Abstract
Ginkgo (Ginkgo biloba L.) has not changed over 121 million years. There may be unknown special strategy for the survival. Gingko litter inhibited the growth of weed species ryegrass (Lolium multiflorum L.). The inhibition was greater with the litter of the close position than that of the far position from the gingko tree. A phytotoxic substance, 2-hydroxy-6-(10-hydroxypentadec-11-enyl)benzoic acid (HHPEBA) was isolated in the litter. HHPEBA concentration was greater in the litter of the close position than that of the far position from the tree. HHPEBA inhibited the ryegrass growth at concentrations greater than 3 μM. HHPEBA was estimated to be able to cause 47–62% of the observed growth inhibition of ryegrass by the gingko litter. Therefore, HHPEBA may contribute to the inhibitory effect caused by ginkgo litter and may provide a competitive advantage for gingko to survive through the growth inhibition of the neighboring plants.
Keywords: allelopathy, Ginkgo biloba, growth inhibitor, litter, phytotoxicity
Ginkgo (Ginkgo biloba L.; Maidenhair tree) appeared in the Jurassic period and is only one surviving species in the family of Ginkgoaceae. It has not changed over 121 million years according to paleontological studies,1 which indicates that the species may have unknown special survival strategies.
A novel phytotoxic substance with allelopathic activity, 2-hydroxy-6-(10-hydroxypentadec-11-enyl) benzoic acid (HHPEBA) has recently been isolated from Ginkgo leaves.2 The activity of HHPEBA was 10- to 52-fold greater than that of nonanoic acid (pelargonic acid), which occurs naturally as esters in the oil of pelargonium, and its ammonium salt, ammonium nonanoate, is used as a herbicide.3 The allelopathic substances can provide a competitive advantage for host-plants to survive through the growth inhibition of the other plants in the local ecosystems.4-6
Ginkgo is a deciduous tree, and the tree drops their leaves in the late autumn. The leaf fall accumulates on the forest floor as litter and becomes one of the main components of the soil of the forest floor.7,8 In this report, it was investigated whether gingko litter contains HHPEBA, and HHPEBA serves as an allelopathic substance, which is able to provide a competitive advantage.
Allelopathic activity of ginkgo litter
Gingko litter was taken with soil in the depth of 5 cm from the surface at the position of 1, 2, 3, and 4 min from gingko tree (Fig. 1) on November 2011. The litter was then extracted with 80% aqueous methanol, and the biological activity of the extract was determined by weed species ryegrass (Lolium multiflorum Lam) as described previously.2 All extracts of ginkgo litter inhibited coleoptile and root growth of ryegrass (Fig. 1). However, the inhibitory activity of the litter was the greatest at 1 min from the gingko tree, and the smallest at 4 min. The result suggests that ginkgo litter may have allelopathic activity and contain allelopathic active substances.
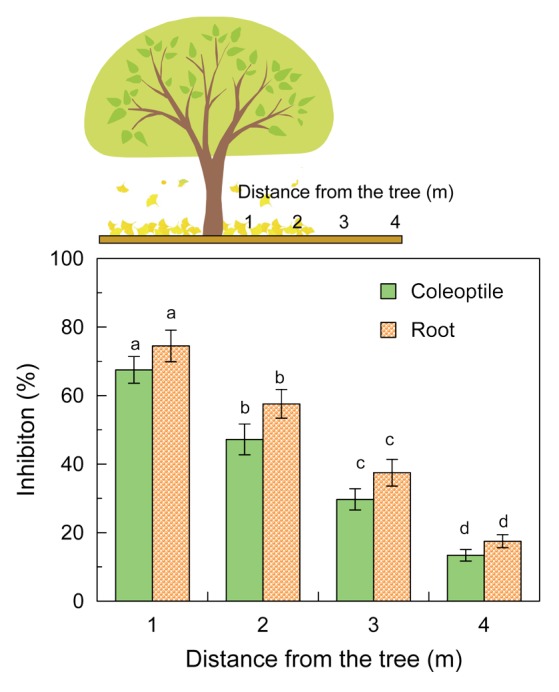
Figure 1. Effect of aqueous methanol extracts of ginkgo litter on the growth of ryegrass. Ginkgo litter (100 g) was extracted with 1 L of 80% (v/v) aqueous methanol. Extract (10 mL) was evaporated and added on the filter paper the petri dishes. After evaporation of the solvent, the filter paper was moistened with 1 mL of a 0.05% (v/v) aqueous solution of Tween 20. The biological activity of the extract was then determined by ryegrass seedlings as described previously.2 After the incubation of 48 h in darkness at 25 °C, the length of ryegrass coleoptiles and roots was measured and inhibition % was determined by the formula: [(control plant length – plant length incubated with the extract) / control plant length] x 100. Control ryegrass was incubated on the medium in the absence of the extract. Different letters indicate significant differences (P < 0.05) according to the Tukey test.
Concentration of HHPEBA
A phytotoxic substance, HHPEBA, was found in the bioassay medium of all gingko litter (Table 1). The concentration of HHPEBA was the highest (9.2 μM) in the medium of the litter at 1 min from the gingko tree and the lowest (1.3 μM) in that at 4 min (Table 1). The result indicates that the litter contained HHPEBA since the medium was prepared only from gingko litter extracts as described in Figure 1.
