Abstract
The plant cuticle, a dynamic interface between plants and their environment, is formed by the secretion of hydrophobic lipids and waxes into the outer wall of aerial epidermal cells. Cuticle formation is such a ubiquitous feature of epidermal cells, and is of such fundamental importance for plant survival, that identifying and understanding specific developmental roles for this structure has been a major challenge for plant scientists. In recent work, we have tried to understand the functional relationships between a signaling feedback loop required for epidermal cell specification in developing plant embryos, and a seed specific signaling cascade, involving components localized both in the embryo and in the embryo surrounding endosperm, and necessary for embryo cuticle function. Analysis of the strongly synergistic genetic relationships between these 2 independent pathways, combined with mathematical simulations of the behavior of the signaling feedback loop, have allowed us to propose an important, and hitherto unsuspected, role for the embryonic cuticle as an apoplastic diffusion barrier, necessary for preventing the excessive diffusion of developmentally important signaling molecules away from developing embryo into surrounding tissues.
Keywords: endosperm, embryo, cuticle, signaling, epidermis, apoplast, diffusion barrier
The plant epidermis is a highly specialized cell layer which covers all aerial plant surfaces, and which is characterized by a number of fundamental traits of which one of the most important is the secretion of lipids and waxes into its outer cell wall (reviewed in 1). The continuous hydrophobic layer thus formed, called the cuticle, has been proposed to fulfil a multitude of different functions, including protecting the plant from uncontrolled water loss2 and from damage by both biotic and abiotic factors.3,4 In addition the cuticle plays an important developmental function by preventing adhesion and fusion of developing organs (reviewed in 1,5). Because the cuticle is a ubiquitous feature of aerial epidermal surfaces, and is essential for plant survival, it has been very difficult to functionally separate cuticle production and function from epidermal fate specification in genetic studies.
In this context, the GASSHO1 (GSO1) and GSO2 receptor kinases are of particular interest, as recent studies have shown that they affect cuticle function specifically during embryogenesis.6,7 Double gso1 gso2 mutants produce seedlings which are highly permeable to hydrophilic dyes, strongly desiccation sensitive, and which show cotyledon fusion at rates of between 50 and 80%. These mutants can be rescued to produce fertile plants, by culturing seedlings under highly humid conditions. Consistent with the fact that GSO1 and GSO2 are only necessary for cuticle formation in the developing embryo, recent work has shown that they act in the same signaling pathway as 2 endosperm specific proteins, the transcription factor ZHOUPI (ZOU)8 and the subtilisin serine protease ABNORMAL LEAF SHAPE1 (ALE1),9 leading to the hypothesis that formation of a functional embryonic cuticle necessitates signals derived from the endosperm in the seed context.7
In Arabidopsis thaliana, epidermal fate specification during early embryogenesis is controlled by the largely redundant activity of the protoderm specific transcription factors ARABIDOPSIS THALIANA MERISTEM L1 (ATML1) and (PROTODERMAL FACTOR 2) PDF2,10 and it has generally been assumed that these proteins, as has been shown for other members of the HDZIP IV transcription factor family, regulate cuticle biosynthetic processes as well as other epidermal traits.10-13 In a recent study14 we have shown that these proteins act together with the receptor kinase ARABIDOPSIS CRINKLY4 (ACR4)15–17 in a feedback loop which is necessary for the maintenance of epidermal identity during post embryonic growth. In this work14 we show that ACR4, probably acting together with other receptors17 is necessary for maintaining the expression levels of ATML1 and PDF2. During post germinative growth, these transcription factors in turn feed-back directly, and negatively, on their own expression and that of ACR4, a situation which mathematical modeling suggests would provide robust epidermal cell fate maintenance in the face of fluctuations in signaling input. Interestingly however, results both from our work, and from previous studies,10 suggest that ATML1 and PDF2, potentially together with other members of the HDZIP IV transcription factor family, provide a net positive rather than negative regulation of their own expression and that of ACR4 during early embryogenesis. When this scenario was modeled, it gave a bi-stable situation in which a decreased signaling intensity can lead to irreversible loss of epidermal cell fate.14 Such a bi-stable situation in globular embryos is consistent with the observed rapid loss of epidermal identity markers, including ATML1 transcripts, in the central cells of the dermatogen stage embryo after periclinal divisions in the octant embryo,18 reviewed in 1.
