Abstract
1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) is the key enzyme in ethylene biosynthesis, catalyzing the conversion of S-adenosylmethionine (AdoMet) to ACC, which is the immediate precursor of ethylene. The regulation of ACS protein stability plays an important role in controlling ethylene biosynthesis. We have recently shown that 14-3-3 positively regulates ACS protein stability by both a direct effect and via downregulation of the stability of the E3 ligases regulating its turnover, Ethylene Overproducer1 (ETO1)/ETO1-like (EOL). Here, we report that treatment of etiolated Arabidopsis seedlings with light rapidly increases the stability of ACS5 protein. In contrast, light destabilizes the ETO1/EOLs proteins, suggesting that light acts to increase ethylene biosynthesis in part through a decrease in the level of the ETO1/EOL proteins. This demonstrates that the ETO1/EOLs are regulated in response to at least one environmental cue and that their regulated degradation may represent a novel input controlling ethylene biosynthesis.
Keywords: Ethylene, 14-3-3, ACC Synthase, E3 ligase, light, protein turnover
The phytohormone ethylene plays an important role in many plant processes and its biosynthesis is highly regulated by a diverse array of endogenous and exogenous inputs.1-4 This regulation of ethylene biosynthesis most often converges on ACC synthase (ACS), which catalyzes the first committed and generally rate-limiting step in ethylene biosynthesis. ACS is encoded by a multigene family in Arabidopsis whose transcription is differentially regulated by various developmental and environmental cues.3,5,6 In addition to this transcriptional control, the stability of subsets of ACS proteins are regulated by cytokinin, brassinosteroid, and pathogens.7-9
The control of protein stability plays a key role in many plant processes, including the regulation of phytohormone signaling and biosynthesis. E3 ligases are key components regulating protein turnover, and they are encoded by a large family of genes in plants. A subset of ACS proteins have been shown to be targeted for degradation via the 26S proteasome by a small family of plant-specific E3 ligases called ETO1, ETO1-like1 (EOL1), and EOL2.10,11 These E3 ligases, which contain a BTB/TRP domain, interact with the CULLIN3 protein to direct the ubiquitination of type-2 ACS proteins.11 Disruption of ETO1/EOLs, CUL3, or the domain in the type-2 ACS proteins that ETO1/EOLs interact with reduces the turnover of these ACS proteins, leading to an increase in the biosynthesis of ethylene.7,10-13
Despite their importance, relatively little is known regarding how E3 ligases themselves are regulated in plants. Recently, we have shown that the 14-3-3 proteins bind to both the ACS proteins and to the ETO1/EOL E3 ligases to regulate their stability.14 14-3-3s generally interact specifically with phosphorylated proteins to control their function by changing their localization, activity or stability.15-18 We demonstrated that 14-3-3 stabilizes ACS proteins via 2 distinct mechanisms. First, 14-3-3 binds directly to ACS proteins to reduce their turnover via an ETO1/EOL-independent mechanism. Second, 14-3-3s also bind to the ETO1/EOL E3 ligases to increase their degradation in an ubiquitin/proteasome-dependent manner, thereby increasing the stability of type-2 ACS proteins.
There are 3 classes of ACS proteins based primarily on their C-terminal domains, which impart distinct regulatory controls on the stability of the respective ACS proteins.2 In our previous study, we demonstrated that 14-3-3 interacts will all 3 classes of ACS proteins.14 Further, it was shown that both type-2 and type-3 ACS proteins are destabilized in response to disruption of the interaction with 14-3-3 using the R18 peptide, which acts as a strong competitive inhibitor of 14-3-3-interacting proteins.19 In order to extend this analysis, we examined the effect of the 14-3-3 interaction on a type-1 ACS protein. Consistent with the effect on the type-2 and type-3 ACS proteins, treatment of seedlings expressing a myc-epitope tagged version of ACS2 (a type-1 ACS protein) with R18 peptide resulted in a substantial destabilization of the fusion protein (Fig. 1). This destabilization did not occur in response to a mutant version of the R18 peptide (R18Lys) that eliminates its ability to disrupt 14-3-3 interactions. Together with our previous study, this result indicates that 14-3-3s are positive regulators of all 3 classes of ACS proteins.
