Abstract
Under stress conditions that bring about excessive absorption of light energy in the chloroplasts, the formation of singlet oxygen (1O2) can be strongly enhanced, triggering programmed cell death. However, the 1O2 signaling pathway can also lead to acclimation to photooxidative stress, when 1O2 is produced in relatively low amounts. This acclimatory response is associated with a strong downregulation of the jasmonate biosynthesis pathway and the maintenance of low jasmonate levels, even under high light stress conditions that normally induce jasmonate synthesis. These findings suggest a central role for this phytohormone in the orientation of the 1O2 signaling pathway toward cell death or acclimation. This conclusion is confirmed here in an Arabidopsis double mutant obtained by crossing the 1O2-overproducing mutant ch1 and the jasmonate-deficient mutant dde2. This double mutant was found to be constitutively resistant to 1O2 stress and to display a strongly stimulated growth rate compared with the single ch1 mutant. However, the involvement of other phytohormones, such as ethylene, cannot be excluded.
Keywords: 1O2 acclimation, high light, ch1 mutant, jasmonate, phytohormone signaling
The sedentary nature of terrestrial plants obliges them to compensate their reduced mobility by a high plasticity in order to rapidly and efficiently adjust their metabolism and maintain their growth performance in a wide range of environmental conditions. Acclimation of plants to environmental challenges involves a variety of genetically controlled physiological and metabolic changes that allow the organism to cope with the new environment. Among the main environmental factors that can affect agricultural crop productivity, sunlight appears to be one of the most crucial and intensively studied, revealing a large variety of responses occurring in different time ranges from rapid biophysical adjustments to slower changes in gene expression and reorganization of the photosynthetic apparatus.1-4 A major problem occurs when light energy is absorbed by plants in excess of what can be used by the photosynthetic processes, e.g., when the photosynthetic activity is inhibited by stress. Excess excitation/electron can leak to O2, leading to the formation of reactive O2 species (ROS).5 In particular, under high light stress, conversion of singlet excited chlorophylls (1Chl*) to the triplet excited state (3Chl*) by intersystem crossing is favored and, because of its relatively long lifetime, 3Chl* can react with O2 to form singlet oxygen (1O2) by triplet annihilation.6 This ROS is toxic, causing damage to macromolecules,7,8 and is believed to play a major destructive role during the execution of ROS-induced cell death in leaf tissues.9,10
A recent study performed on the chlorina1 mutant (ch1) of Arabidopsis thaliana has shown that vascular plants have the capacity to acclimate to 1O2.11 A similar phenomenon was previously reported in the microalga Chlamydomonas.12 The Arabidopsis ch1 mutant is characterized by a lack of chlorophyll b, leading to a complete deficiency in PSII chlorophyll-protein antenna complexes (LHCII) and to PSII units restricted to their reaction centers.13 This mutant is highly photosensitive,9,13 due to a selective increase in the release of 1O2 by the “naked” PSII centers, as measured by different techniques including 1O2-specific fluorescent probes, EPR spectroscopy of spin traps, HPLC analyses of 1O2 lipidic markers, and gene expression profiles.11,14 As illustrated in Figure 1, increasing photon flux density (PFD) from 200 μmol photons m–2 s–1 (growth conditions) to 1000 μmol m–2 s–1 caused leaf bleaching (Fig. 1A), lipid peroxidation (Fig. 1B and C) and loss of PSII activity (Fig. 1D) in ch1 plants. These effects were not observed in the wild type under similar conditions (not shown).
