Abstract
Lectins are clusters of glycoproteins of nonimmune foundation that combine specifically and reversibly to carbohydrates, mainly the sugar moiety of glycoconjugates, resulting in cell agglutination and precipitation of glycoconjugates. They are universally distributed in nature, being established in plants, fungi, viruses, bacteria, crustacea, insects, and animals, but leguminacae plants are rich source of lectins. The present review reveals the structure, biological properties, and application of plant lectins.
Keywords: lectins, leguminous plant, biological possessions, glycoprotein
Introduction
Lectins are omnipresent proteins that are possibly there in all eukaryotic and numerous bacterial species as well as in several viruses. They play a vital function in the plants resistance against insect pests and have been found to be deadly to viruses, bacteria, fungi, insects, and prominent animals. Lectins are sugar-binding proteins that are extremely specific for their sugar molecules. They are carbohydrate-binding proteins of non-immune temperament.1,2 Lectins are extensively dispersed in nature and ample in plants (Table 1). Lectins are established in diverse species of major taxonomical groups and most of as seed storage proteins.3,4 It is usually thought that the initial report of a lectin was given by Peter Stillmark.5 First lectin to be purified on a large scale and accessible on a trade basis is concanavalin A. Lectins also originated in vegetative organs like roots, leaves, rhizomes, bulbs, tubers, corms, stems, bark, flowers, fruits, phloem sap, latex, and nectar.6,7 Plant lectins possess analogous biological activities and chemical properties.8 Lectins authenticate composite association and dissociation reactions, which is manifold form of the same lectins this is called as isolectin, e.g., lentil,9 soybean,10 garden pea,11 wheat germ,12 Griffonia simplicifolia,13 Viccia graminae,14 Madura pomifera,15 Hura crepitans,16 Psophocarpus tetragonolobus,17 and Abrus precatorius.18
Table 1. Listing of plant lectins.
| Lectin icon | Lectin name | Source | |
|---|---|---|---|
| ConA | Concanavalin A | Canavalia ensiformis | |
| GNA | Snowdrop lectin | Galanthus nivalis | |
| RCA | Ricinus communis Agglutinin, | Ricinus communis | |
| AIL | Jacalin | Artocarpus integrifolia | |
| VVL | Hairy vetch lectin | Vicia villosa | |
| WGA | Wheat Germ agglutinin | Triticum vulgaris | |
| SNA | Elderberry lectin | Sambucus nigra | |
| MAL | Maackia amurensis leukoagglutinin | Maackia amurensis | |
| MAH | Maackia amurensis hemoagglutinin | Maackia amurensis | |
| UEA | Ulex europaeus agglutinin | Ulex europaeus | |
| PNA | Peanut agglutinin | Arachis hypogaea | |
| LCH | Lentil lectin | Lens culinaris | |
| GalPhL | Galactose specific lectin | Phaseolus lunatus | |
| ManPSL | Mannose specific lectin | Pisum sativum | |
| GalDBL | Galactose specific lectin | Dolichos biflorus | |
| GalCaL | Galactose specific lectin | Caragana arborescens | |
A variety of the plant lectins reveals the ribosome inactivating property that depurinates the rRNA, thus deleterious ribosomes and arresting the protein synthesis19,20 are called as Ribosome Inactivating proteins (RIPs). The physiological, biochemical, cellular, and molecular characteristics of lectin show its involvement in plant defense mechanism. Lectins are steady over broad pH range, by means of resistant to insect and animal proteases.21,22
The plant lectins combine to glycoconjugates of other organisms.23 Plant lectin has capability to detect molecules in a cell, among cells, or organisms. Lectin has physiological roles to recognize nitrogen-fixing bacteria at the surface of roots and transport of sugars, hormones, and glycoproteins.24,25 Legumes lectins are one of the chief lectin families out of 70 lectins were reported. The legume lectins are a family of carbohydrate binding lectins found in the seeds of plants belonging to the Fabacaea family. The accurate role of the legume lectins in vivo is unknown but they are perhaps implicated in the defense of plants against predators.26
Structure of Plant Lectin
The secondary structure of legume lectin is characterized by 2 anti-parallel β sheets and the presence of strongly bound calcium and manganese ions. In β strands of legume lectin aspatate and aspargine amino acid are highly conserved; these 2 particular amino acids play important role in carbohydrate recognition and participate in hydrogen bonding with the sugar.27