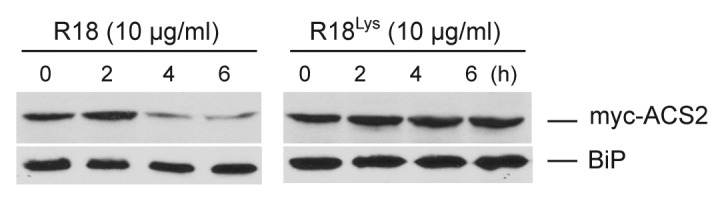
Figure 1. R18 peptide treatment results in a decrease in the steady-state level of type-1 ACS2 protein. Three-day-old etiolated seedlings expressing myc-ACS2 were treated with 10 µg/mL R18 and R18Lys for the indicated times. Total protein extracts from these seedlings were analyzed by immunoblotting using an anti-myc antibody or an anti-BiP antibody as a loading control as described.14
As the 3 classes of ACS proteins have distinct C-terminal domains, these results suggest that 14-3-3 acts through the conserved catalytic domain of the ACS proteins. Consistent with this, 14-3-3 interacts with the eto2 version of ACS5, which disrupts the C-terminal domain of ACS5.14 Further, the eto2 and eto3 versions of the type-2 ACS proteins ACS5 and ACS9, both of which lack the TOE domain necessary for interaction with ETO1/EOLs,20 are stabilized in vivo by the phytohormones cytokinin and brassinosteroid,8 indicating that there is a mechanism to control these proteins in a C-terminal independent manner. This alternative pathway regulating ACS stability may act through the 14-3-3 pathway.
In addition to the direct effect on all 3 classes of ACS proteins, 14-3-3 also stabilizes ACS proteins by increasing the degradation of the ETO1/EOL proteins. Disruption of the interaction with R18 caused a substantial increase in ETO1/EOL protein stability and increased ethylene biosynthesis, and conversely, overexpression of 14-3-3 resulted in decreased ETO1/EOL protein levels and reduced ethylene.14 This suggests the level of ETO1/EOL proteins plays a role in regulating the level of ethylene biosynthesis. Consistent with this, there is a quantitative effect of disruption of the 6 copies of the ETO1/EOL1/EOL2 genes on ethylene production in etiolated and light-grown seedlings.10,14 If modulation of ETO1/EOL levels is a mechanism by which plants regulate ethylene biosynthesis, then the level of ETO1/EOL proteins should be regulated by a subset of factors known to regulate ethylene biosynthesis. To test this, we examined the effect of light on ETO1/EOL proteins as light has a substantial effect on ethylene biosynthesis in various plant species, including Arabidopsis.21-23 Light treatment of 3-day-old etiolated seedlings caused an increase in the level of myc-ACS5 protein within 2 h (Fig. 2A). This increase in ACS5 protein is not associated with a change in myc-ACS5 transcript (not shown), suggesting that light acts by increasing ACS5 stability. Light treatment had an inverse effect on EOL2 protein levels, causing a substantial decrease within 2 h after treatment (Fig. 2B). This result supports the hypothesis that the level of ETO1/EOL proteins is regulated in response to environmental cues, and that the altered level of the ETO1/EOL proteins likely acts as an input for the control of ACS protein stability. A subset of 14-3-3 isoforms have also been shown to be involved in a light signaling pathway regulating flowering transition, phytochrome response, and stomatal opening,24,25 suggesting that 14-3-3 proteins may play an important role in multiple light responses.
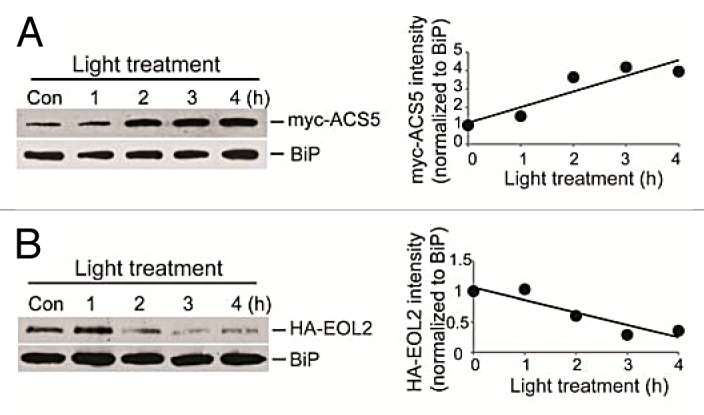
Figure 2. Light stabilizes myc-ACS5 protein, but destabilizes HA-EOL2 protein. Left: Immunoblot of protein extracts from 3-day-old seedlings expressing either a myc-ACS5 (A) or an HA-EOL2 (B) fusion protein after treatment with light. Seedlings were grown in the dark for 3 days, moved to light for the indicated times, proteins extracted and analyzed by immunoblotting using an anti-myc, anti-HA, or an anti-BiP antibody as described.14 Right: The myc and HA signal intensities were quantified and normalized to the BiP loading control.