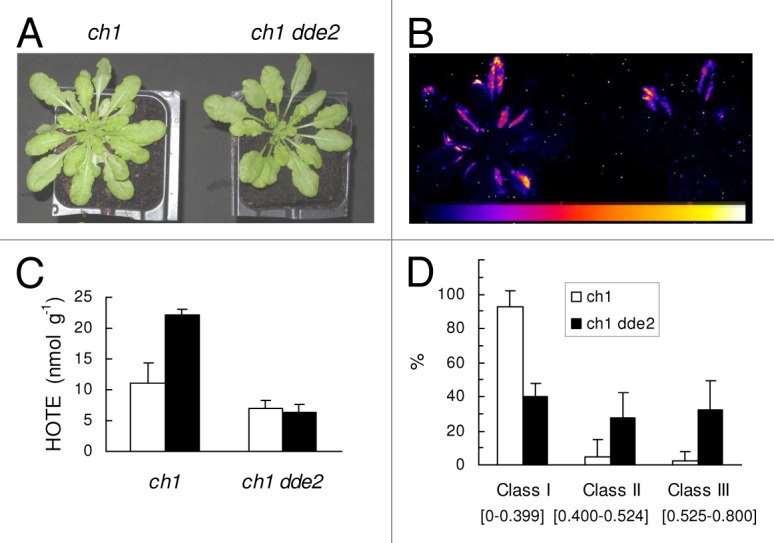
Figure 1. Photooxidative stress in the ch1 single mutant and the ch1 dde2 double mutant exposed for 27 h to high light (1000 µmol photons m–2 s–1) and low temperature (10°C). (A) Plants after the light/cold treatment. (B) Autoluminescence imaging of lipid peroxidation after the light/cold treatment. Color scale indicates signal intensity from 0 (black) to saturation (white); (C) ROS-induced lipid peroxidation (HOTE levels) before (white bars) and after the treatment (black bars). Data are mean values of 3 separate experiments + SD (D) PSII photochemical efficiency (Fv/Fm) after the treatment. The plot represents the % of leaves in 3 classes of Fv/Fm values.
Pre-exposure to a moderately elevated PFD (e.g., 400 μmol m–2 s–1) for a few days, which induced a low production of 1O2, was shown to markedly enhance the tolerance of ch1 plants to photooxidative stress.11 This increased phototolerance was not associated with a decreased rate of 1O2 formation and was accompanied by the induction of many genes, particularly in the categories related to metabolism, oxidative stress responses and regulation of transcription.11 Thus, moderately elevated PFDs oriented the 1O2 signaling pathway toward acclimation and prevented 1O2-induced programmed cell death (PCD). A striking phenomenon associated with this acclimation process was a downregulation of the jasmonate (JA) biosynthesis pathway, with most of the genes involved in this pathway being strongly repressed.11 This was accompanied by a very low concentration of JA in the acclimated leaves, which contrasted with the marked stimulation of JA synthesis in non-acclimated leaves exposed to high light stress. A block of JA synthesis during acclimation to 1O2 is consistent with previous data obtained on the Arabidopsis flu mutant15 that have shown that JA accumulates and promotes cell death under 1O2 stress.16-18 This is also consistent with previous observations showing that leaf tissue or suspension-cultured cells undergo PCD upon exogenous addition of methyl JA.19 Actually, the involvement of JA in the regulation of PCD in plants has been reported in various plant species in response to different stressors.20,21 In the flu mutant, the JA precursor, 12-oxo phytodienoic acid (OPDA), was found to antagonize the JA activity.18 However, OPDA did not increase in ch1 leaves under light stress or under acclimatory conditions,11 suggesting that modulation of the sensitivity to 1O2 in ch1 was not attributable to changes in OPDA levels.
To further study the role of JA biosynthesis in the acclimation of the ch1 mutant to 1O2, we crossed this mutant with the dde2 Arabidopsis mutant (delayed-dehiscence 2) to create a 1O2-producing mutant deficient in JA. The dde2 mutant is unable to metabolize 13-hydroperoxy linolenic acid (HOTE) and to synthesize JA, due to the breakdown of the gene encoding allene oxide synthase.22 The ch1 dde2 double mutant was noticeably more resistant to high-light stress than the ch1 single mutant (Fig. 1). Indeed, we observed a marked attenuation of leaf bleaching after a 27 h-treatment under a high light regime (Fig. 1A) and a lower level of lipid peroxidation in the ch1 dde2 mutant compared with the single mutant ch1, as imaged by plant autoluminescence (Fig. 1B) or indicated by the HOTE levels (Fig. 1C). Moreover, after high light stress, the chlorophyll fluorescence parameter Fv/Fm was < 0.4 in most ch1 leaves while the majority of ch1 dde2 leaves had a Fv/Fm ratio above 0.4 (Fig. 1D). This indicates less inhibition of PSII photochemical efficiency in the double mutant. Clearly, inhibition of JA synthesis is beneficial to plants exposed to 1O2 stress, precluding the development of PCD. Accordingly, applications of exogenous JA to acclimated ch1 plants cancelled the increased tolerance to 1O2 stress.11 The initial production of 1O2 linked to the ch1 mutation should be equivalent in both mutants, and therefore the maintenance of low levels of lipid peroxidation in the absence of JA can be explained by a block of PCD and the activation of defense and detoxification mechanisms, limiting oxidative damage.