The finest advanced structure of legume lectin is that of concanavaline A (Figure 1). The quaternary structure of concanavalin A is having tetrameric form.28 The quaternary structure of lectins form complex with cell surfaces and matrix glycoconjugates. It contains multiple binding sites, which play important role in the biological activities of plant lectins. The number of vander Waals and hydrogen bonding involve in the dimmers formation. This 2 dimmer rotate in the different angle to each other and shows the different hydrogen bonding scheme on the dimmer–dimmer interface.29
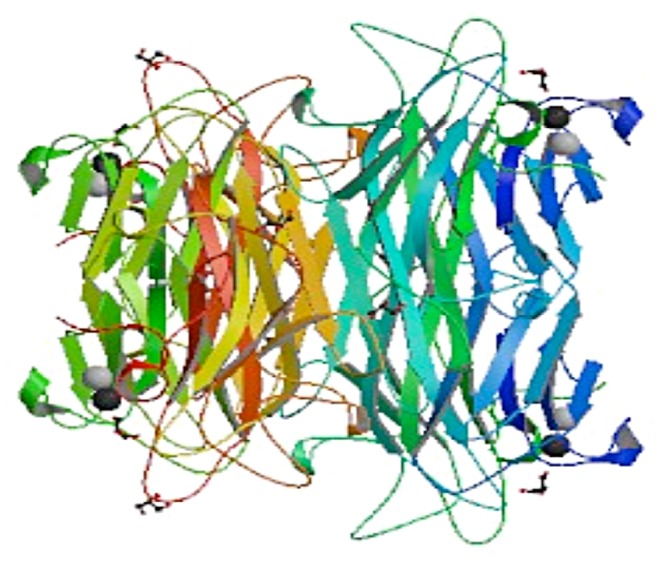
Figure 1. Structure of concanavaline A Canavalia ensiformis.
Biological Properties of Lectins
Lectins has very important role in biomedical research. Lectins are used in expansive applications such as agglutination, mitogenic stimulation, toxicity to cells, inhibition of fungal, bacterial, and viral growth, insecticidal property, anti-HIV property, anti-cancer and nuclease like activity.30-32 Application of lectin in the biomedical field are mitogenic activity on human lymphocytes,33 antifungal activity,34 and activity against the human cancer cell.35 The lectin from Moringa oleifera seeds show coagulant properties, antioxidant activity, and antimicrobial properties.36 The biological possessions of lectins are as follows:
Agglutination
Agglutination is the most effortlessly measurable manifestation of the interface of a lectin with lymphocytes cells. This technique used to identify the occurrence of lectin in a biological source. The aptitude of lectin to agglutinate lymphocytes cells are useful to discriminate between lectins and other sugar binding macromolecules. Agglutination arises while lectin bounds and forms multiple cross bridges among lymphocytes cells. The lectins are blood group specific. The lectins isolated from Sophora japonica, Calpumea aurea, and Dolichos biflorus show specificity to Blood group A and B and Lectin from Erythrina velutina seeds is A, B, and O blood group-specific.37-39 Lectin extracted from Iris amara is M blood group specific and Vicia graminae and Bauhinia purpurea lectins are N blood group specific, while Parkia javanica lectin agglutinates RBC of rabbit and rat.40,41 Haemagglutinating activity of the lectin for human erythrocytes increases when crosslinking of soybean agglutination with glutaraldehyde takes place.42
Mitogenic stimulation of lymphocytes
The earliest mitogenic activity of lectin was demonstrated in PHA from Phaseolus vulgaris. The ordinary spectacular effects in the interface of lectins with lymphocytes cells is mitogenic stimulation, it means that occurrence of dormant, non-dividing lymphocytes from the situation of development and propagation.43 Concanavaline-A found to be the first lectin mitogen whose activity might be eagerly inhibited in a reversible way by low concentrations of simple sugars. A number of lectins have been accepted to have mitogenic activity, which includes Wistaria floribunda, Hura crepitans, Lathyrus sativus, Abrus precatorius, and all of these mitogens are inhibited by simple sugars.44-46
WGA, formerly considered as a non-mitogenic lectin, under appropriate conditions stimulates obviously both human B-lymphocytes47 and T-lymphocytes.48
Induction of suppressor cells
Lectins are competent to provoke the suppressor lymphocytes cells that deter the activities of both T and B-cells. The aptitude to build up suppressor cell activity leading the conduct with Con A, in humans seems to be a variety of normal lymphoid cells. The production of suppressor cells is reduces in side-line blood lymphocytes of patients with immunodeficiency diseases. Lectins offer a basis for using the measurement of mitogen-induced suppressor cell activity as a suitable clinical test for assessing the level of the immune fitness of patients.42
Toxicity
