Abstract
There are many various diseases in the bone and joint infections, and we tried to make antimicrobial treatment guidelines for common infectious diseases based on available data for microbiology and clinical trials. This guidelines focused on the treatment of osteomyelitis and septic arthritis, which can be experienced by physicians at diverse clinical settings. This guidelines is not applicable to diabetic foot infections, postoperative infections or post-traumatic infections which need special considerations. The guidelines for those conditions will be separately developed later. Surgical treatment of bone and joint infections, pediatric bone and joint infection, tuberculous bone and joint infection, and prophylactic antibiotic use were not included in this guideline.
Keywords: Osteomyelitis, Septic arthritis, Antimicrobial treatment
Introduction
1. Background and purpose
In recent years, the clinical practice of treating bone and joint infection has changed. Despite new therapeutic modalities being developed, physicians still consider bone and joint infections difficult to cure due to the high rate of treatment failure and recurrence. Osteomyelitis and septic arthritis usually refer to infections of the upper and lower extremities of bones, spinal bone structures, and joints. These guidelines recommend antimicrobial therapy for the treatment of osteomyelitis and septic arthritis for primary physicians, trainees of teaching hospitals, medical specialists, orthopedic surgery specialists, and neurosurgery specialists.
2. Scope
These guidelines suggest antimicrobial therapy for the treatment of osteomyelitis and septic arthritis based on evidence acquired from the domestic and foreign literature. We will develop differentially further medical guidelines on the diagnosis of osteomyelitis and septic arthritis, diabetic foot infection, surgical site infection including arthroplasty, and post-trauma infection.
The surgical treatment of osteomyelitis and arthritis, pediatric osteomyelitis and arthritis, and tubercular osteomyelitis and arthritis, and the prophylactic use of preoperative antibiotics, are excluded from these guidelines.
3. Guideline development methods
1) Establish a committee for developing the guidelines
Through multidisciplinary cooperation, the committee was composed of 12 people, including infectious disease specialists, orthopedic surgery specialists, and preventive medicine specialists.
2) Define the scope of the guidelines
The guideline development committee created these criteria based on the five considerations of PIPOH (Population, Intervention, Professionals, Outcomes, Healthcare setting). We defined the scope of the guidelines in a committee that discussed the state of the disease in a population, therapeutic interventions, target professionals, patient outcomes (focused on increasing survival rate or improving quality of life), and the customization of individual healthcare settings.
3) Framing key questions
Defining key questions was the first stage; it involved collecting evidence and data and conducting evaluations. After reviewing domestic and foreign medical guidelines and literature, the committee identified the development of these guidelines as the key question.
4) Searching for evidence
We searched the PubMed (www.pubmed.gov) and KoreaMed (www.koreamed.org) databases for articles and guidelines published between January 1975 and December 2012 using the keywords "Arthritis, Infectious" [MeSH] or "septic arthritis" and "guideline" or "systematic". The committee assessed studies for potential eligibility and selected articles from the literature.
5) Writing the guidelines and clarifying the strength of the recommendations
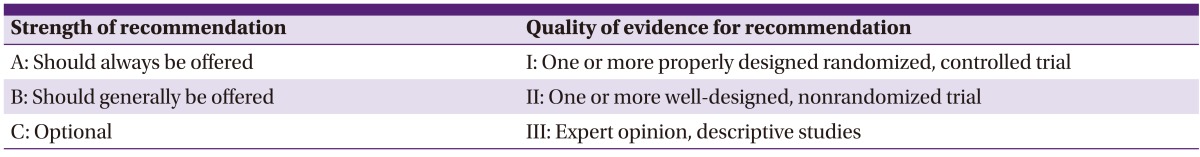
The guideline development committee drafted the guidelines and classified the evidence according to three criteria (I, II, III) after reviewing the literature based on each key question. We classified evidence as A, B, or C when determining the strength of a recommendation. Our development committee applied the strength of the recommendation and the quality of evidence for the recommendation according to information from the Infectious Diseases Society of America (Table 1).
Table 1.
Strength of recommendation and quality of evidence for recommendation
6) External specialists review and approval
The guidelines were presented to the Korean Society for Chemotherapy on April 14, 2012. Specialist groups and society members provided feedback freely. Based on their opinions, we revised and finalized our guidelines.
Practice guidelines
1. Osteomyelitis
1) Epidemiology and classification
Osteomyelitis is defined as inflammatory changes of bone tissue and accompanying bone destruction due to pyogenic organisms [1]. Once symptoms appear and as time progresses, the disease can be classified as acute, subacute, or chronic. However, osteomyelitis is a complicated condition as it is influenced by not only the time of disease onset, but also pathogenesis, affected site, and local blood supply. Thus, classification methods were used when considering these points.
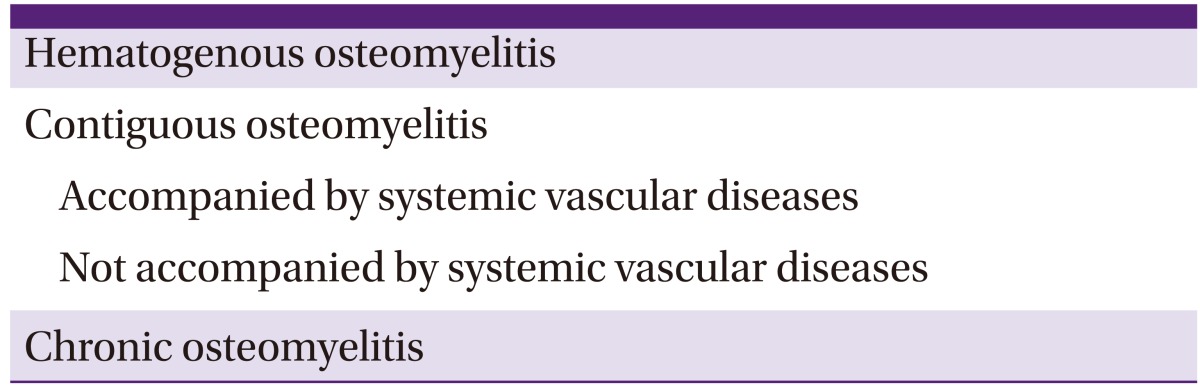
Waldvogel's classification was originally presented in 1970, dividing osteomyelitis into hematogenous, contiguous, and chronic osteomyelitis categories according to pathogenesis and the time of disease onset (Table 2). Contiguous osteomyelitis was classified according to the presence or absence of systemic vascular disease. Waldvogel's classification system was organized according to pathogenesis; thus, it is helpful to make assumptions about infecting pathogens and choose the appropriate empirical antibiotic treatment [2]. Hematogenous osteomyelitis accounts for approximately 20% of all osteomyelitis cases; it usually develops in children. In cases of osteomyelitis related to parenteral drug abuse, the spine is commonly involved [3]. The number of hematogenous osteomyelitis cases has been decreasing thanks to social and economic changes as well as the advancement of medical technology, whereas the number of contiguous osteomyelitis cases has been rising because of increased numbers of traffic accidents and an increased number of arthroplasty operations. Contiguous osteomyelitis accounts for 80% of all osteomyelitis cases. It develops in soft tissues or secondary to surgery or trauma, and occurs more often in adults than in children. It is commonly associated with vascular insufficiency of the infected area or prosthesis. In such cases, it is hard to be cured.
Table 2.