Based on these results, 2 different signaling pathways directly impact epidermal development during embryogenesis: the ZOU/GSO1/GSO2/ALE1 pathway, and the ACR4/ATML1/PDF2 feedback loop (Fig. 1). Several studies8,17 have shown that acr4 mutants have strong synergistic interactions (leading to loss of epidermal fate and embryo lethality) with zou, ale1 and gso1 gso2 mutants, suggesting that the 2 signaling pathways are distinct. We therefore tested the hypothesis that the 2 pathways converge to regulate the expression of ATML1 and PDF2. Surprisingly, contrary to what had previously been proposed in the literature, we showed that the ZOU/GSO1/GSO2/ALE1 pathway in fact controls embryonic cuticle properties independently of the activities of ATML1 and PDF2 .
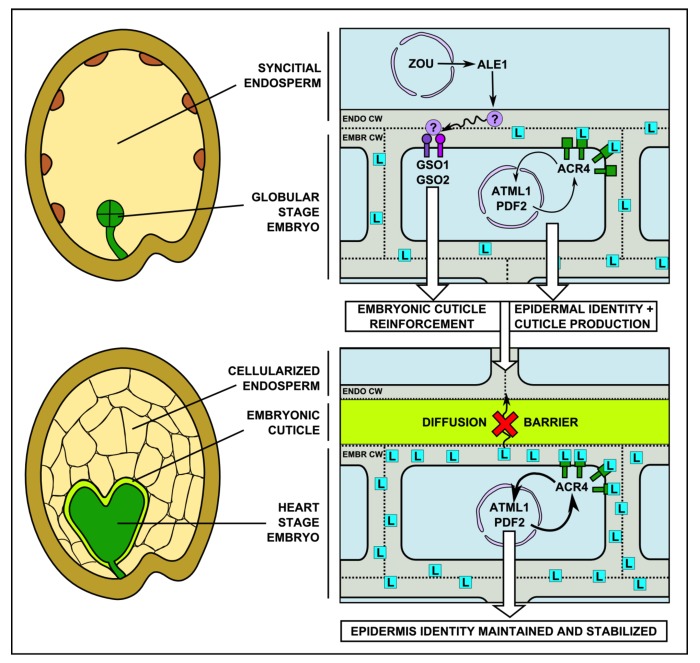
Figure 1. Illustration of a potential mechanism for the observed role for an apoplastic diffusion barrier (the embryonic cuticle) in robust epidermal cell fate establishment during Arabidopsis embryogenesis. A signaling feedback loop within the embryo, implicating the receptor kinase ACR4, the hypothetical ACR4 ligand (L) and the transcription factors ATML1 and PDF2, is established during very early embryogenesis and is necessary for the maintenance of epidermal fate in the outer cell layer of the embryo. This maintenance depends upon levels of Ligand L which, in the absence of an apoplastic barrier, is free to move away from the embryo in the apoplast. At around the late globular stage in development, as the endosperm starts to cellularize, a second signaling pathway involving the receptor-like kinases GSO1 and GSO2 and the endosperm-specific subtilisin protease ALE1 (produced under the control of the bHLH transcription factor ZOU), mediates a reinforcement of the embryonic cuticle, effectively sealing off the embryo to permit maintenance of high levels of ligand L within the embryo and thus stabilize epidermal cell fate. Solid arrows represent positive regulation which can be either direct or indirect. ENDO CW = Endosperm Cell Wall, EMBR CW = Embryo Cell Wall.