There are a number of lectins viz. Con A, WGA, PHA, and the lectin from Robinia pseudoacacia are toxic to mammalian cells both in vitro and in vivo.49 In meticulous, transformed cells are normally much more sensitive to the cytotoxic effects of lectins than normal cells. The lectins like ricin, abrin, and modeccine are toxic and move down through neuronal processes to the cell body where they inactivate ribosomes resulting in neuronal death.50 The defensive effect of ricin,51,52 Con A,53 and Griffonia simplicifolia lectin54 against tumor growth in experimental animals has been depicted. The shielding effect of ricin and abrin in humans against tumor growth and in grouping with anticancer drugs has also been illustrated.46,55
Cytotoxicity
The phenomenon, lectin dependent cytotoxicity, can be explained by interaction of lectin with killing of target cells by cytotoxic T-lymphocytes through the specific recognition by the affected cells, mediated by lectin. The mitogenic lectins encompasses no immune specificity to both effected and target cells.56,57 The lectins do not participate in the intercellular recognition, but modifying the surface of the target cells.58 Lectins from wheat germ, Ghffonia simplicifolia, hold the capacity to mediate carbohydrate explicit binding of mouse macrophages and tumor cells and to induce killing of the tumor cells by the macrophages.59,60 Lectin mediated cytotoxicity may take place in vivo, by intraperitoneal injection of Griffonia simplicifolia.61
Lectin Phagocytosis
The precise identification concerning phagocytes and their targets can be consummate by using lectins on the surface of cell that unite with definite sugar on the surface of another cell resembling a lock and key model. This type of acknowledgment has been termed as lectinophagocytosis.62 WGA noticeably increases the binding and phagocytosis of bacteria such as Staphylococcus aureus H, Staphylococcus albus, and Micrococcus luteus. Ingestion of the bacteria take place through an encapsulated E. coli cells when attached to human polymorphonuclear leukocytes by bridging with Con A.63
The antifungal activity
Plant lectins binding to carbohydrates present on the surface of the fungal cell wall is promising. The chitin-binding lectins projected to have a role in the plant's protection against fungi from stinging nettle (Urtica dioica) inhibited the growth of Botrytis cinerea, Trickoderma kamatum, and Pkycomyces blakesleeanus.64 WGA inhibited spore germination and hyphal growth of Trichoderma viride which designate that lectin is vital defense protein in plants.65 The plant lectins ease can be considered fungicidal proteins are the chimerolectins well in to the class I chitinases.66 A number of lectins has been purified and many of lectin shows antifungal activity (Table 2).
Table 2. Lectins and inhibited fungi.
| Source of lectin | Fungal species inhibited |
|---|---|
| Amaranthus viridis |
Botrytis cinerea, Fusarium oxysporum |
| Astragalus mongholicus |
Borrytis cinerea, Colletrichum sp, Droschslara turia, Fusarium oxysporum |
| Capparis spinosa | Valsa mali |
| Capsicum frutescens |
Aspergillus flavus, Fusarium moniliforme |
| Curcuma amarissima |
Colectrotrichum cassiicola, Exserohilum turicicum, Fusarium oxysporum |
| Dendrobium findlayanum | Alternaria alternata, Colletrichum |
| Phaselous vulgaris | Mycosphaerella arachidicola |
| Phaseolus coccineus seeds |
Gibberalla sanbinetti, Helminthosporium maydis, Rhizoctonia solani, Sclerotinia sclerotiorum |
| Pouteria torta |
Saccharomyces carevisiae, C. musae, Fusarium oxysporum |
| Talisia esculenta | Microsporum canis |
| Withania somnifera |
Fusarium moniliforme, Macrophomina phaseolina |
The antiviral activity
The plant lectins contribution in inhibiting the viral infection has reported by Balzarini et al., 1992.67 A amount of plant lectins are persuasive inhibitors in vitro of animal and human viruses, which have glycoproteins in their virions. The first report of lectin as a antiviral drug is that D-mannose-specific lectin from Gerardia savaglia to prevent infection of H9 cells with human immunodeficiency virus (HIV)-1. The lectin inhibited syncytium formation in the HTLV and HIV-1 by reacting with the oligosaccharide side chains of the HIV-1 gp120 envelop molecule.68
Lectins like concanavalin A, wheat germ agglutinin, Lens culinaris agglutinin, Vicia faba agglutinin, Pisum sativum agglutinin, and phytohaem (erythro) agglutinin were found to bind to gp120. They were able to inhibit fusion of HIV-infected cells with CD4 cells by a carbohydrate-specific interaction with the HIV-infected cells.69 Plant lectins exhibited anti-coronaviral activity, especially mannose-binding lectins, in severe acute respiratory syndrome coronavirus.70 Banana (Musa acuminata) lectin inhibited HIV replication.71 Extra long autumn purple bean lectin72 and mushroom Russula delica lectin73 are capable to inhibit HIV-1 reverse transcriptase. Lectin is able to inhibit HIV-1 reverse transcriptase. Consequently, lectins are potential drugs for treatment of AIDS. Some plant lectins may have an indirect antiviral role.74