Waldvogel's osteomyelitis classification system
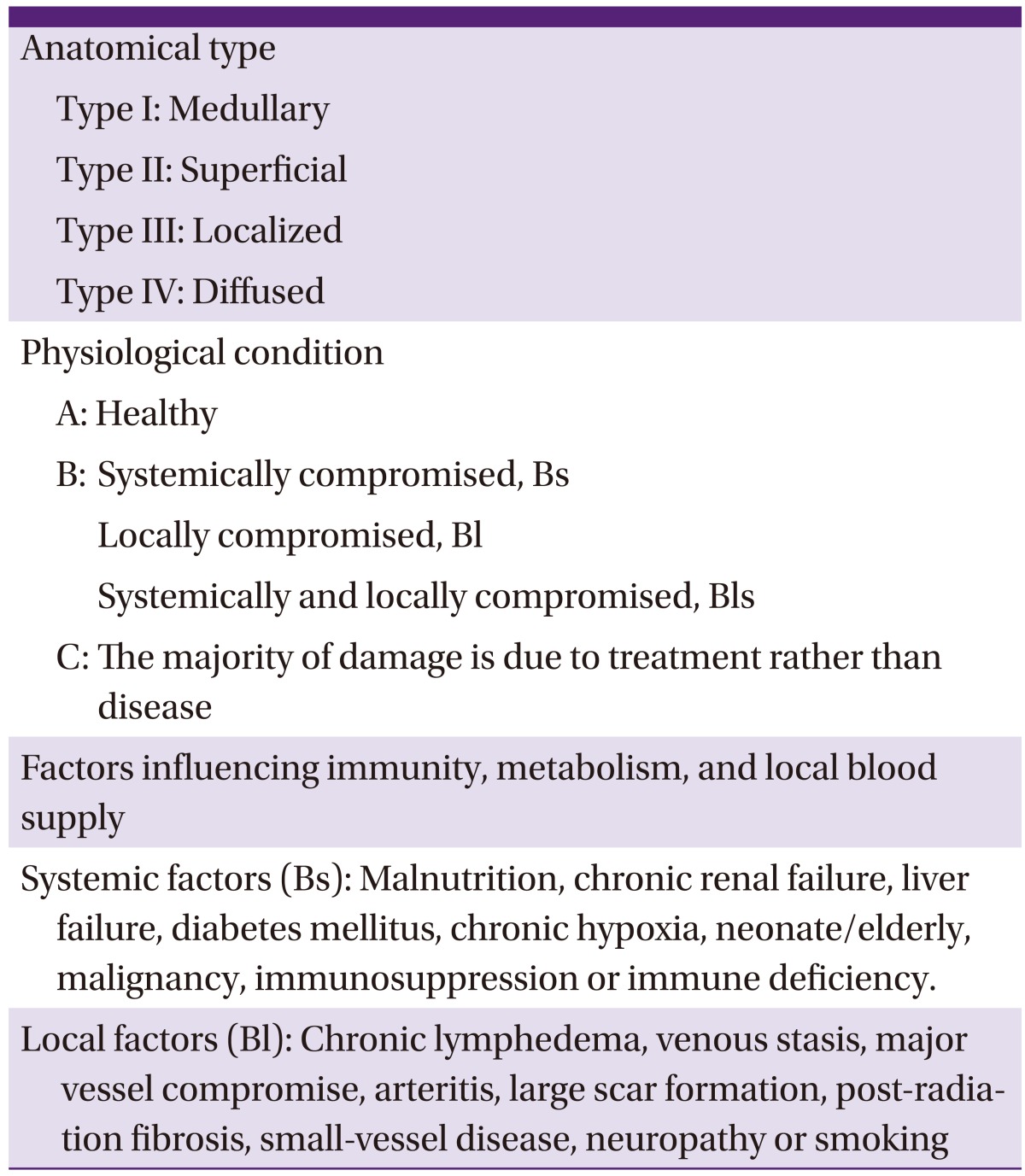
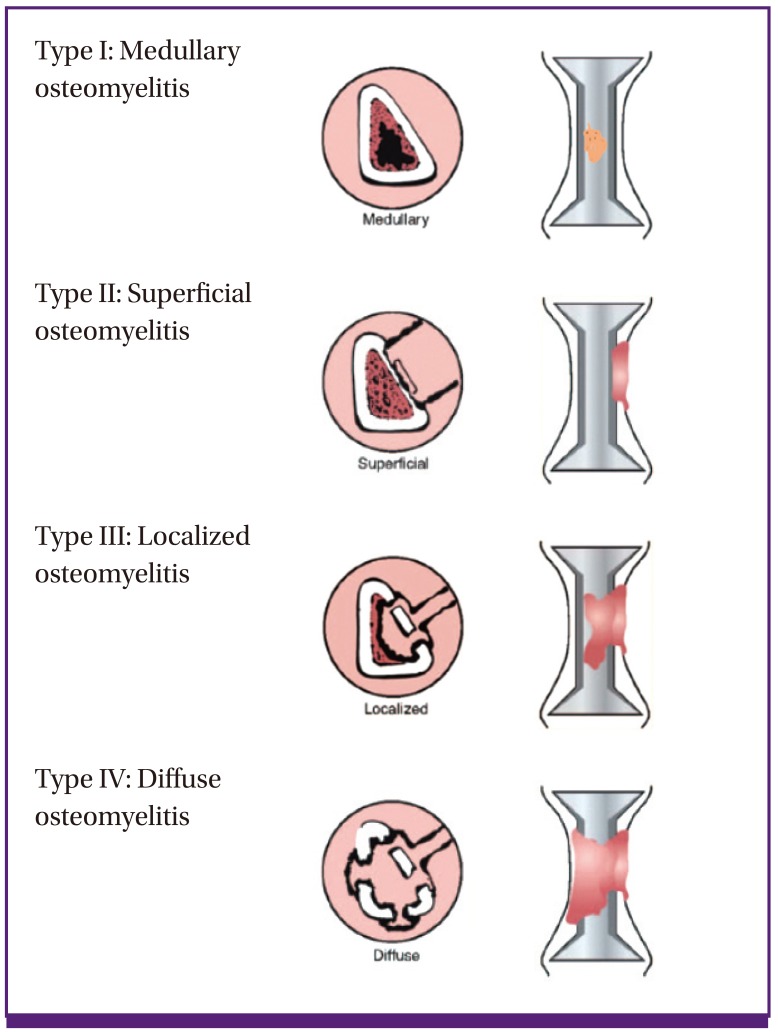
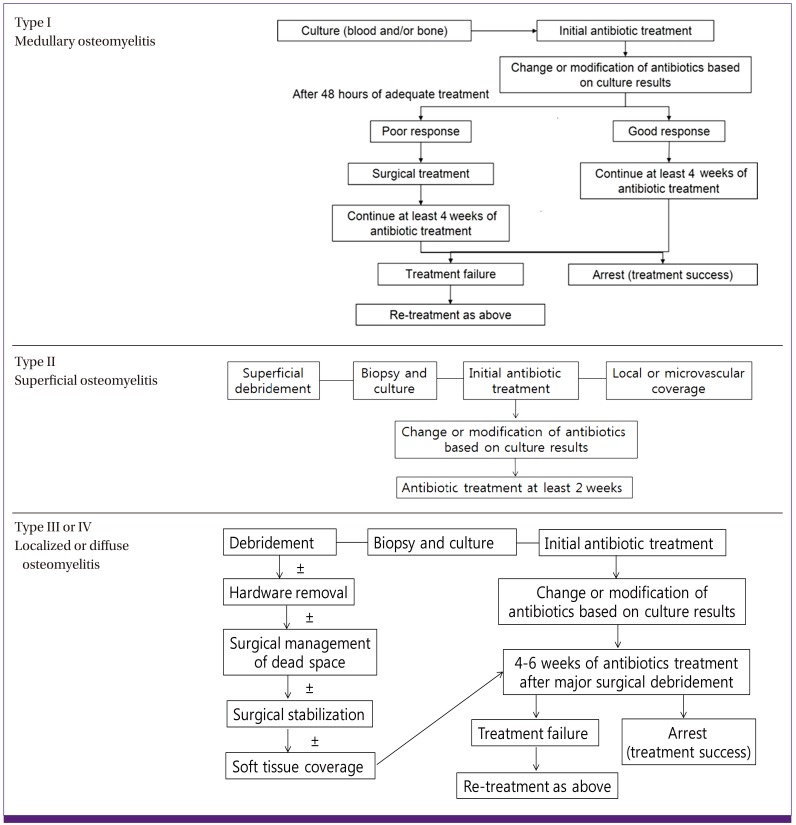
Cierny-Mader's classification system does not categorize the disease according to its duration (i.e., acute or chronic). Rather, it classifies the disease based on the anatomical extent of necrosis of the infected bone sites, the patient's physiological state and the systemic or local impact of disease on function (Table 3, Fig. 1) [4]. Cierny-Mader's classification system is frequently used in clinical practice because it is especially helpful for determining treatment and prognosis in patients with long bone osteomyelitis. It is useful as a tool for determining whether surgery is appropriate, and for selecting surgical methods.
Table 3.
Cierny-Mader's osteomyelitis classification system
Figure 1.
Cierny-Mader classification of osteomyelitis according to the anatomical extent.
Osteomyelitis Type I indicates that the infection is limited to the medulla; it includes primary hematogenous infections. Osteomyelitis Type II mostly occurs through a direct inoculation or a contiguous focus of infection. Osteomyelitis Type III usually involves cortical bone. Although the stability of the bone is maintained, necrotic areas need to be removed. Osteomyelitis Type IV indicates that all layers of bone are infected and that all necrotic bone should be removed. Therefore, the structural stability of the bone is compromised. However, it is important to note that the osteomyelitis categories of the Cierny-Mader's classification system can change dynamically according to the patient's condition, administration of antibiotic therapy, and other treatments. In addition, this classification system would not be applicable to osteomyelitis in special situations, such as peri-prosthetic osteomyelitis and vertebral osteomyelitis.
2) Distribution of causative pathogens
Isolation of the causative pathogen in osteomyelitis is very important for determining the diagnosis and selecting effective treatments. Data from other countries indicate that Staphylococcus aureus is the most common causative pathogen in most types of osteomyelitis. Enterobacteriaceae, coagulase-negative staphylococci (CoNS), and streptococci (bite wounds, bedsores, and diabetic foot infections) are also common causative pathogens [1, 5, 6]. Pseudomonas aeruginosa is a common causative pathogen when the disease is acquired in hospitals. The number of tuberculous osteomyelitis patients has recently been growing in parallel with an increasing number of AIDS patients, while fungal infection cases remain rare.
