterial P. syringae infection by 60%. In inoculated leaves, Val, Leu, Ile, Lys, and aromatic AAs massively increased by 5- to 14-fold, while their common precursor Asp decreased.14 Because Lys strongly feedback-inhibits the Asp-pathway, transcriptional downregulation of key biosynthetic steps leading to Lys and the joint upregulation of the lysine catabolism were consistently observed in microarray data from similar experiments on leaves inoculated with P. syringae15 (Fig. 1C). Furthermore, several amino acid transporter genes were also rapidly transcriptionally upregulated, including CAT1, LYSINE HISTIDINE TRANSPORTER1 (LHT1), and LHT7. Interestingly, this upregulation of AA transporter genes preceded that of other genes and was even observed upon treatments with elicitors, even in the absence of the pathogens.5 Such stimulation was not observed with the metabolic enzymes and a second, larger set of amino acid transporter genes that were later upregulated (Fig. 1C).5 This may indicate that besides the stimulation of the AA biosynthesis/catabolism, uptake into the leaf cells occurs, as reduced amounts of Lys, Thr, and Ile in petiole exudates were found.14 Among the catabolites of Lys that were upregulated about 70-fold was the cyclic, non-protein amino acid pipecolic acid (Pip). Pip biosynthesis occurs via the ALD1-dependent pipecolate pathway and in ald1 mutants, the precursor Lys accumulates after pathogen infection.14 The detailed analyses with mutants and marker genes showed that Pip acts as a critical metabolic regulator and intensifier of defense responses and thereby mediates systemic acquired resistance establishment, while salicylic acid is required for the local amplification of the signal.12,14 Exogenous application of Pip induced ALD1 expression and primed plant defense responses,14,16 but its precursor lysine was unable to activate SAR. Interestingly, although many metabolites (including Pip) accumulated in the inoculated leaf, only Pip was enriched in the respective leaf exudates. This suggests a specific transport mechanism of Pip out of pathogen-inoculated leaves, but whether transport from inoculated to distal leaves occurs, is still not fully established. Exogenous Pip increased the resistance only when applied via the roots, but not when infiltrated into the leaf apoplast and it was speculated that it is not efficiently taken up at the plasma membrane into the cytosol.
Besides this strong importance of the Asp-derived amino acid biosynthesis/catabolism, increasing evidence suggests that also the transport of AAs is of high relevance. The decrease in the export of AAs from the leaves upon pathogen infection and an accumulation of Asp-derived AAs in the leaf already pointed to the importance of AA transport and distribution.14 However, because most AA-transporters have a broad substrate spectrum, it is less clear whether the transport of specific amino acids, the bulk transport of AAs, or even AA-derivatives or AA-conjugates are relevant. The major high affinity transporter for uncharged and acidic amino acids in the mesophyll plasma membrane, LHT1, is not only important for the supply of amino acids into the leaf mesophyll cells, but is also important for the plant pathogen resistance. In a loss-of-function mutant (lht1) of this H+-coupled AA importer, glutamine deficiency and an alternation of the redox status were observed, which lead to an increased pathogen resistance via the SA pathway.17 Unfortunately, the name LYSINE HISTIDINE TRANSPORTER1 is misleading and the detailed analysis of the substrate spectrum of LHT1 clearly indicated that Lys and histidine are not transported under physiological conditions. This plasma membrane protein transports a broad spectrum of neutral and acidic AAs.18,19 In lht1, only a minor decrease in the total leaf AAs was found, which was restricted to the more flexible and highly abundant glutamine (Gln) and proline (Pro).17 In another AA-transport mutant that overexpresses a small membrane protein with unclear function (gdu1–1), the Gln translocated from the roots to the shoots via the xylem is not taken up efficiently into the leaf, leading to accumulation of total leaf AAs and even secretion of Gln at the hydathodes.20 The increased AA level in that mutant lead to enhanced reactive oxygen production, PR-1 expression and improved the disease resistance.17 Finally, the ectopic overexpression of CAT1 increased PR-1 expression and enhanced the pathogen resistance via a constitutively activated salicylic acid pathway.5 While this phenotype is not seen with the ectopic overexpression of other plasma membrane H+-coupled amino acid transporters, the substrate specificity and affinity toward basic amino acids (or other not yet identified transported substrates) may be relevant for this phenotype. As the total and apoplastic AA composition was only marginally changed and the AA amount was insignificantly increased, it is speculated that CAT1 overexpression may have induced the SA signaling via high Lys, Pip and/or another metabolite at an earlier growth stage. It should be noted that the soluble AAs generally decrease strongly during vegetative growth with plant age, by a factor of about 3 from young to older leaves in maize.21 Furthermore, the relevance of the diurnal and dynamic internal storage of AAs, e.g., in the vacuole, remains unclear, but key genes for that vacuolar compartmentation have recently been identified.22
Manipulation of AA pools and signaling by fertilization?