The antibacterial activity
The main function of lectin to prevent microorganism into the cytoplasm and involve in plant defense mechanism. Lectins play vital role in the plant’s defense against bacteria; it is the indirect mechanism that based on interactions of lectin with cell wall carbohydrates or extracellular glycans.75 The second indirect defense mechanism of lectin is the blocking of the movements of normally motile bacteria at the air-water interface by the Datura stramonium seed lectin.76 The binding of plant lectins to bacterial cell wall peptidoglycans reveals that lectins strongly interact with muramic acid, N-acetylmuramic acid, and muramyl dipeptide, lectins involve in the plant's defense against microbes.77 The cell wall of bacteria can recognized the glycoconjugates and carbohydrate-binding proteins on their membrane. The many strains of E. histolytica express a surface lectin that are immunogenic. This lectin is linked by disulfide bonds and is required for adherence to human intestinal epithelial cells and contact-dependent killing of immune effector cells.78 Dietary lectins may also affect the intestinal flora,79 and bacterial lectins in turn can activate intestinal cells80,81 suggested that the b-galactoside binding lectin present in the granular glands around keratinocytes and proposed that skin lectins might mediate mechanisms associated with host defense. Riera et al.82 detected b-galactoside-binding lectins from Bufo arenarum skin. The antibacterial activity of these lectins may provide an effective defense against invading microbes in the amphibian Bufo arenarum.
The anti-insect activity
Lectins attach to the glycan receptors there on the surface facing the insect gut to influence the survival and growth of insect pests.83 There are many purified lectin from different sources show the anti-insect properties, which includes; the chitin-binding lectins from rice (Oryza sativa) and stinging nettle also inhibited larva1 growth of the (Callosobruckus maculates) cowpea weevil.84 It was found that Bauhinia monandra leaf lectin produced mortality in Zabrotes subfaciatus and Callosobruchus maculatus when incorporated into an artificial diet.85 The seeds of leguminous plants (Amburana cearensis, Anadenanthera macrocarpa, Dioclea megacarpa, Enterolobium contortisiliquum, and Piptadenia) are promising sources of primary metabolites, especially lectins and trypsin inhibitors and secondary metabolites with larvicidal activity against A. aegypti with low toxicity to mammals.86 The leguminous crops have also suffered greatly due to various diseases and pests for example, the plant parasitic nematodes. Control of root knot nematode would be possible using lectins.87 The lectins from seeds of Canavalia brasiliensis, Dioclea grandiflora, D. rostrata, and Cratylia floribunda were shown to protect artificial seeds against the beetle Callosobruchus maculatus.88 The plant lectins are the most potent agents against insect pests of variety of crops including wheat, rice, tobacco, and potatoes. The first lectin has been reported to possess insecticidal activity was haemagglutinating lectin (PHA). The presence of PHA, the haemagglutinating lectin in the seeds protects the seeds of Phaseolus vulgaris from the attack of cowpea bruchid beetle, Callosobruckus maculatus.89-92 Maudlin and Welburn93 have been demonstrated that the lectins in the gut and hemolymph of tsetse flies are function as signaling factors of maturation for African trypanosome species. Protease inhibitors, lipoxygenases, α-amylase inhibitors which can inhibit insect digestive enzymes, and carbohydrate binding lectin proteins in plants considered as a potential control mechanisms against herbivorous insects.94,95 The lectin extracted from Arisaema helleborifolium exhibited anti-insect activity toward the second instars larvae of B. cucurbitae.96 The emergence of Helicoverpa armigera larvae into adults inhibited by Dioscorea batatas lectin by avidly binding to larval brush border and peritrophic membrane.97 Majumder et al.98 demonstrated that the lectin of Arum maculatum tuber that causes Lipaphis erysimi and Aphis craccivora to succumb, by binding to the gut brush border membrane vesicle proteins. The lectin extracted from Olneya tesota to midgut glycoconjugates and microvillae of Zabrotes subfasciatus larvae.99 Annona coriacea which is rich in lectin displayed toxicity in Anagasta kuehniella which apparently resulted from a change in the gut membrane environment and consequent disruption of digestive enzyme recycling mechanisms by binding to midgut proteins.100