According to a domestic article dealing exclusively with spondylitis, causative pathogens were isolated in 71 of 101 cases; the results indicated that S. aureus was the most common pathogen, accounting for 36.6% of isolates. Meanwhile, 19.2% of S. aureus isolates were methicillin-resistant S. aureus (MRSA). Viridans-group streptococci, Streptococcus agalactiae, and Streptococcus pneumoniae accounted for 18.3%, 8.5%, and 4.2% of isolates, respectively. Gram-negative pathogens accounted for 18.3% of isolates, and included Escherichia coli (9.9%) and P. aeruginosa (4.2%) [7]. Other domestic data indicated that S. aureus accounted for the largest proportion (39.8%) at 37 cases among 93 cases of vertebral osteomyelitis; 37.8% of which was MRSA. Meanwhile, S. epidermidis accounted for 12.9% of isolates, and streptococci accounted for 16.1%. Gram-negative pathogens accounted for 24.7% of all vertebral osteomyelitis cases. E. coli was the most common (12.9%), followed by Klebsiella pneumoniae (3.2%) and P. aeruginosa (2.2%). No significant differences were detected among the studies [8].
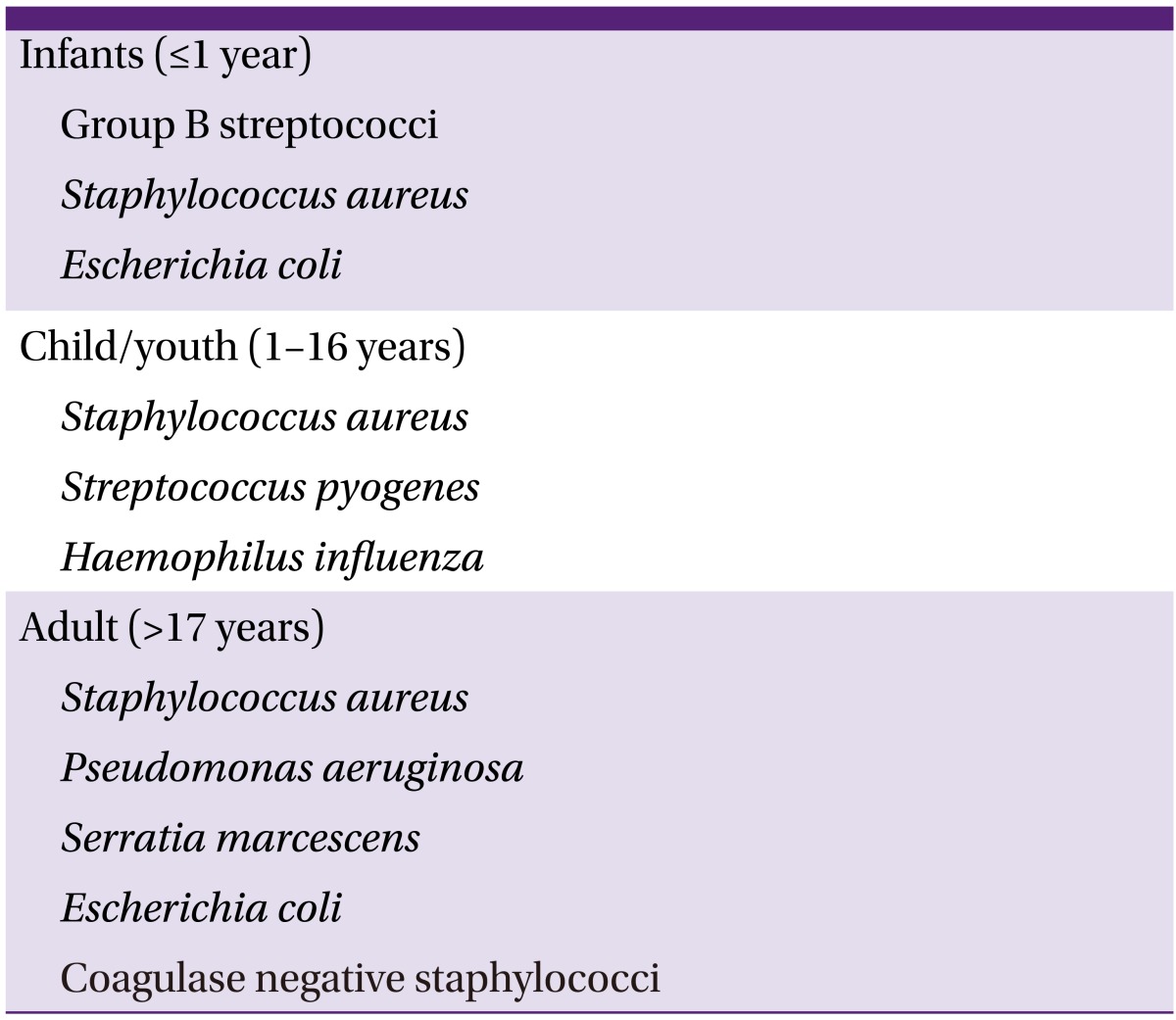
The distribution of causative pathogen in bacterial osteomyelitis differs according to patient age, route of disease dispersion, clinical situation, and the severity of clinical manifestation [6, 9]. Considering age, 90% of hematogenous osteomyelitis occurring in healthy children develops as a result of S. aureus infection. Hematogenous osteomyelitis due to Haemophilus influenzae is also common in children who have not received H. influenzae type B vaccination (Table 4) [2, 5, 6]. S. aureus, S. epidermidis, P. aeruginosa, Serratia marcescens, and E. coli are the common causative pathogens in adults with osteomyelitis.
Table 4.
Major causative organisms according to patient age
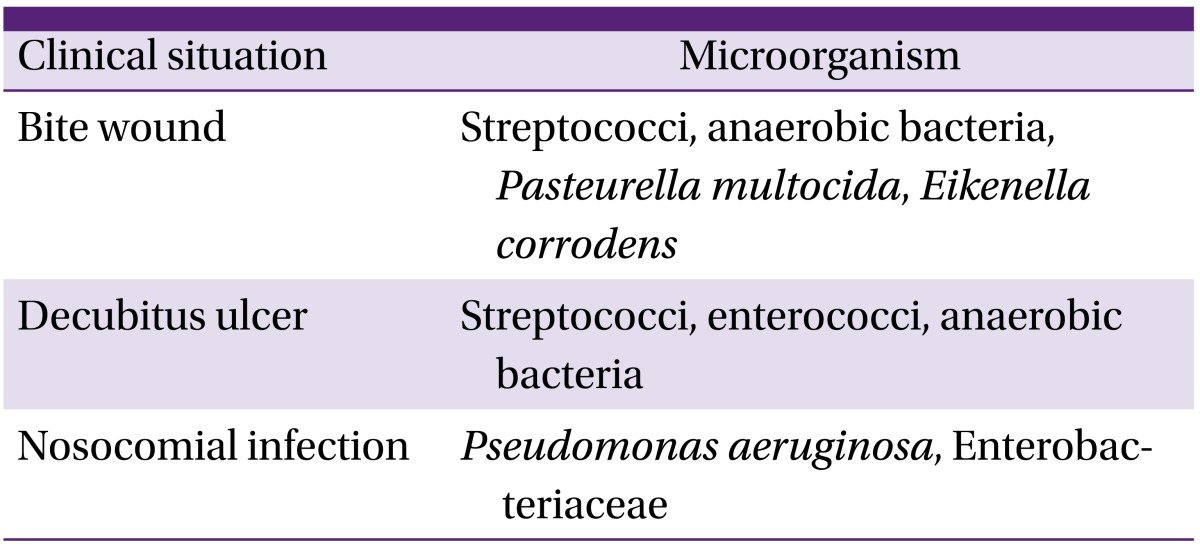
Hematogenous osteomyelitis mainly occurs as a result of one pathogen, but contiguous osteomyelitis can occur through infection with a single pathogen or multiple pathogens [5]. Patients with vascular insufficiency frequently develop mixed infection with S. aureus, CoNS, Enterobacteriaceae, streptococci, enterococci, and anaerobic pathogens. In patients with osteomyelitis related to prosthesis, the causative pathogen is most commonly S. aureus. In such patients, we should consider the possibility of infection by S. epidermidis, P. aeruginosa, and Propionibacterium spp. (Table 5) [1, 5].
Table 5.
Major causative organisms according to clinical conditions
On the other hand, we also have to consider the possibility of infection with Brucella spp., Coxiella burnetii (Q fever), and fungal infection, depending on the patient's immunity, occupation, and traveling history. If patients have risk factors for candida infection, such as a history of treatment with broad-spectrum antibiotics, use of central catheters, and repeated isolation of Candida spp. in the absence of another pathogen, we must suspect Candida osteomyelitis.