Although novel, this finding leaves the strong synergistic interaction observed between the GSO1/GSO2/ALE1 pathway, and the ACR4/ATML1/PDF2 feedback loop unexplained. Although it has been shown that most “between pathway” mutant combinations (for example acr4 ale1, gso1 gso2 acr4 or gso1 gso2 pdf2) are embryo lethal,14,17 a possible clue to the question resided in the double pdf2 ale1 mutants. Single mutants in either gene produce normal looking plants and seedlings with extremely subtle defects in cuticle permeability. In contrast, about 20% of double mutant embryos arrest early in embryo development showing serious epidermal disorganization, while the rest produced abnormal seedlings which were highly permeable to hydrophilic dyes, and showed cotyledon stunting and notching. Despite these defects, if transferred to soil under humid conditions, these seedlings gave rise to normal looking plants. This mutant combination thus reveals a developmental threshold which occurs at around the globular stage in embryogenesis, and at which epidermal identity appears to be either stabilized to allow subsequent development, or irretrievably lost. Interpreted in the light of our mathematical models, it therefore appears that even weak defects in cuticle reinforcement at this specific stage are enough to flip the bistable switch to the “no epidermis” state in the outer cell layer of young embryos in which the ACR4/ATML1/PDF2 feedback loop establishing epidermal identity is compromised.
In order to understand why embryonic epidermis formation is particularly sensitive to cuticular perturbations, it is necessary to consider its developmental context. We have previously proposed that the specific need for cuticle reinforcement in zygotic embryos could be engendered by the fact that they develop surrounded by the developing endosperm, which is not thought to be cuticularized7,19 However, the developmental role of this reinforcement early in embryogenesis has remained unclear. Our study may shed light on this issue, since the activity of the ACR4/ATML1/PDF2 feedback loop in our mathematical models is assumed to involve an ACR4-binding, apoplastically located, ligand which to date remains unidentified. Because this feedback loop is active both during and after embryogenesis, it is reasonable to assume that the ACR4 ligand must be produced within the embryo and accumulate within the embryonic apoplast. We propose then, that the stochastic loss of epidermal identity in a background in which both the ACR4/ATML1/PDF2 feedback loop and cuticle formation are compromised, could reflect a role for the embryonic cuticle in preventing the diffusion of apoplastic signaling molecules, including the ACR4 ligand, out of the developing embryo, and into apoplast of the surrounding endosperm. Thus, by cutting apoplastic bridges between the embryo proper and the endosperm, the cuticle could play a critical role in concentrating the ACR4 ligand within the embryonic tissues, and maintaining ACR4 signaling activity at high enough levels to permit the maintenance of epidermal cell fate specification in developing embryos, even in backgrounds where the signaling loop is compromised (Fig. 1)
A logical prediction from this model is that loss of embryonic cuticle biosynthesis alone should critically compromise the maintenance of epidermal identity. Cuticle defects in ale1–4 or gso1 gso2 double mutants do not appear to significantly destabilize an uncompromised ACR4/ATML1/PDF2 feedback loop. Interestingly, however, loss of function alleles in the Acetyl-Coenzyme A Carboxylase encoding ACC1 gene, which has recently been shown to play a non-redundant role predominantly in the biosynthesis of cuticular waxes in Arabidopsis,20 show embryo developmental defects which are startlingly similar to defects reported for double mutants between certain alleles of ATML1 and PDF2,10 including lack of cotyledon initiation and disruption of cell organization in apical regions of the embryo.21 However, a loss of epidermal identity in these mutants remains to be proven.
In summary, although further studies will be required to confirm our hypothesis, including quantitative expression analysis of epidermal markers specifically during the early stages of embryogenesis in a variety of backgrounds, our results have permitted us to put forward one of the first propositions for a concrete molecular mechanism underlying the developmental role of an apoplastic diffusion barrier in plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Moussu S is supported by a grant from the Rhône Alpes Region (France). San-Bento R was supported by a Marie Curie ITN network (SIREN). This work also forms part of an ANR (France) Chaire D’Excellence awarded to Ingram G and supporting Creff A and Galletti R.