As the 14-3-3 proteins generally act as dimers, they may increase ETO1/EOL degradation by blocking the access to the ACS substrates and promoting ETO1/EOL dimerization, leading to auto-ubiquitination. Further biochemical analyses should help clarify the mechanism by which ETO1/EOL degradation is increase by 14-3-3 and may reveal additional regulatory inputs into ETO1/EOL degradation. As 14-3-3s generally bind phosphorylated substrates, it is likely that both the ETO1/EOL proteins and the catalytic domain of ACS proteins are phosphorylated by as yet unidentified kinases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
This project was supported by National Science Foundation grant MCB 1021704 to J.J.K.
Glossary
Abbreviations:
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACS
ACC synthase
- ETO1
Ethylene Overproducer1
- EOL
ETO1-like
References
- 1.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–89. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 2.Chae HS, Kieber JJ. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–6. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Argueso CT, Hansen M, Kieber JJ. Regulation of ethylene biosynthesis. J Plant Growth Regul. 2007;26:92–105. doi: 10.1007/s00344-007-0013-5. [DOI] [Google Scholar]
- 4.Lyzenga WJ, Stone SL. Regulation of ethylene biosynthesis through protein degradation. Plant Signal Behav. 2012;7:1438–42. doi: 10.4161/psb.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller GE, Kieber JJ. Ethylene. In: Somerville CR, Meyerowitz EM, eds. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists, 2002. [Google Scholar]
- 7.Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci U S A. 1998;95:4766–71. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen M, Chae HS, Kieber JJ. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009;57:606–14. doi: 10.1111/j.1365-313X.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–99. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christians MJ, Gingerich DJ, Hansen M, Binder BM, Kieber JJ, Vierstra RD. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2009;57:332–45. doi: 10.1111/j.1365-313X.2008.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KL-C, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–50. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- 12.Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003;15:545–59. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomann A, Lechner E, Hansen M, Dumbliauskas E, Parmentier Y, Kieber J, Scheres B, Genschik P. Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms. PLoS Genet. 2009;5:e1000328. doi: 10.1371/journal.pgen.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon GM, Kieber JJ. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell. 2013;25:1016–28. doi: 10.1105/tpc.113.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darling DL, Yingling J, Wynshaw-Boris A. Role of 14-3-3 proteins in eukaryotic signaling and development. Curr Top Dev Biol. 2005;68:281–315. doi: 10.1016/S0070-2153(05)68010-6. [DOI] [PubMed] [Google Scholar]
- 16.Oecking C, Jaspert N. Plant 14-3-3 proteins catch up with their mammalian orthologs. Curr Opin Plant Biol. 2009;12:760–5. doi: 10.1016/j.pbi.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Freeman AK, Morrison DK. 14-3-3 Proteins: diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol. 2011;22:681–7. doi: 10.1016/j.semcdb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117:1875–84. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Yang H, Liu YC, Jelinek T, Zhang L, Ruoslahti E, Fu H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry. 1999;38:12499–504. doi: 10.1021/bi991353h. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Wang KL, Chang C-M, Mori K, Uchida E, Ecker JR. The ACC synthase TOE sequence is required for interaction with ETO1 family proteins and destabilization of target proteins. Plant Mol Biol. 2006;62:427–37. doi: 10.1007/s11103-006-9029-7. [DOI] [PubMed] [Google Scholar]
- 21.Jiao XZ, Yip WK, Yang SF. The effect of light and phytochrome on 1-aminocyclopropane-1-carboxylic Acid metabolism in etiolated wheat seedling leaves. Plant Physiol. 1987;85:643–7. doi: 10.1104/pp.85.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goeschl JD, Pratt HK, Bonner BA. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol. 1967;42:1077–80. doi: 10.1104/pp.42.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel JP, Schuerman P, Woeste KW, Brandstatter I, Kieber JJ. Isolation and characterization of Arabidopsis mutants defective in the induction of ethylene biosynthesis by cytokinin. Genetics. 1998;149:417–27. doi: 10.1093/genetics/149.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayfield JD, Folta KM, Paul A-L, Ferl RJ. The 14-3-3 Proteins mu and upsilon influence transition to flowering and early phytochrome response. Plant Physiol. 2007;145:1692–702. doi: 10.1104/pp.107.108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng TS, Whippo C, Hangarter RP, Briggs WR. The role of a 14-3-3 protein in stomatal opening mediated by PHOT2 in Arabidopsis. Plant Cell. 2012;24:1114–26. doi: 10.1105/tpc.111.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]