3) Clinical findings
Hematogenous osteomyelitis is frequently seen in children, while contiguous osteomyelitis occurs in adults. Patients with acute osteomyelitis, especially hematogenous osteomyelitis, present localized pain of several days. In young children, systemic symptoms in particular, such as fever, irritability, and lethargy, are accompanied by local symptoms such as tenderness, local heating, and swelling [1]. However, in the case of vertebral, hip joint, and pelvic osteomyelitis, specific symptoms and signs may be absent, except pain. In addition, acute osteomyelitis commonly occurs on the metaphysis in children and the diaphysis in adults. Occasionally, the disease spreads to nearby joints and progresses to septic arthritis. In most cases, the infected bones are tender, and the range of motion around the joints can be limited. Patients with subacute osteomyelitis usually shows mild bony tenderness of several weeks, whereas fever and systemic symptoms are rarely accompanied.
In general, patients with chronic osteomyelitis complain of only chronic pain in the infected areas. Such patients could have mild fever. Bone defects, sequestration, osteosclerosis, and sinus tract formation are also common characteristics of chronic osteomyelitis [1]. Chronic osteomyelitis can be detected in a static state, but it can also slowly progress. In cases where there is obstruction of the sinus tract, local abscess or acute soft tissue infection are often identified.
4) Diagnosis
Acute hematogenous osteomyelitis occurs with rapid onset within a few days, and is usually accompanied by pain or tenderness over the affected bone and generalized symptoms such as fever or chills. The onset of chronic osteomyelitis is insidious. Symptoms of chronic osteomyelitis are subtle, but include mild generalized symptoms, pain or tenderness over the affected bone for long periods, and sinus tract involvement. Osteomyelitis must be suspected in such cases (AIII).
After an intensive review and physical examination, plain radiographs and blood tests including complete blood count (CBC) with differential counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels must be performed to confirm diagnosis (AIII).
Magnetic resonance imaging (MRI) is considered the best modality for the early detection of osteomyelitis (AI). Three-phase bone scintigraphy can be used for patients for whom computed tomography or MRI approaches are difficult (BIII).
In principle, cultures of blood, abscess, and bony tissue specimens must be obtained before the institution of antimicrobial agents (AIII).
Swab culture results from sinus discharge are often inaccurate for the detection of causative microorganisms, and thus cannot be relied upon. Culture from surgical or percutaneous bone biopsy is effective and should be performed (AII). Along with bony tissue culture, a histopathological examination can be performed to increase diagnostic sensitivity (BII).
5) Treatment
(1) General principles of treatment (Fig. 2)
Figure 2.
Treatment algorithm for adult long bone osteomyelitis (Figures modified from Lazzarini et al. [10] Reprinted with permission from The Journal of Bone and Joint Surgery).
In cases of acute osteomyelitis, appropriate antimicrobial agents should be given promptly to limit bacteremia, bone necrosis and bone destruction (AI).
Surgical treatment should be considered in cases of acute osteomyelitis when there is abscess formation or radiologic evidence of necrosis, or when the patient does not respond to antimicrobial agents (AII).
A multidisciplinary team approach is needed for the treatment of chronic osteomyelitis (AIII). Surgical interventions including adequate debridement of necrotic tissue, stabilization of bony structures, management of dead space, and reconstruction of soft tissue are needed. It is essential that the selected antimicrobial agents are appropriate for the isolated organism and that dosage and treatment duration is adequate.
Patient factors, such as improving nutritional state, stopping smoking, controlling glucose levels, and restoring blood flow, should be optimized as a part of treatment in patients with chronic osteomyelitis (AIII).
Surgical modalities and duration of antimicrobial agents are determined based on the Cierny-Mader's classification. In general, we recommend antimicrobial treatment of 4-6 weeks after the last major debridement. However, treatment must be tailored according to the stage and condition of the individual patient (AI).
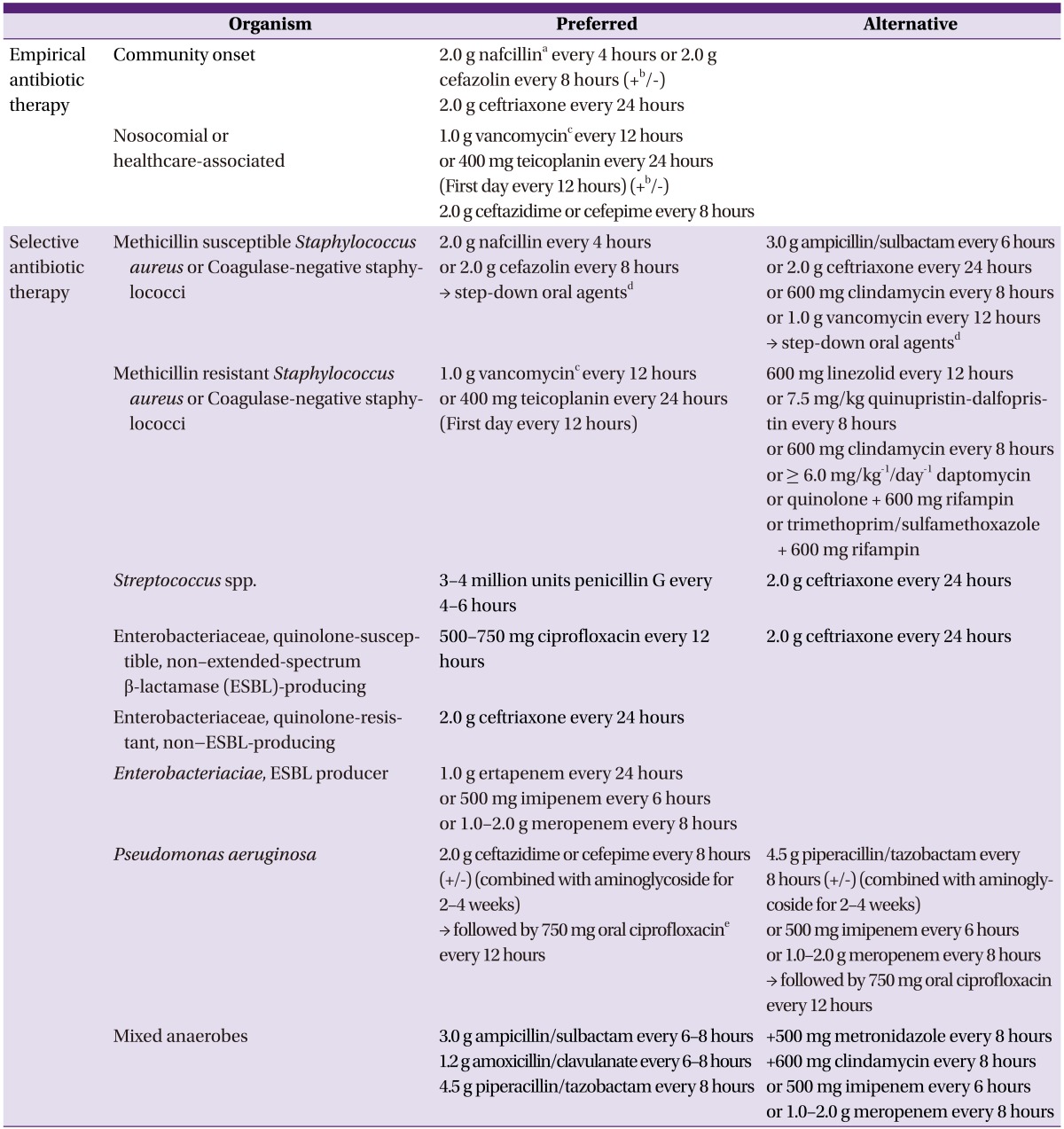
(2) Antimicrobial therapy (Table 6)
Table 6.
Suggested regimens for antimicrobial therapy of osteomyelitis
aIn patients with delayed hypersensitivity to nafcillin, cefazolin can be used. In patients with immediate hypersensitivity, penicillins should be replaced by vancomycin or clindamycin. S. aureus isolates that are clindamycin-susceptible but erythromycin-resistant should be tested for inducible clindamycin resistance using the D-test.
bCombination therapy can be considered before the causative organism is identified in some conditions, i.e., preceding bacteremia when associated with urinary tract infection or intra-abdominal infection, or in the immunocompromised or elderly.
cThe trough concentration of vancomycin should be 15-20 µg/mL.
dCombination therapy with drugs to which the organism is susceptible should be used for the treatment of osteomyelitis caused by S. aureus. 500-750 mg ciprofloxacin every 12 houres + 600 mg rifampin every 24 houres/750 mg levofloxacin + 600 mg rifampin every 24 houres/trimethoprim/sulfamethoxazole 80/400 mg single strength, 2 tablets every 12 houres + 600 mg rifampin every 24 houres.
eQuinolone monotherapy is no longer considered adequate because of the high risk for the emergence of resistance during the high bacterial burden that exists in the initial stages of the disease; however, it can be used as an oral step down therapy after initial combination therapy with β-lactam agent and aminoglycoside.
-
Empirical antimicrobial agents must be administered after obtaining cultures from blood, abscess, and bone tissue specimens. Prior to obtaining a definitive Gram stain and culture result, the clinician must select an appropriate antimicrobial agent considering the epidemiologic factors of the community, local susceptibility rates, the origin of infection (community or hospital), the general condition of the patient, and the primary cause of osteomyelitis.