Because the N fertilization increases the internal amino acid pools of crops, one may ask whether the amount and N-form of fertilizer affects the critical Asp-derived amino acids and their potential signaling derivatives, such as Pip. The total AAs differed by roughly a factor of 2 in leaves in N-fertilized and unfertilized maize, but very low variation in the minor amino acids, such as Lys, is typically seen.21 The nitrogen form, timing, and amount can change the free amino acid profiles, but inconsistent effects on the disease resistance of crops were reported. The susceptibility appeared mainly affected by the lifestyle of the pathogen and the tolerance to facultative parasites (which damage or kill the host plant cells and feed from senescing tissues) is mostly increased by higher metabolic activities in N-fertilized plants, as this delays senescence. On the contrary, an increase in severity of the infection is frequently observed with obligate parasites (of viral, bacterial, oomycete, or fungal origin) with N supply,23 with notable exceptions.24 The hemibiotrophic P. syringae requires nitrogen for successful invasion, colonization and growth, and amino auxotroph mutants of P. syringae were unable to confer disease symptoms in tomato.25 This argues that amino acids are a rich and economic nitrogen source for the pathogen. For fungal pathogens it was concluded that in the majority of crops and pathogens, N-fertilization negatively impacts on plant disease resistance.24 Although N-fertilization likely has only a minor impact on Lys-derived signaling metabolites, it may be worthwhile to re-analyze such reports with respect to the potential impact of Asp-derived amino acid levels. Furthermore, the large number of mutants in different species with increased soluble Lys or other Asp-derived AAs that had been generated for improving the nutritional value of certain plant products are waiting to be tested for their pathogen resistance, in order to further elucidate the Pip/Lys signaling pathway. The Lys catabolite Pip appears to be a missing link with SAR and Asp-derived amino acid homeostasis, but whether Pip is transported by the same transporters as are proteinogenic amino acids for the distribution via long-distances between infected and uninfected tissues, or whether Pip uses a specific transporter, is an interesting open question. Furthermore, the relevance of amino acid conjugates in SAR signaling and the overall relevance of the amino acid homeostasis is only beginning to be understood. These are interesting topics to address in the near future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Yan Liu for critical reading of the manuscript and the Deutsche Forschungsgemeinschaft (DFG) for financial support.
Glossary
Abbreviations:
- SAR
systemic acquired resistance
- AA
amino acid
- Asp
aspartate
- Lys
lysine
- Val
valine
- Met
methionine
- Thr
threonine
- Ile
isoleucine
- Tyr
tyrosine
- Gln
glutamine
- Pro
proline
- N
nitrogen
- SA
salicylic acid
- Pip
pipecolic acid
References
- 1.Azevedo RA, Lancien M, Lea PJ. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids. 2006;30:143–62. doi: 10.1007/s00726-005-0245-2. [DOI] [PubMed] [Google Scholar]
- 2.Long X, Liu Q, Chan M, Wang Q, Sun SS. Metabolic engineering and profiling of rice with increased lysine. Plant Biotechnol J. 2013;11:490–501. doi: 10.1111/pbi.12037. [DOI] [PubMed] [Google Scholar]
- 3.Ufaz S, Galili G. Improving the content of essential amino acids in crop plants: goals and opportunities. Plant Physiol. 2008;147:954–61. doi: 10.1104/pp.108.118091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter H, Robinson DG, Heldt HW. Subcellular Volumes and Metabolite Concentrations in Barley Leaves. Planta. 1993;191:180–90. doi: 10.1007/BF00199748. [DOI] [Google Scholar]
- 5.Yang H, Postel S, Kemmerling B, Ludewig U. Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ. 2014 doi: 10.1111/pce.12244. [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Foster J, Chen J, Voll LM, Weber AP, Tegeder M. AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J. 2007;50:305–19. doi: 10.1111/j.1365-313X.2007.03045.x. [DOI] [PubMed] [Google Scholar]