Strikingly, when grown under standard conditions of light, the double mutant ch1 dde2 was also characterized by a much higher growth rate compared with the single mutant ch1 (Fig. 2), suggesting a negative effect of JA on growth, even in the absence of high light stress. The effect was spectacular with almost a doubling of the size of the JA-deficient ch1 plants compared with the JA-producing plants. Analyses of 1O2-specific oxidation products of linolenic acid and β-carotene have shown that 1O2 is produced in plant leaves even in low light, resulting in a chronic oxidation of lipids and carotenoids, and this phenomenon is exacerbated in the ch1 mutant due to the enhanced formation of 1O2.9,23 JA is an essential plant hormone that regulates defense responses and development processes.21,24 Moreover, it is known that JA and light signaling are interconnected for the regulation of growth and development, with their interaction determining the metabolic balance between growth and defense.25 Our observation that JA synthesis regulates negatively plant growth under conditions of chronic 1O2 formation supports those previous conclusions.
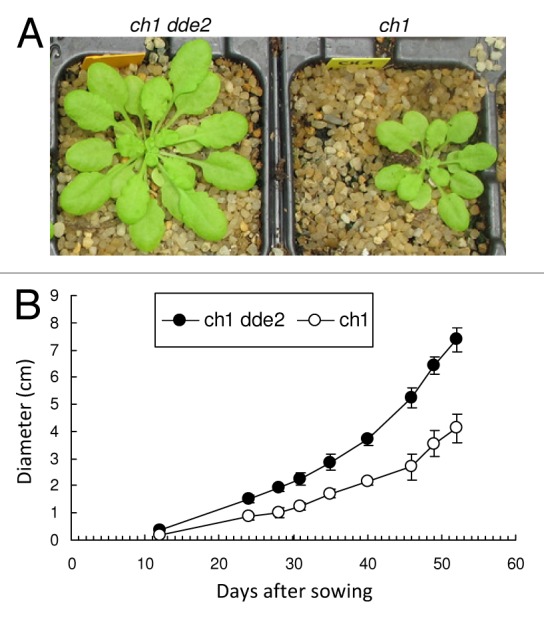
Figure 2. Growth characteristics of the ch1 single mutant and the ch1 dde2 double mutant. (A) Plants aged 49 d. (B) Growth curve (rosette diameter) of plants. Plants were grown under standard conditions: 200 µmol photons m–2 s–1; 8 h photoperiod; 20/18°C, day/night temperature. Data are mean values of 7 and 14 measurements (ch1 and ch1 dde2, respectively) +SD.