Definitive antimicrobial therapy must be tailored according to the Gram stain results, the susceptibility of the isolated microorganism, and the degree of bone penetration (AII).
-
Empirical antimicrobial treatment
1) In cases of community-acquired osteomyelitis, nafcillin or cefazolin is recommended as an empirical agent, given that the most commonly isolated organism is methicillin-susceptible S. aureus (MSSA) (AI).
2) If gram-negative bacteria cannot be ruled out as the causative agents in patients with community-acquired osteomyelitis, ceftriaxone can be combined with nafcillin or cefazolin for coverage of both staphylococci and gram-negative pathogens (CIII).
3) In cases of healthcare-associated or hospital-acquired osteomyelitis, and in cases that have responded poorly to antistaphylococcal treatment, vancomycin or teicoplanin can be considered to cover for MRSA (CIII).
-
Antimicrobial treatment for specific organisms
1) Nafcillin or cefazolin should be administrated to treat osteomyelitis caused by MSSA (AI).
2) Vancomycin is not recommended for the treatment of osteomyelitis caused by MSSA because of the high rate of recurrence of MSSA (AII).
3) Either vancomycin or teicoplanin is recommended as first-line therapy for MRSA osteomyelitis (BII).
4) The trough concentration of vancomycin should be 15-20 µg/mL when treating MRSA osteomyelitis (BIII).
-
Role of combination antimicrobial treatment and switching to oral agents
1) Rifampin-containing regimen at early stage is generally not recommended for the treatment of osteomyelitis (CIII).
2) Combinations regimens can be used as oral step-down treatments for osteomyelitis caused by S. aureus, if susceptible. They include rifampin + ciprofloxacin, rifampin + levofloxacin, and rifampin + trimethoprim/sulfamethoxazole (BII).
3) Oral first-generation cephalosporin agents such as cefadroxil, cephalexin, and cefradine are generally not recommended (CIII).
4) For the treatment of osteomyelitis caused by P. aeruginosa, monotherapy with a quinolone is not adequate because of the high risk for the development of resistance during the high bacterial burden that exists in the initial stages of disease. Therefore, combination therapy with a β-lactam agent and an aminoglycoside should be initiated at early stage of treatment (BIII).
(3) The role of adjuvant therapy
The local delivery of antimicrobial agents (antibiotic impregnated cement) can be used in the treatment of chronic osteomyelitis as an adjuvant method of systemic antimicrobial treatment (BII).
(4) Treatment of vertebral osteomyelitis
Vertebral osteomyelitis is associated primarily with hematogenous monobacterial infection, and requires appropriate selective antimicrobial treatment (AII).
Indications for surgery include obtainment of specimen for microbiological and histological diagnosis, resolution of compression of neural elements, stabilization of instability due to extensive bone destruction, prevention or correction of biomechanical deformity such as severe kyphosis, drainage of clinically significant abscesses, or management of intractable pain (BIII). Spinal cord compression due to epidural abscess is a surgical emergency; the abscess must be surgically decompressed within 24-36 hours of the development of neurologic deficits (AI).
The recommended duration of antimicrobial treatment is usually 6-12 weeks. However, treatment must be individualized for each patient according to clinical response, course of improvement, antimicrobial susceptibility of the causative organism, and the presence or absence of an implant (BIII).
6) The role of adjuvant therapy
Inflammatory markers such as ESR and CRP level should be checked regularly to evaluate the response to treatment (AIII).
2. Septic arthritis
Septic arthritis is the major infectious disease in joints. However, because of its characteristics, most published studies involved a retrospective design or were case reports; as a result, it is difficult to develop clear clinical guidelines [11, 12]. However, it is critical that physicians are able to accurately distinguish septic arthritis from other conditions that have similar symptoms, such as joint pain, erythema, and swelling.
1) Epidemiology
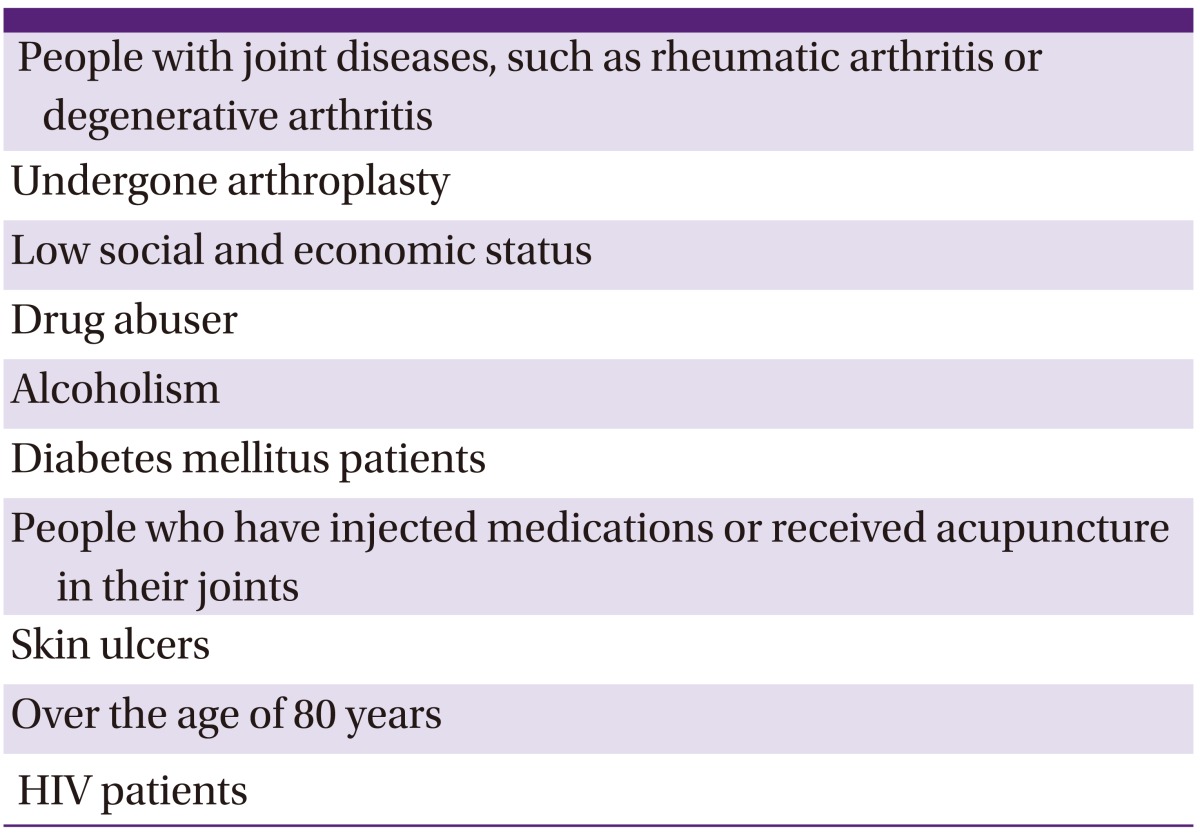
The following are at high risk for septic arthritis: patients with degenerative osteoarthritis, people who abuse drugs, people with alcoholism, patients with diabetes, people receiving injections or acupuncture in their joints, people with skin ulcers, people aged 80 years or older, HIV patients, people of lower social and economic status, and those who have undergone arthroplasty surgery [13, 20] (Table 7). Current Korean studies are lacking, but some retrospective studies have reported that infections proceeding from joint acupuncture or intra-articular injections represent 50% of septic arthritis cases [19].
Table 7.
Groups at high risk for septic arthritis
The number of patients with chronic illness who are in long-term care facilities has recently been increasing; consequently, the number of patients with septic arthritis has also increased. It is important to consider the possibility that patients living in medical facilities who have catheters, foot ulcers, drug addictions, or who have recently undergone orthopedic surgery may have contracted MRSA [21]. In the US and Europe in particular, the rates of community-associated MRSA (CA-MRSA) infections have been gradually increasing [22, 23]. In Korea, studies examining CA-MRSA have been conducted at multiple medical centers; however, only one case involved a patient with septic arthritis [24].