Table 1. Concentration of HHPEBA in the bioassay medium.
| Position (m) | Concentration (μM) |
|---|---|
| 1 | 9.2 ± 1.7 |
| 2 | 6.2 ± 1.5 |
| 3 | 3.4 ± 1.1 |
| 4 | 1.3 ± 0.5 |
Concentration of HHPEBA in the bioassay medium of Figure 1 was determined by HPLC. Means ± SE from 5 independent experiments are shown.
HHPEBA had been isolated from ginkgo leaves2 and the leaves are the main source of plant litter.7,8 Therefore, HHPEBA in ginkgo leaves may be delivered onto gingko forest floor by the defoliation and accumulate in the litter.
Inhibitory activity of HHPEBA
HHPEBA inhibited the growth of ryegrass coleoptiles and roots at concentrations greater than 3 μM (Fig. 2). When the inhibition of coleoptiles and roots was plotted against the logarithmic concentration of HHPEBA as described by Streibig,9 there were the significant logistic functions. The equations of HHPEBA were Y = [(4.024–89.276)/{1+(X/12.515)1.487}]+89.27; (r2 = 0.998) and Y = [(3.449–86.252)/ {1+(X/10.091)1.062}]+86.252; (r2 = 0.997) for coleoptiles and roots, respectively. Y is the growth inhibition (%) and X is the HHPEBA concentration (μM) as shown in Figure 2.
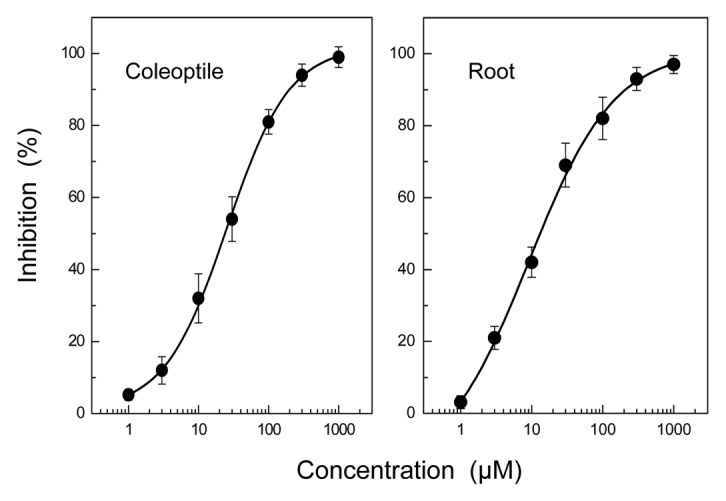
Figure 2. Effect of 2-hydroxy-6-(10-hydroxypentadec-11-enyl)benzoic acid (HHPEBA) on coleoptile and root growth of ryegrass seedlings. Ryegrass seeds were incubated in aqueous solution of HHPEBA for 48 d, and the length of coleoptiles and roots of ryegrass seedlings were measured as described previously.2 Inhibition % was then determined as described in Figure 1.
Contribution of HHPEBA to allelopathy of gingko litter
The potential ability of HHPEBA in the bioassay medium for the growth inhibition was estimated by the equations obtained by inhibitory activity of HHPEBA (Fig. 2) and substituting X values with HHPEBA concentrations in the bioassay medium (Table 1). The estimated values in Table 2 suggest that HHPEBA in the medium of the litter at 1 min has the ability to inhibit ryegrass coleoptile and root by 37.1 and 42.8%, respectively, and that at 4 min has the ability to inhibit the coleoptile and root growth by 6.3 and 10.6%, respectively.
Table 2. Potential ability of HHPEBA in the medium to growth inhibition.
| Potential growth inhibition (%) | ||
|---|---|---|
| Position (m) | Coleoptile | Root |
| 1 | 37.1 | 42.8 |
| 2 | 26.2 | 34.4 |
| 3 | 14.8 | 23.3 |
| 4 | 6.3 | 10.6 |
The contribution of HHPEBA to the growth inhibition by ginkgo litter was calculated with the formula: [potential ability of HHPEBA (Table 2)] / [growth inhibition observed (Fig. 1)] x 100. The calculated values in Table 3 suggest that HHPEBA explains 47.0–55.5% and 50.0–62.1% of the observed growth inhibition of ryegrass coleoptiles and roots (Fig. 1) by the gingko litter, respectively. Thus, HHPEBA may account for 47–62% of the growth inhibitory activity of the gingko litter.
Table 3. Contribution of HHPEBA to the growth inhibition by gingko litter.
| Contribution (%) | ||
|---|---|---|
| Position (m) | Coleoptile | Root |
| 1 | 55.0 | 57.4 |
| 2 | 55.5 | 50.0 |
| 3 | 49.8 | 62.1 |
| 4 | 47.0 | 60.6 |
The ginkgo litter showed growth inhibitory activity on weed species ryegrass (Fig. 1) and a phytotoxic substance, HHPEBA, existed in the bioassay medium of all gingko litter (Table 1). Judging from the activity of HHPEBA for growth inhibition (Fig. 2) and the concentration of HHPEBA in the gingko litter (Table 1), HHPEBA may be one of important contributors to the growth inhibitory effect of the litter (Table 3).