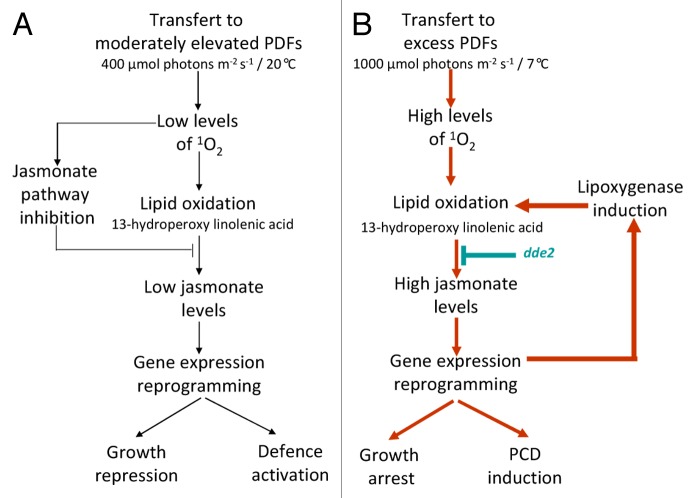
The findings described above suggest a central role for jasmonate in the 1O2 signaling networks and in the orientation of plant metabolism toward PCD or acclimation to 1O2. JA appears to be one of the decision makers in this choice, with the increased synthesis of this hormone favoring the “growth arrest/cell death” options (Fig. 3). The jasmonate pathway has been predicted in Chlamydomonas,26,27 and therefore it would be interesting to check the link between jasmonate and 1O2 acclimation in this species. However, we cannot exclude the involvement of other phytohormones in this process. Although the concentrations of both OPDA and salicylic acid remain stable in ch1 leaves under various light conditions,11 microarray-based transcriptomic analyses revealed the induction of genes related to ethylene such ACS2 (1-aminocyclopropane-1-carboxylic acid synthase) and ERF (ethylene response factor) during 1O2 stress. No such induction was observed during acclimation.11 Induction of ethylene synthesis by high light is documented in photosynthetic organisms.28 Therefore, one cannot exclude the involvement of both JA and ethylene in the regulation of 1O2-induced cell death. This interaction was actually reported in tomato for cell death induced by a toxin.29
Figure 3. Schematic representation of 1O2 signaling in the ch1 mutant under (A) acclimatory or (B) photooxidative stress conditions.
Oxylipin hormones are known to function as regulators of defense against herbivores and pathogens.30,31 Therefore, as recently pointed out by Demmig-Adams et al.,32 the inhibition of jasmonate synthesis in plants acclimated to photooxidative stress may have negative effects on the tolerance to biotic stresses. Accordingly, Arabidopsis mutants deficient in lipid-soluble antioxidants were found to accumulate oxylipin hormones and to have superior defenses against insect herbivores.33 Thus, there could be a compatibility issue between tolerance toward abiotic stresses and defense against biotic stresses. This important point deserves to be addressed in future research.
Acknowledgments
We would like to thank the Agence Nationale de la Recherche within the project Photox (Program 'blanc') and the Commissariat à l’Energie Atomique et aux Energies Alternatives (Cadarache) for the funding that made this work possible. Many thanks also to the GRAP platform for growing the plants.
Glossary
Abbreviations:
- 1O2
singlet oxygen
- Chl
chlorophyll
- PSII
photosystem II
- ROS
reactive oxygen species
- PFD
photon flux density
- PCD
programmed cell death
- JA
jasmonic acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Walters RG. Towards an understanding of photosynthetic acclimation. J Exp Bot. 2005;56:435–47. doi: 10.1093/jxb/eri060. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–70. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 3.Karpiński S, Szechyńska-Hebda M, Wituszyńska W, Burdiak P. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ. 2013;36:736–44. doi: 10.1111/pce.12018. [DOI] [PubMed] [Google Scholar]
- 4.Kouřil R, Wientjes E, Bultema JB, Croce R, Boekema EJ. High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta. 2013;1827:411–9. doi: 10.1016/j.bbabio.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–60. doi: 10.1146/annurev.arplant.58.032806.103844. [DOI] [PubMed] [Google Scholar]
- 6.Fischer BB, Hideg É, Krieger-Liszkay A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid Redox Signal. 2013;18:2145–62. doi: 10.1089/ars.2012.5124. [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305:761–70. doi: 10.1016/S0006-291X(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 8.Sies H, Menck CF. Singlet oxygen induced DNA damage. Mutat Res. 1992;275:367–75. doi: 10.1016/0921-8734(92)90039-R. [DOI] [PubMed] [Google Scholar]