References
- 1.Javelle M, Vernoud V, Rogowsky PM, Ingram GC. Epidermis: the formation and functions of a fundamental plant tissue. New Phytol. 2011;189:17–39. doi: 10.1111/j.1469-8137.2010.03514.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin SM, Jenks MA, eds. Plant Cuticle function as a barrier to water loss. In: Plant Abiotic Stress. Jenks MA and Hasegawa PM. Blackwell Publishing Inc: Oxford, UK: 2005 [Google Scholar]
- 3.Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JM, Métraux JP, Nawrath C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007;26:2158–68. doi: 10.1038/sj.emboj.7601658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZY, Xiong L, Li W, Zhu JK, Zhu J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell. 2011;23:1971–84. doi: 10.1105/tpc.110.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuels L, Kunst L, Jetter R. Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol. 2008;59:683–707. doi: 10.1146/annurev.arplant.59.103006.093219. [DOI] [PubMed] [Google Scholar]
- 6.Tsuwamoto R, Fukuoka H, Takahata Y. GASSHO1 and GASSHO2 encoding a putative leucine-rich repeat transmembrane-type receptor kinase are essential for the normal development of the epidermal surface in Arabidopsis embryos. Plant J. 2008;54:30–42. doi: 10.1111/j.1365-313X.2007.03395.x. [DOI] [PubMed] [Google Scholar]
- 7.Xing Q, Creff A, Waters A, Tanaka H, Goodrich J, Ingram GC. ZHOUPI controls embryonic cuticle formation via a signalling pathway involving the subtilisin protease ABNORMAL LEAF-SHAPE1 and the receptor kinases GASSHO1 and GASSHO2. Development. 2013;140:770–9. doi: 10.1242/dev.088898. [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Johnston N, Talideh E, Mitchell S, Jeffree C, Goodrich J, Ingram G. The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development. 2008;135:3501–9. doi: 10.1242/dev.026708. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y. A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development. 2001;128:4681–9. doi: 10.1242/dev.128.23.4681. [DOI] [PubMed] [Google Scholar]
- 10.Abe M, Katsumata H, Komeda Y, Takahashi T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;130:635–43. doi: 10.1242/dev.00292. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Katsumata H, Abe M, Yabe N, Komeda Y, Yamamoto KT, Takahashi T. Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis. Plant Physiol. 2006;141:1363–75. doi: 10.1104/pp.106.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew W, Hrmova M, Lopato S. Role of Homeodomain Leucine Zipper (HD-Zip) IV Transcription Factors in Plant Development and Plant Protection from Deleterious Environmental Factors. Int J Mol Sci. 2013;14:8122–47. doi: 10.3390/ijms14048122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takada S, Takada N, Yoshida A. ATML1 promotes epidermal cell differentiation in Arabidopsis shoots. Development. 2013;140:1919–23. doi: 10.1242/dev.094417. [DOI] [PubMed] [Google Scholar]
- 14.San-Bento R, Farcot E, Galletti R, Creff A, Ingram G. Epidermal identity is maintained by cell-cell communication via a universally active feedback loop in Arabidopsis thaliana. Plant J. 2013 doi: 10.1111/tpj.12360. [DOI] [PubMed] [Google Scholar]
- 15.Gifford ML, Dean S, Ingram GC. The Arabidopsis ACR4 gene plays a role in cell layer organisation during ovule integument and sepal margin development. Development. 2003;130:4249–58. doi: 10.1242/dev.00634. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Tanaka H, Watanabe D, Machida C, Machida Y. The ACR4 receptor-like kinase is required for surface formation of epidermis-related tissues in Arabidopsis thaliana. Plant J. 2004;39:298–308. doi: 10.1111/j.1365-313X.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Watanabe M, Sasabe M, Hiroe T, Tanaka T, Tsukaya H, Ikezaki M, Machida C, Machida Y. Novel receptor-like kinase ALE2 controls shoot development by specifying epidermis in Arabidopsis. Development. 2007;134:1643–52. doi: 10.1242/dev.003533. [DOI] [PubMed] [Google Scholar]
- 18.Lu P, Porat R, Nadeau JA, O’Neill SD. Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell. 1996;8:2155–68. doi: 10.1105/tpc.8.12.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters A, Creff A, Goodrich J, Ingram G. “What we’ve got here is failure to communicate”: zou mutants and endosperm cell death in seed development. Plant Signal Behav. 2013;8:e24368. doi: 10.4161/psb.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lü S, Zhao H, Parsons EP, Xu C, Kosma DK, Xu X, Chao D, Lohrey G, Bangarusamy DK, Wang G, et al. The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis. Plant Physiol. 2011;157:1079–92. doi: 10.1104/pp.111.185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C. Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J. 2003;33:75–86. doi: 10.1046/j.1365-313X.2003.016010.x. [DOI] [PubMed] [Google Scholar]