Plant litter is able to affect the microbial community, and the physical and chemical properties of the soil of the plant rhizosphere.10-12 In addition, plant litter has positive and negative influences on the growth of neighboring plants. These effects are related to several mechanisms such as nutrient cycling, reducing light penetration, physical impediment, and releasing allelopathic substances.11,13,14
The plant rhizosphere is a very competitive area where plant root systems fight each other to get sufficient nutrients, minerals, and water as well as space.10,15,16 Allelopathic substances with inhibitory activity in plant litter are involved in the competitive ability of host-plants to survive because of their effects on the seed germination, seedling establishment, plant growth, and distribution.7,17-19 Therefore, HHPEBA may serve for ginkgo survival strategy as an allelopathic active substance that is able to give an advantage in such competition for gingko to survive through the growth inhibition of neighboring plants in the local ecosystems.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Royer DL, Hickey LJ, Wing SL. Ecological conservatism in the 'living fossil' Ginkgo. Paleobiology. 2003;29:84–104. doi: 10.1666/0094-8373(2003)029<0084:ECITLF>2.0.CO;2. [DOI] [Google Scholar]
- 2.Kato-Noguchi H, Takeshita S, Ohno O, Suenaga K. A novel allelopathic active substance in Ginkgo biloba. J Plant Physiol. 2013;170:1593–1599. doi: 10.1016/j.jplph.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Lide DR. CRC Handbook of Chemistry and Physics. 70. Boca Raton, FL: CRC Press, 1990. [Google Scholar]
- 4.Duke SO, Dayan FE, Romagni JG, Rimando AM. Natural products as sources of herbicides, current status and future trends. Weed Res. 2000;40:99–111. doi: 10.1046/j.1365-3180.2000.00161.x. [DOI] [Google Scholar]
- 5.Belz RG. Allelopathy in crop/weed interactions--an update. Pest Manag Sci. 2007;63:308–26. doi: 10.1002/ps.1320. [DOI] [PubMed] [Google Scholar]
- 6.Macías FA, Molinillo JMG, Varela RM, Galindo JC. Allelopathy--a natural alternative for weed control. Pest Manag Sci. 2007;63:327–48. doi: 10.1002/ps.1342. [DOI] [PubMed] [Google Scholar]
- 7.Rice EL. Allelopathy. 2. Orlando: Academic Press, 1984. [Google Scholar]
- 8.Lonsdale WM. Predicting the amount of litterfall in forests of the world. Ann Bot (Lond) 1988;61:319–24. [Google Scholar]
- 9.Streibig JC. Herbicide bioassay. Weed Res. 1988;28:479–84. doi: 10.1111/j.1365-3180.1988.tb00831.x. [DOI] [Google Scholar]
- 10.Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–66. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 11.Bonanomi G, Sicurezza MG, Caporaso S, Esposito A, Mazzoleni S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006;169:571–8. doi: 10.1111/j.1469-8137.2005.01611.x. [DOI] [PubMed] [Google Scholar]
- 12.Weidenhamer JD, Callaway RM. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J Chem Ecol. 2010;36:59–69. doi: 10.1007/s10886-009-9735-0. [DOI] [PubMed] [Google Scholar]
- 13.Blum U, Shafer SR, Lehman ME. Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: concepts vs an experimental model. Crit Rev Plant Sci. 1999;18:673–93. doi: 10.1016/S0735-2689(99)00396-2. [DOI] [Google Scholar]
- 14.Zhao H, Peng S, Chen Z, Wu Z, Zhou G, Wang X, Qiu Z. Abscisic acid in soil facilitates community succession in three forests in China. J Chem Ecol. 2011;37:785–93. doi: 10.1007/s10886-011-9970-z. [DOI] [PubMed] [Google Scholar]
- 15.McCully ME. Roots in soil: unearthing the complexities of roots and their rhizospheres. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:695–718. doi: 10.1146/annurev.arplant.50.1.695. [DOI] [PubMed] [Google Scholar]
- 16.Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–33. doi: 10.1016/S1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- 17.Reigosa MJ, Sanchez-Moriras A, Gonxalez L. Ecophysiological approach in allelopathy. Crit Rev Plant Sci. 1999;18:577–608. doi: 10.1016/S0735-2689(99)00392-5. [DOI] [Google Scholar]
- 18.Caboun V. Tree-tree allelopathic interactions in middle European forests. Allelopathy J. 2006;17:17–31. [Google Scholar]
- 19.Fernandez C, Santonja M, Gros R, Monnier Y, Chomel M, Baldy V, Bousquet-Mélou A. Allelochemicals of Pinus halepensis as drivers of biodiversity in Mediterranean open mosaic habitats during the colonization stage of secondary succession. J Chem Ecol. 2013;39:298–311. doi: 10.1007/s10886-013-0239-6. [DOI] [PubMed] [Google Scholar]