Antitumor activity
The proper mechanism of the antitumor effect of plant lectins is not clear, although many have been proposed, including reduction of cell division, increasing the number of macrophages, increasing the susceptibility of tumor cells to macrophage attack, serving as a bridge between tumor cells and macrophages, and improving the immuno-competence of tumor-bearing animals. Lectins are potent biomarkers that has been used in a variety of studies such as histochemical, biochemical, and functional techniques for cancer cell characterization.101 It has been suggested that plant lectins may have antitumor and anticarcinogenic activities that could be beneficial in cancer treatment.102,103 Liener (1991)104 first time reported that lectin shows antitumor activity is the soybean agglutinin which capable to inhibit the growth of a transplanted tumor in rats. Xia and Ng105 have reported the dark red kidney bean hemagglutinin exerted an antiproliferative activity toward leukemia L1210 cells. There are some other investigation shows that lectin has excellent antitumor activity that includes Banana lectin retarded proliferation of (L1210) cells and hepatoma (HepG2) cells. Also autumn purple bean lectin inhibited the proliferation of hepatoma HepG2 cells by inducing the production of apoptotic bodies.106,107 Some kinds of plant lectins have been identified which induce apoptosis activity in tumor cells, viz. Viscum album L. and garlic-lectin induced tumor cell apoptosis.108 On the other hand, there was one kind of mushroom lectin with tumor cell apoptosis-inducing activity, isolated from the edible mushroom Kurokawa (Boletopsis leucomelas).109 The fungal lectins exhibit antitumor activities, e.g., Volvariella volvacea against sarcoma cells.110 The lectin from Agrocybe aegerita, which exerts its effects via apoptosis induction and DNase activity.111 The lectin from Agaricus bisporus show antiproliferation activities against human colon cancer cell line and breast cancer cell line, and Tricholoma mongolicum lectin inhibits mouse mastocytoma cells in vitro and sarcoma cells in vivo.112,113
Toxicity of lectin
Elevated concentration of lectin in foods, such as beans, cereal grains, seeds, and nuts may be injurious to human and animals. Unfavorable possessions may restrain nutritional deficiencies, gastrointestinal distress, immune allergic reactions, and food poisoning.114 Lectin can be a source of leptin resistance. Such leptin resistance may decipher into diseases, particularly it could be liable for obesity in humans who have high levels of leptin.115 Lectin as well basis of rigorous inflammation and devastation of epithelial cells, edema, hyperemia, and hemorrhages in the lymphatic tissues are very ordinary. Lectin when injected to animals local necrosis and fatty changes can be detect in the liver tissue whereas hemorrhages are observed in the stomach, the intestinal wall, and other organs.116
Conclusion
Lectin is well-studied plant proteins that subsisted a significant prototype of evenness and variability of their binding regions. Lectin used in extensive relevance such as agglutination, blood typing, mitogenic stimulation, toxicity to cells, inhibition of fungal, bacterial and viral growth, insecticidal property, anti-HIV property, anti-cancer, neurosciences, and recently reported to have nuclease like activity. The high concentration of lectin causes toxicity, food poisoning, and tissue damage and leptin resistance. In this review, we focused on plant lectin, especially legume lectin, its structure, biological activity and applications. Lectin has broad spectrum of biomedical application in cancer therapy, HIV treatment, and now recently neurons study.