- 7.Forsum O, Svennerstam H, Ganeteg U, Näsholm T. Capacities and constraints of amino acid utilization in Arabidopsis. New Phytol. 2008;179:1058–69. doi: 10.1111/j.1469-8137.2008.02546.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonner CA, Rodrigues AM, Miller JA, Jensen RA. Amino acids are general growth inhibitors of Nicotiana silvestris in tissue culture. Physiol Plant. 1992;84:319–28. doi: 10.1111/j.1399-3054.1992.tb04671.x. [DOI] [Google Scholar]
- 9.Song JT, Lu H, McDowell JM, Greenberg JT. A key role for ALD1 in activation of local and systemic defenses in Arabidopsis. Plant J. 2004;40:200–12. doi: 10.1111/j.1365-313X.2004.02200.x. [DOI] [PubMed] [Google Scholar]
- 10.Song JT, Lu H, Greenberg JT. Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and death2 and AGD2-LIKE DEFENSE RESPONSE PROTEIN1, encoding novel aminotransferases. Plant Cell. 2004;16:353–66. doi: 10.1105/tpc.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson AO, Singh BK, Leustek T, Gilvarg C. An LL-diaminopimelate aminotransferase defines a novel variant of the lysine biosynthesis pathway in plants. Plant Physiol. 2006;140:292–301. doi: 10.1104/pp.105.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeier J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013;36:2085–103. doi: 10.1111/pce.12122. [DOI] [PubMed] [Google Scholar]
- 13.Stuttmann J, Hubberten HM, Rietz S, Kaur J, Muskett P, Guerois R, Bednarek P, Hoefgen R, Parker JE. Perturbation of Arabidopsis amino acid metabolism causes incompatibility with the adapted biotrophic pathogen Hyaloperonospora arabidopsidis. Plant Cell. 2011;23:2788–803. doi: 10.1105/tpc.111.087684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Návarová H, Bernsdorff F, Döring AC, Zeier J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell. 2012;24:5123–41. doi: 10.1105/tpc.112.103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C, et al. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol. 2007;17:1116–22. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Vogel-Adghough D, Stahl E, Návarová H, Zeier J. Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal Behav. 2013;8:8. doi: 10.4161/psb.26366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Ji Y, Bhuiyan NH, Pilot G, Selvaraj G, Zou J, Wei Y. Amino acid homeostasis modulates salicylic acid-associated redox status and defense responses in Arabidopsis. Plant Cell. 2010;22:3845–63. doi: 10.1105/tpc.110.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirner A, Ladwig F, Stransky H, Okumoto S, Keinath M, Harms A, Frommer WB, Koch W. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell. 2006;18:1931–46. doi: 10.1105/tpc.106.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svennerstam H, Ganeteg U, Bellini C, Näsholm T. Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol. 2007;143:1853–60. doi: 10.1104/pp.106.092205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilot G, Stransky H, Bushey DF, Pratelli R, Ludewig U, Wingate VP, Frommer WB. Overexpression of GLUTAMINE DUMPER1 leads to hypersecretion of glutamine from Hydathodes of Arabidopsis leaves. Plant Cell. 2004;16:1827–40. doi: 10.1105/tpc.021642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirel B, Martin A, Tercé-Laforgue T, Gonzalez-Moro MB, Estavillo JM. Physiology of maize. I. A comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiol Plant. 2005;124:167–77. doi: 10.1111/j.1399-3054.2005.00510.x. [DOI] [Google Scholar]
- 22.Yang H, Krebs M, Stierhof YD, Ludewig U. Characterization of the putative amino acid transporter genes AtCAT2, 3 &4: The tonoplast localized AtCAT2 regulates soluble leaf amino acids. J Plant Physiol. 2014;171:594–601. doi: 10.1016/j.jplph.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Dordas C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agronomy for Sustainable Development. 2008;28:33–46. doi: 10.1051/agro:2007051. [DOI] [Google Scholar]
- 24.Veresoglou SD, Barto EK, Menexes G, Rillig MC. Fertilization affects severity of disease caused by fungal plant pathogens. Plant Pathol. 2013;62:961–9. doi: 10.1111/ppa.12014. [DOI] [Google Scholar]
- 25.Cuppels DA. Generation and Characterization of Tn5 Insertion Mutations in Pseudomonas syringae pv. tomato. Appl Environ Microbiol. 1986;51:323–7. doi: 10.1128/aem.51.2.323-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