2) Distribution of causative pathogens
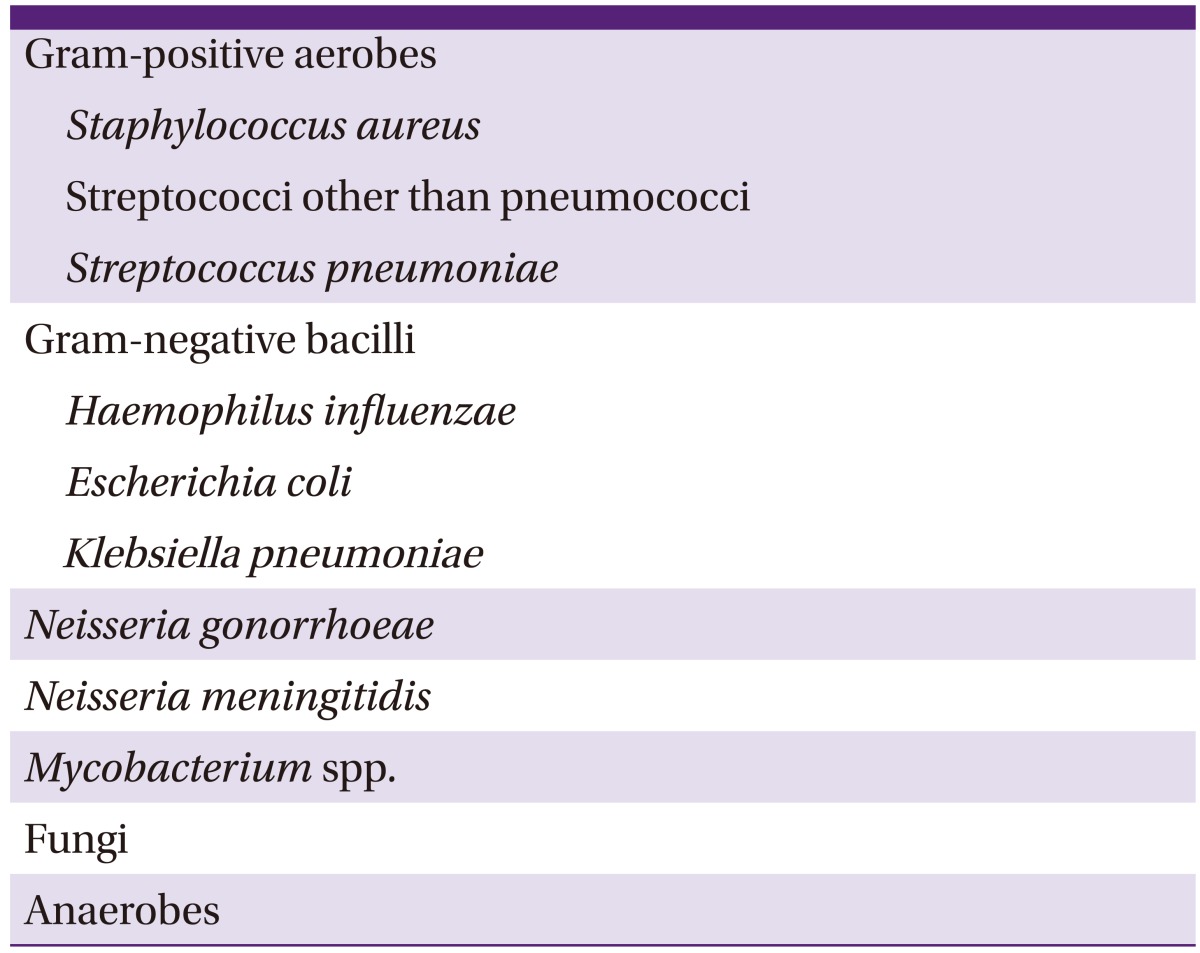
The most common causative pathogens are S. aureus and streptococci, which account for 60-90% of total septic arthritis cases (Table 8) [15, 16, 20, 25, 26]. In retrospective studies in Korea, 43 of 122 septic knee arthritis patients had positive culture results. Of those, 25 (58.1%) were due to S. aureus, while two (4.7%) were due to streptococci. Another Korean study reported that 20 of 80 patients had positive culture results; of those, 10 (50%) were due to S. aureus and four (20%) were due to CoNS [27]. Among young adults who engage in frequent sexual contact, arthritis can result from infection with gonococci, and septic arthritis with trauma can develop due to anaerobic pathogens [13, 28, 29, 30]. When risk factors such as old age, repeated urinary tract infections, recent abdominal surgery history, and immune suppression are present, infection with gram-negative bacilli should be considered [20, 31, 32]. Studies have found that between 4.5% and 64% of septic arthritis cases are culture-negative; the selection of antibiotics for such patients is difficult [19, 33].
Table 8.
Causative pathogens of septic arthritis
3) Clinical opinions
Septic arthritis is associated with symptoms that include a sensation of heat, tenderness, and limited movement at the joint; in most cases, these characteristics progress rapidly within 2 weeks [15, 28]. In cases of low-pathogenic or tuberculous arthritis, symptoms can develop slowly; in arthroplasty infections, the symptoms are mostly acute, although it is possible that symptoms emerge slowly over an extended period [34]. Patients with septic arthritis can also exhibit systemic symptoms, such as fever [15]. Studies have reported that 60% of septic arthritis patients experience fever (over 37.5℃) [13, 15, 16, 35].
Septic arthritis usually occurs in the large joints, especially the knee and hip joints, which accounts for 60% of all cases of septic arthritis [20]. Shemerling and Jeng reported that in prospective studies, 8-27% of patients who complained of monoarticular heating sensation, tenderness, and edema had septic arthritis (according to bacterial culture results) [35, 36]. In most cases, the causative pathogens affected single joints, but approximately 22% of cases had multiple joint involvement. Gonococci and meningococci were the most common pathogens involved in infections in multiple joints [13, 16].
4) Diagnosis
-
Joint fluid analysis
1) In patients with suspected septic arthritis, joint fluid analysis should be immediately conducted before antimicrobial agents are administered (AII).
2) Joint fluid should be collected for Gram staining and culture, and the culture test should be performed using liquid agar, or after centrifuging, agar plates (AII).
3) Septic arthritis patients taking warfarin should still undergo joint fluid analysis (BIII).
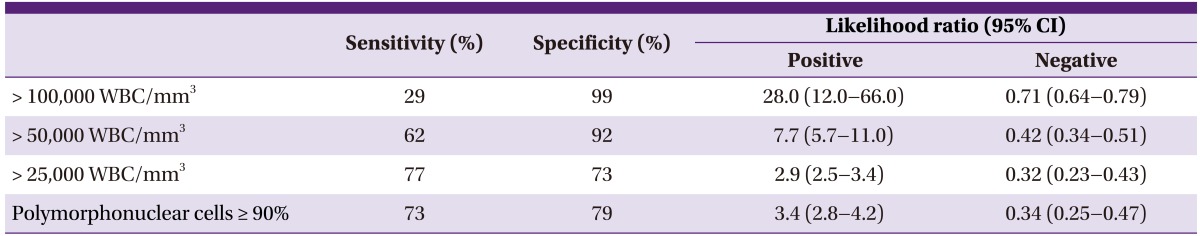
4) Total white blood cell (WBC) and differential counts in joint fluid should be checked (AII) (Table 9).
5) To identify the causative pathogens, a polymerase chain reaction (PCR) can be conducted (CIII).
6) In cases of suspected tuberculosis, an acid-fast bacilli stain, culture, and PCR test can be conducted (BIII).
7) In cases of suspected fungal infection, fungal infection tests can be conducted (BIII).
8) To confirm the crystallization of joint fluid, polarizing microscopy should be performed. The joint fluid should be stored at room temperature (AII). If crystallization of joint fluid is confirmed, septic arthritis still cannot be ruled out.
-
Laboratory tests
1) Uric acid level analysis is not helpful when diagnosing gout or septic arthritis (BII).
2) Before antimicrobial agents are administered, a blood culture test should be conducted (AII).
3) WBC count, CBC, ESR, and CRP tests should be conducted (AII). However, even if WBC count, ESR, and CRP are not increased, septic arthritis may still be diagnosed.
-
Radiology examination
1) Septic arthritis cannot be diagnosed by plain radiography of infected joints; however, such methods can be used for distinguishing septic arthritis from other diseases (BII).
2) MRI is helpful for checking for osteomyelitis and skin and soft tissue infection in areas near septic arthritis, and for determining the need for surgery (BII).
3) A bone scan can be used as an additional method for confirming septic arthritis (BIII).
-
Tissue biopsy and culture
A biopsy and tissue culture should be obtained during irrigation or curettage (AIII).
