Abstract
Intermittent exposure during a period of 3 weeks of undamaged Arabidopsis plants to trace amounts of volatiles emitted by freshly damaged Arabidopsis plants resulted in an increase of subsequent artificial-damage-induced production of (Z)-3-hexen-1-yl acetate and (Z)-3-hexen-1-ol in the exposed Arabidopsis plants when compared with Arabidopsis plants exposed to undamaged Arabidopsis plant volatiles (control plants). We previously showed that (Z)-3-hexen-1-yl acetate attracts a parasitic wasp, Cotesia glomerata. Thus, the induced production of this volatile explained our previously reported finding that, when artificially damaged, the exposed plants were more attractive to C. glomerata than control plants.
Keywords: Arabidopsis thaliana, plant-plant signalling, green leaf volatiles, (Z)-3-hexen-1-yl acetate, (Z)-3-hexen-1-ol
Undamaged plants are known to become more defensive against biotic stresses (e.g., herbivory, pathogen infections) when exposed to volatiles from neighboring plants that are either infested, infected, or artificially damaged.1,2 One of the crucial questions that remains to be answered is how sensitive the receiver plant is to such volatiles.3 We recently showed that intermittent exposure of trace amounts (less than 140 pptV) of green leaf volatiles emitted by a freshly damaged Arabidopsis plant to undamaged cospecific neighboring plants for 3 wks induced physiological responses in the exposed plants.4 These results demonstrated that plants can respond to long-term repeated exposure to subcritical amounts of chemical signals. To further support our results, we conducted headspace analyses of undamaged and artificially damaged Arabidopsis Ler plants that were exposed to either artificially damaged conspecific plants or undamaged ones.
In this study, we damaged a plant by cutting one 5-mm slit in a leaf using ophthalmologic surgical scissors. Three-week-old emitter plants were used. We damaged them twice per week (at 10 AM on Monday and Friday) for 3 wks. Two different treatments of receiver plants denoted as follows were performed during these 3 wk: R-(damaged E) were receiver plants (R) exposed to volatiles from damaged emitter plants (E); R-(undamaged E) were receiver plants exposed to volatiles from undamaged emitter plants. After the 3-wk exposure period, 5 receiver plants from each treatment group were used for headspace analysis. We cut 4 leaves of each receiver plant (6 wks old) as the same way of the emitter plants. We used the damaged receivers for chemical analyses immediately after injury. Undamaged plants were used as control. We compared volatiles emitted from undamaged R-(undamaged E) vs undamaged R-(damaged E), and damaged R-(undamaged E) vs damaged R-(damaged E). Headspace sampling and GC-MS analysis of the headspace of the receiver plants were performed according to the methods previously reported.4 All experiments were repeated 3 or 4 times.
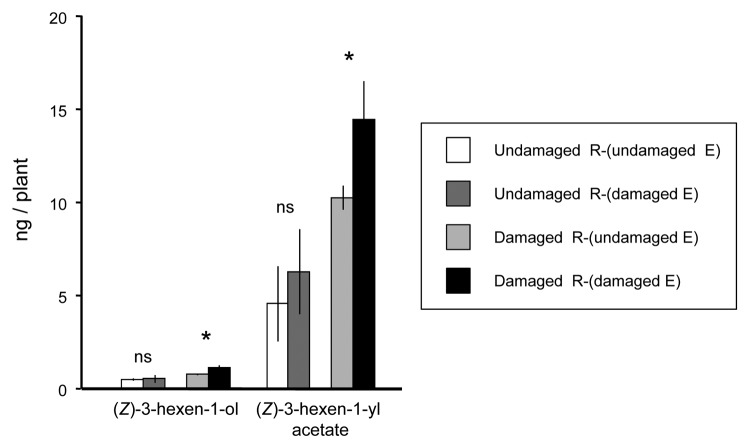
(Z)-3-Hexen-1-yl acetate and (E)-2-hexenal emitted from infested Arabidopsis plants attract Cotesia glomerata, a parasitoid of cabbage white butterfly (Pieris rapae) larvae, while (Z)-3-hexen-1-ol does not attract the wasp.5 The wasps show equal preference between undamaged R-(damaged E) and undamaged R-(undamaged E), but prefer damaged R-(damaged E) to damaged R-(undamaged E).4 Thus, we hypothesized that the amounts of (Z)-3-hexen-1-yl acetate and (E)-2-hexenal, were not significantly different between undamaged R-(damaged E) and undamaged R-(undamaged E), while the amounts in damaged R-(damaged E) were significantly higher than those in damaged R-(undamaged E). (Z)-3-Hexen-1-ol and (Z)-3-hexen-1-yl acetate were recorded in the headspace of odor source plants used in this study (Fig. 1). Aldehyde GLVs were under the detectable levels probably because the aldehydes were largely converted into corresponding alcohols and acetates as shown in our previous study.6 The amount of (Z)-3-hexen-1-yl acetate from undamaged R-(undamaged E) (3.72 ± 2.13 ng / plant) was not significantly different from that from undamaged R-(damaged E) (5.55 ± 2.42 ng / plant; P = 0.61, t test). Thus, the possibility that R-(damaged E) adsorbed GLVs of emitter plant origin on the surface of their leaves and re-emitted them was ruled out.7 By contrast, the amount of (Z)-3-hexen-1-yl acetate from damaged R-(damaged E) (14.29 ± 2.19 ng / plant) was significantly higher than that from damaged R-(undamaged E) (9.79 ± 0.69 ng / plant; P = 0.049, t test). The same trends were also found for (Z)-3-hexen-1-ol: a significant increase was detected between damaged R-(damaged E) (1.10 ± 0.17 ng / plant) over damaged R-(undamaged E) (0.74 ± 0.02 ng / plant; P = 0.038, t test) but not between undamaged R-(undamaged E) (0.49 ± 0.08 ng / plant) and undamaged R-(damaged E) (0.52 ± 0.21 ng / plant; P = 0.91, t test) (Fig. 1).