- 9.Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008;148:960–8. doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triantaphylidès C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–28. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Ramel F, Ksas B, Akkari E, Mialoundama AS, Monnet F, Krieger-Liszkay A, Ravanat JL, Mueller MJ, Bouvier F, Havaux M. Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell. 2013;25:1445–62. doi: 10.1105/tpc.113.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledford HK, Chin BL, Niyogi KK. Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot Cell. 2007;6:919–30. doi: 10.1128/EC.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havaux M, Dall’Osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007;145:1506–20. doi: 10.1104/pp.107.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dall’Osto L, Cazzaniga S, Havaux M, Bassi R. Enhanced photoprotection by protein-bound vs free xanthophyll pools: a comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol Plant. 2010;3:576–93. doi: 10.1093/mp/ssp117. [DOI] [PubMed] [Google Scholar]
- 15.Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2001;98:12826–31. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsenbein C, Przybyla D, Danon A, Landgraf F, Göbel C, Imboden A, Feussner I, Apel K. The role of EDS1 (enhanced disease susceptibility) during singlet oxygen-mediated stress responses of Arabidopsis. Plant J. 2006;47:445–56. doi: 10.1111/j.1365-313X.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 17.Przybyla D, Göbel C, Imboden A, Hamberg M, Feussner I, Apel K. Enzymatic, but not non-enzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 2008;54:236–48. doi: 10.1111/j.1365-313X.2008.03409.x. [DOI] [PubMed] [Google Scholar]
- 18.Danon A, Miersch O, Felix G, Camp RG, Apel K. Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J. 2005;41:68–80. doi: 10.1111/j.1365-313X.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 19.Repka V, Carna M, Pavlovkin J. Methyl jasmonate-induced cell death in grapevine requires both lipoxygenase activity and functional octadecanoid biosynthetic pathway. Biologia. 2013;68:896–903. doi: 10.2478/s11756-013-0220-4. [DOI] [Google Scholar]
- 20.Reinbothe C, Springer A, Samol I, Reinbothe S. Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 2009;276:4666–81. doi: 10.1111/j.1742-4658.2009.07193.x. [DOI] [PubMed] [Google Scholar]
- 21.Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111:1021–58. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Malek B, van der Graaff E, Schneitz K, Keller B. The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta. 2002;216:187–92. doi: 10.1007/s00425-002-0906-2. [DOI] [PubMed] [Google Scholar]
- 23.Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat JL, Havaux M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012;158:1267–78. doi: 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–11. doi: 10.1016/S1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- 25.Kazan K, Manners JM. The interplay between light and jasmonate signalling during defence and development. J Exp Bot. 2011;62:4087–100. doi: 10.1093/jxb/err142. [DOI] [PubMed] [Google Scholar]
- 26.Lee DY. Biochemical and physiological network responses of Chlamydomonas reinhardtii on abiotic stress, PhD thesis, University of California, Davis, 2009; 124 pages; 3362494. [Google Scholar]
- 27.Li W, Liu B, Yu L, Feng D, Wang H, Wang J. Phylogenetic analysis, structural evolution and functional divergence of the 12-oxo-phytodienoate acid reductase gene family in plants. BMC Evol Biol. 2009;9:90. doi: 10.1186/1471-2148-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plettner I, Steinke M, Malin G. Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ. 2005;28:1136–45. doi: 10.1111/j.1365-3040.2005.01351.x. [DOI] [Google Scholar]
- 29.Zhang L, Jia C, Liu L, Zhang Z, Li C, Wang Q. The involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin-induced tomato cell death. J Exp Bot. 2011;62:5405–18. doi: 10.1093/jxb/err217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaler JS, Owen B, Higgins VJ. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol. 2004;135:530–8. doi: 10.1104/pp.104.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 32.Demmig-Adams B, Cohu CM, Amiard V, Zadelhoff G, Veldink GA, Muller O, Adams WW., 3rd Emerging trade-offs - impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytol. 2013;197:720–9. doi: 10.1111/nph.12100. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel M, Külheim C, Jänkänpää HJ, Skogström O, Dall’Osto L, Ågren J, Bassi R, Moritz T, Moen J, Jansson S. Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol. 2009;9:12. doi: 10.1186/1471-2229-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]