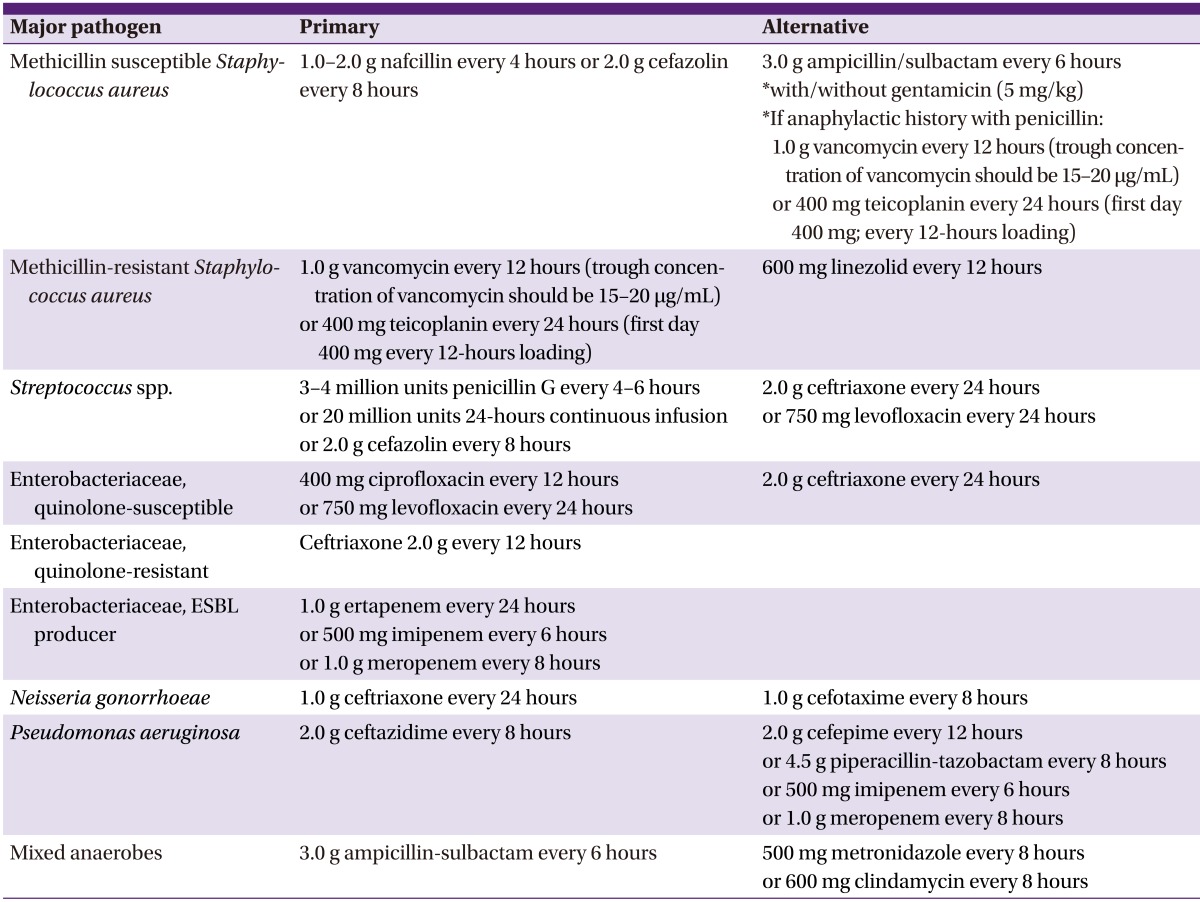
5) Treatment (Table 10, 11, 12)
Table 10.
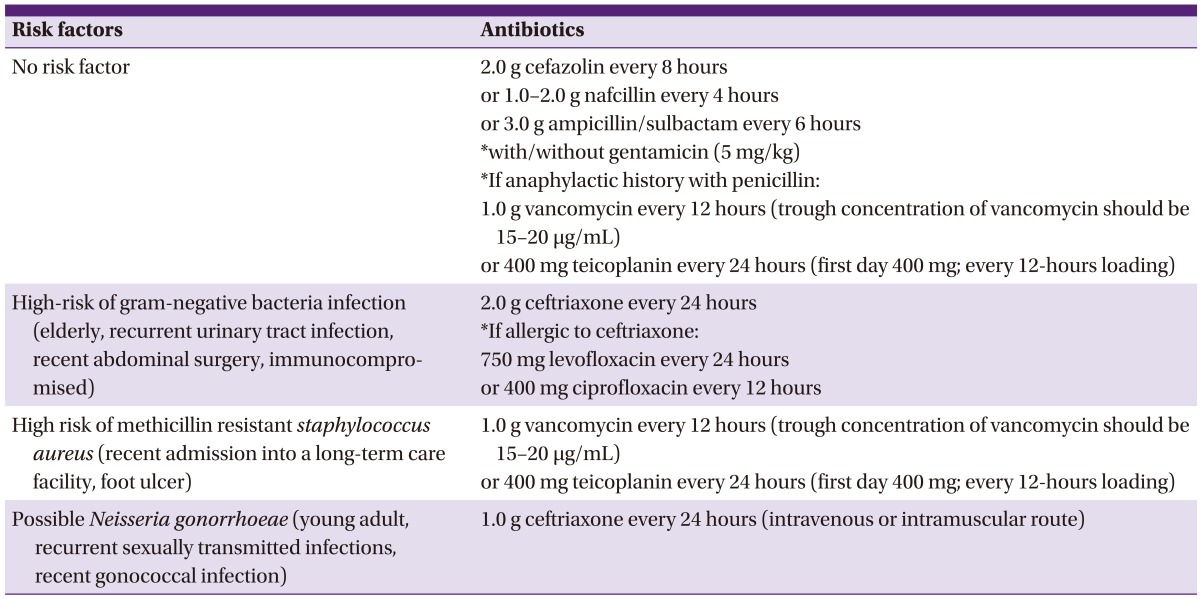
Selection of empirical antimicrobial agents for the treatment of septic arthritis according to risk factors
Table 11.
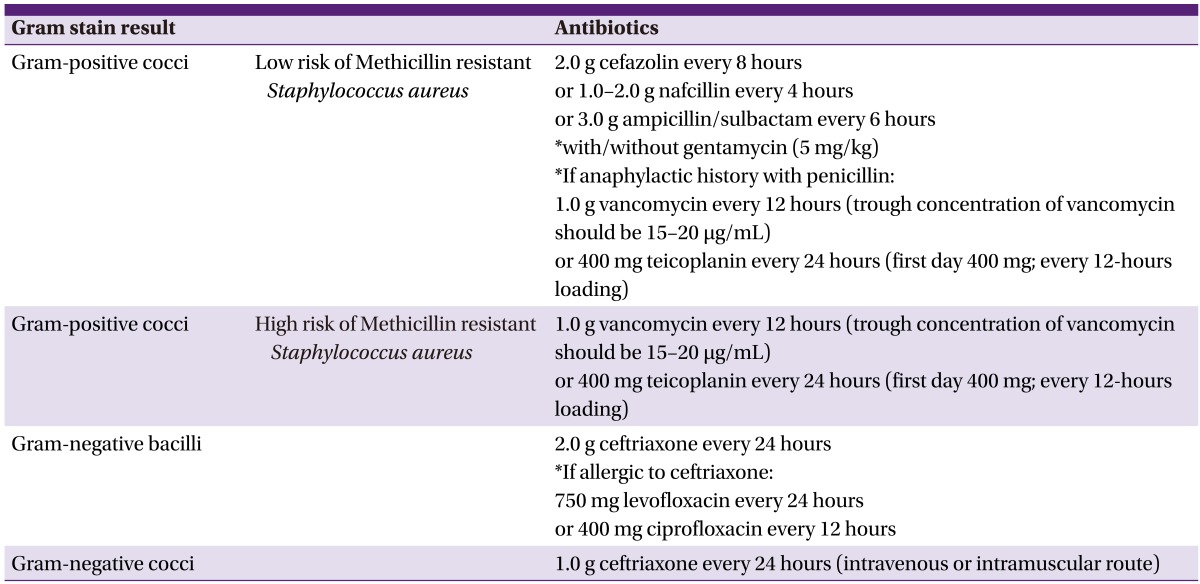
Selection of antimicrobial agents based on Gram stain results
Table 12.
Selection of antimicrobial agents based on the results of bacterial culture and antibiotic susceptibility testing
-
If septic arthritis is suspected, joint fluid and blood culture samples should be collected and empirical - antimicrobial agents should be instituted, and then switched to selective antimicrobial agents according to the results of the Gram stain, as follows:
1) In cases without risk factors, cefazolin, ampicillin/sulbactam, or nafcillin should be used.
2) In cases with risk factors for MRSA infection, vancomycin or teicoplanin should be used.
3) In cases with risk factors for gram-negative bacterial infection, ceftriaxone should be used.
4) In cases with risk factors for gonococcal infection, ceftriaxone should be used.
Bactericidal drugs should be chosen for empirical therapy (BII).
As soon as septic arthritis is diagnosed, sufficient draining should be conducted immediately (AII).
Early joint aspiration is performed for septic joints. After 24 to 48 hours that joint aspiration is done, repeated procedure of joint aspiration and antimicrobial agents therapy will be ineffective. In this case, surgical procedures will be necessary. If joint aspiration is unavailable, surgical procedure will be necessary (AIII).
According to the antibiotic susceptibility of the cultivated strain, targeted antimicrobial agents should be maintained or changed (AII).
In general, the total antimicrobial agents treatment period should be about 4-6 weeks, with injectable antimicrobial agents being adminstered for at least 2 weeks. After 2 weeks, treatment may be switched to oral antimicrobial agents if the symptoms improve (CIII).
By monitoring the results of ESR and CRP tests as indicators of acute infection as well as the clinical manifestations of joint symptoms, the end-point for antibiotic therapy can be determined (CIII).
If the culture result is negative and septic arthritis remains suspected, antimicrobial agents treatment should be maintained. Empirical antimicrobial agents should be continuously administered for as long as joint responses improve. Antimicrobial agents treatment can be terminated based on joint symptoms, ESR, CRP level, and clinical manifestations (BII).