Figure 1. The amounts of green leaf volatiles emitted from Arabidopsis plants subjected to the following treatments: plants exposed to volatiles from undamaged emitter plants were not damaged [undamaged R-(undamaged E)], plants exposed to volatiles from damaged emitter plants were not damaged [undamaged R-(damaged E)], plants exposed to volatiles from undamaged emitter plants were damaged [damaged R-(undamaged E)], and plants exposed to volatiles from damaged emitter plants were damaged [damaged R-(damaged E)]. See text for details.
This study showed that the artificial-damage-induced production of (Z)-3-hexen-1-ol and (Z)-3-hexen-1-yl acetate was enhanced by 3-wk periodic exposure of trace amounts (less than 140 pptV) of GLVs emitted by a freshly damaged Arabidopsis plant. Such increased production in response to traces of GLVs is considered to be priming.8-10 The phytooxylipin pathway is involved in the production of GLVs in plants.11 This pathway is probably primed by the exposure. Hirao et al. (2012) reported that, when Arabidopsis (Col-0) was treated with GLVs, the anthocyanin content was significantly increased by a subsequent treatment of methyl jasmonate.12 Their data suggest that GLVs enhance the response to MeJA in Arabidopsis. As shown in our previous report, 1-wk exposure did not prime the exposed plants to be more attractive to C. glomerata while 3-wk exposure did.4 Comprehensive molecular analyses of Arabidopsis plants with no exposure, with 1-wk exposure, and with 3-wk exposure would be needed to clarify the mechanisms involved in this highly sensitive priming.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported in part by JSPS Grant-in-Aid for Scientific Research (S) (No. 19101009), by JSPS Core-to-Core Project, by Global Center of Excellence Program A06 “Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem” of Kyoto University, Japan, by the Grants to Excellent Graduate Schools program of MEXT, Japan, by JSPS Grant-in-Aid for Young Scientists (B) (No. 23770020) and by JSPS Grant-in-Aid for Scientific Research (B) (No. 23405007)
References
- 1.Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol. 2010;25:137–44. doi: 10.1016/j.tree.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 2009;50:911–23. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- 3.Girón-Calva PS, Molina-Torres J, Heil M. Volatile dose and exposure time impact perception in neighboring plants. J Chem Ecol. 2012;38:226–8. doi: 10.1007/s10886-012-0072-3. [DOI] [PubMed] [Google Scholar]
- 4.Shiojiri K, Ozawa R, Matsui K, Sabelis MW, Takabayashi J. Intermittent exposure to traces of green leaf volatiles triggers a plant response. Sci Rep. 2012;2:378. doi: 10.1038/srep00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiojiri K, Ozawa R, Matsui K, Kishimoto K, Kugimiya S, Takabayashi J. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J Chem Ecol. 2006;32:969–79. doi: 10.1007/s10886-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 6.Matsui K, Sugimoto K, Mano J, Ozawa R, Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS One. 2012;7:e36433. doi: 10.1371/journal.pone.0036433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choh Y, Shimoda T, Ozawa R, Dicke M, Takabayashi J. Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: active or passive process? J Chem Ecol. 2004;30:1305–17. doi: 10.1023/B:JOEC.0000037741.13402.19. [DOI] [PubMed] [Google Scholar]
- 8.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A. 2004;101:1781–5. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008;180:722–34. doi: 10.1111/j.1469-8137.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Felton GW. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013;20:273–85. doi: 10.1111/j.1744-7917.2012.01584.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol. 2006;9:274–80. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Hirao T, Okazawa A, Harada K, Kobayashi A, Muranaka T, Hirata K. Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. J Biosci Bioeng. 2012;114:540–5. doi: 10.1016/j.jbiosc.2012.06.010. [DOI] [PubMed] [Google Scholar]