Notes
1. Limitations
Causative pathogens and antibiotic sensitivities can vary according to geographical area and country; therefore, it is important to accumulate abundant data to allow the development of clinical guidelines reflecting the reality of each particular situation. However, these guidelines have been developed with limited data available for review because the domestic literature is scarce. It will be necessary to revise the guidelines after data from ongoing and future studies are analyzed. These treatment guidelines provide standards for the clinical treatment of patients, yet they do not represent an exclusive standard of care, as the treatments applied for each patient may differ depending on the opinion of each individual physician.
2. Plan for updating the guidelines
The plan is to revise these guidelines every three years. Based on the data collected between January 2013 and June 2016, we will update the guidelines with new content in 2016.
3. Potential conflicts of interest
The development of these treatment guidelines was supported by the Korean Society for Chemotherapy. However, the guideline development committee commits to maintaining its objectivity regardless of the origin of the funds provided. No other research funding was received while developing these guidelines. Moreover, no interest group influenced the development of these guidelines.
Supplement
We searched the PubMed (www.pubmed.gov) and KoreaMed (www.koreamed.org) databases for articles and guidelines published between January 1975 and December 2012 using the keywords "Arthritis, Infectious" [Mesh] or "septic arthritis" and "guideline" or "systematic". The committee assessed studies for potential eligibility and selected 138 articles from the literature.
Table 9.
Sensitivity and specificity based on the results of white blood cell (WBC) counts and fractions [37]
CI, confidence interval.
Supplementary material
Guideline Korean version.
Supplementary material can be found with this article online http://www.icjournal.org/src/sm/ic-46-125-s001.pdf.
References
- 1.Carek PJ, Dickerson LM, Sack JL. Diagnosis and management of osteomyelitis. Am Fam Physician. 2001;63:2413–2420. [PubMed] [Google Scholar]
- 2.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Cierny G, Mader JT, Pennick JJ. A clinical staging system for adult osteomyelitis. Contemp Orthop. 1985;10:17–37. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 5.Esposito S, Leone S, Bassetti M, Borrè S, Leoncini F, Meani E, Venditti M, Mazzotta F Bone Joint Infections Committee for the Italian Society of Infectious Tropical Diseases (SIMIT) Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic joint infections in adults. Infection. 2009;37:478–496. doi: 10.1007/s15010-009-8269-2. [DOI] [PubMed] [Google Scholar]
- 6.Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 7.Kim CJ, Song KH, Park WB, Kim ES, Park SW, Kim HB, Oh MD, Kim NJ. Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother. 2012;56:2122–2124. doi: 10.1128/AAC.05953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YI, Kim SE, Jang HC, Jung SI, Song SK, Park KH. Analysis of the clinical characteristics and prognostic factors of infectious spondylitis. Infect Chemother. 2011;43:48–54. [Google Scholar]
- 9.Dirschl DR, Almekinders LC. Osteomyelitis. Common causes and treatment recommendations. Drugs. 1993;45:29–43. doi: 10.2165/00003495-199345010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86-A:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Weston V, Coakley G British Society for Rheumatology (BSR) Standards, Guidelines and Audit Working Group; British Society for Antimicrobial Chemotherapy; British Orthopaedic Association; Royal College of General Practitioners; British Health Professionals in Rheumatology. Guideline for the management of the hot swollen joint in adults with a particular focus on septic arthritis. J Antimicrob Chemother. 2006;58:492–493. doi: 10.1093/jac/dkl295. [DOI] [PubMed] [Google Scholar]
- 12.Coakley G, Mathews C, Field M, Jones A, Kingsley G, Walker D, Phillips M, Bradish C, McLachlan A, Mohammed R, Weston V British Society for Rheumatology Standards, Guidelines and Audit Working Group. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology (Oxford) 2006;45:1039–1041. doi: 10.1093/rheumatology/kel163a. [DOI] [PubMed] [Google Scholar]
- 13.Brook I, Frazier EH. Anaerobic osteomyelitis and arthritis in a military hospital: a 10-year experience. Am J Med. 1993;94:21–28. doi: 10.1016/0002-9343(93)90115-6. [DOI] [PubMed] [Google Scholar]
- 14.Euba G, Murillo O, Fernández-Sabé N, Mascaró J, Cabo J, Pérez A, Tubau F, Verdaguer R, Gudiol F, Ariza J. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother. 2009;53:2672–2676. doi: 10.1128/AAC.01504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford) 2001;40:24–30. doi: 10.1093/rheumatology/40.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Ispahani P, Weston VC, Turner DP, Donald FE. Septic arthritis due to Streptococcus pneumoniae in Nottingham, United Kingdom, 1985-1998. Clin Infect Dis. 1999;29:1450–1454. doi: 10.1086/313526. [DOI] [PubMed] [Google Scholar]
- 17.Rhee YG, Cho NS, Kim BH, Ha JH. Injection-induced pyogenic arthritis of the shoulder joint. J Shoulder Elbow Surg. 2008;17:63–67. doi: 10.1016/j.jse.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Saraux A, Taelman H, Blanche P, Batungwanayo J, Clerinx J, Kagame A, Kabagabo L, Ladner J, Van de Perre P, Le Goff P, Bogaerts J. HIV infection as a risk factor for septic arthritis. Br J Rheumatol. 1997;36:333–337. doi: 10.1093/rheumatology/36.3.333. [DOI] [PubMed] [Google Scholar]
- 19.Seo SS, Ha DJ, Kim CW, Kim KW, Seo JH. Etiologic transition of septic arthritis of the knee. J Korean Knee Soc. 2008;20:44–49. [Google Scholar]
- 20.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58:214–219. doi: 10.1136/ard.58.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiére JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61:267–269. doi: 10.1136/ard.61.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, Howard C. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 23.Millar BC, Loughrey A, Elborn JS, Moore JE. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA) J Hosp Infect. 2007;67:109–113. doi: 10.1016/j.jhin.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007;60:1108–1114. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]
- 25.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker DJ, Young I, Hassey GA, Smith AM, Goring M, Platt PN. The acute hot joint in medical practice. J R Coll Physicians Lond. 1995;29:101–104. [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Kim J, Ihm C. The usefulness of multiplex PCR for the identification of bacteria in joint infection. J Clin Lab Anal. 2010;24:175–181. doi: 10.1002/jcla.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke CL, Owen DS, Jr, Irby R, Toone E. Gonococcal arthritis. A survey of 54 cases. JAMA. 1971;217:204–205. doi: 10.1001/jama.217.2.204. [DOI] [PubMed] [Google Scholar]
- 29.Rompalo AM, Hook EW, 3rd, Roberts PL, Ramsey PG, Handsfield HH, Holmes KK. The acute arthritis-dermatitis syndrome. The changing importance of Neisseria gonorrhoeae and Neisseria meningitidis. Arch Intern Med. 1987;147:281–283. [PubMed] [Google Scholar]
- 30.Wise CM, Morris CR, Wasilauskas BL, Salzer WL. Gonococcal arthritis in an era of increasing penicillin resistance. Presentations and outcomes in 41 recent cases (1985-1991) Arch Intern Med. 1994;154:2690–2695. doi: 10.1001/archinte.1994.00420230077009. [DOI] [PubMed] [Google Scholar]
- 31.Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997;22:2089–2093. doi: 10.1097/00007632-199709150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Kaandorp CJ, Van Schaardenburg D, Krijnen P, Habbema JD, van de Laar MA. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. 1995;38:1819–1825. doi: 10.1002/art.1780381215. [DOI] [PubMed] [Google Scholar]
- 33.Eberst-Ledoux J, Tournadre A, Mathieu S, Mrozek N, Soubrier M, Dubost JJ. Septic arthritis with negative bacteriological findings in adult native joints: a retrospective study of 74 cases. Joint Bone Spine. 2012;79:156–159. doi: 10.1016/j.jbspin.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Gupta MN, Sturrock RD, Field M. Prospective comparative study of patients with culture proven and high suspicion of adult onset septic arthritis. Ann Rheum Dis. 2003;62:327–331. doi: 10.1136/ard.62.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeng GW, Wang CR, Liu ST, Su CC, Tsai RT, Yeh TS, Wen CL, Wu YQ, Lin CY, Lee GL, Chen MY, Liu MF, Chuang CY, Chen CY. Measurement of synovial tumor necrosis factor-alpha in diagnosing emergency patients with bacterial arthritis. Am J Emerg Med. 1997;15:626–629. doi: 10.1016/s0735-6757(97)90173-x. [DOI] [PubMed] [Google Scholar]
- 36.Shmerling RH, Delbanco TL, Tosteson AN, Trentham DE. Synovial fluid tests. What should be ordered? JAMA. 1990;264:1009–1014. [PubMed] [Google Scholar]
- 37.Margaretten ME, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? JAMA. 2007;297:1478–1488. doi: 10.1001/jama.297.13.1478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Guideline Korean version.
Supplementary material can be found with this article online http://www.icjournal.org/src/sm/ic-46-125-s001.pdf.