Glossary
Abbreviations:
- RIPs
ribosome inactivating proteins
- rRNA
ribosomal RNA
- HIV
human immunodeficiency Virus
- HTLV
Human T lymphotrafic virus
- Con A
Concanavalin A
- WGA
Wheat Germ agglutinin
- PHA
Phytohemagglutinin-
- RBC
Red blood cells
- AIDS
acquired immunodeficiency syndrome
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N. What should be called a lectin? Nature. 1980;285:66. doi: 10.1038/285066b0. [DOI] [Google Scholar]
- 2.Van DammeEJM, PeumansWJ, PusztaiA, BardoczS. Properties and biomedical applications. In: A handbook of plant lectins. Chichester, UK: John Wiley and Sons, 1998. [Google Scholar]
- 3.EtzlerME, LienerIE, SharonN, GoldsteinIJ, eds. Distribution and function of plant lectins. In: The lectins: properties, functions and Application in Biology and Medicine. Orlando, FL USA: Academic Press, 1986: 371-435. [Google Scholar]
- 4.Su LC, Pueppke SG, Friedman HP. Lectins and the soybean-Rhizobium symbiosis. I. Immunological investigations of soybean lines, the seeds of which have been reported to lack the 120 000 dalton soybean lectin. Biochim Biophys Acta. 1980;629:292–304. doi: 10.1016/0304-4165(80)90102-6. [DOI] [PubMed] [Google Scholar]
- 5.StillmarkH. Uber Rizin, ein giftiges ferment aus dem Samen von Ricinus communis L Und einigen anderen Euphorbiaceen. Inaug. Diss, Dorpat, 1888. [Google Scholar]
- 6.Peumans WJ, Van Damme EJ. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–52. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seshagirirao K, Prasad MN. Purification and partial characterization of a lectin from Euphorbia neriifolia latex. Biochem Mol Biol Int. 1995;35:1199–204. [PubMed] [Google Scholar]
- 8.Entlicher G, Koštíř JV, Kocourek J. Studies on phytohemagglutinins III: Isolation and characterization of hemagglutinins from the pea (Pisum sativum L.). Biochemical Biophysica Acta (BBA)- Protein Structure. 1970;221:272–81. doi: 10.1016/0005-2795(70)90267-9. [DOI] [PubMed] [Google Scholar]
- 9.Howard IK, Sage HJ. Isolation and characterization of a phytohemagglutinin from the lentil. Biochemistry. 1969;8:2436–41. doi: 10.1021/bi00834a028. [DOI] [PubMed] [Google Scholar]
- 10.Catsimpoolas N, Meyer EW. Isolation of soyben hemagglutinin and demonstration of multiple forms by isoelectric focusing. Arch Biochem Biophys. 1969;132:279–85. doi: 10.1016/0003-9861(69)90363-4. [DOI] [PubMed] [Google Scholar]
- 11.Entlicher G, Kocourek J. Studies on phytohemagglutinins: XXIV. Isoelectric point and hybridization of the pea (Pisum sativum L.) isophytohemagglutinins. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1975;393:165–9. doi: 10.1016/0005-2795(75)90227-5. [DOI] [PubMed] [Google Scholar]
- 12.Rice RH. Wheat germ agglutinin evidence for a genetic basis of multiple forms. Biochimica et Biophysica Acta (BBA) - General Subjects. 1976;444:175–80. doi: 10.1016/0304-4165(76)90234-8. [DOI] [PubMed] [Google Scholar]
- 13.Wood C, Kabat EA, Murphy LA, Goldstein IJ. Immunochemical studies of the combining sites of the two isolectins, A4 and B4, isolated from Bandeiraea simplicifolia. Arch Biochem Biophys. 1979;198:1–11. doi: 10.1016/0003-9861(79)90389-8. [DOI] [PubMed] [Google Scholar]
- 14.Duk M, Lisowska E, Kordowicz M, Waśniowska K. Studies on the specificity of the binding site of Vicia graminea anti-N lectin. Eur J Biochem. 1982;123:105–12. doi: 10.1111/j.1432-1033.1982.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 15.Bausch JN, Richey J, Poretz RD. Five structurally related proteins from affinity-purified Maclura pomifera lectin. Biochemistry. 1981;20:2618–20. doi: 10.1021/bi00512a039. [DOI] [PubMed] [Google Scholar]
- 16.Barbieri L, Falasca A, Franceschi C, Licastro F, Rossi CA, Stirpe F. Purification and properties of two lectins from the latex of the euphorbiaceous plants Hura crepitans L. (sand-box tree) and Euphorbia characias L. (Mediterranean spurge) Biochem J. 1983;215:433–9. doi: 10.1042/bj2150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kortt AA. Purification and properties of the basic lectin from winged bean seed (Psophocarpus anguina) Biochem Mol Biol Int. 1984;39:243–52. doi: 10.1111/j.1432-1033.1984.tb07946.x. [DOI] [PubMed] [Google Scholar]
- 18.Hegde R, Podder SK. A- and B-subunit variant distribution in the holoprotein variants of protein toxin abrin: variants of abrins I and III have constant toxic A subunits and variant lectin B subunits. Arch Biochem Biophys. 1997;344:75–84. doi: 10.1006/abbi.1997.0177. [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–30. [PubMed] [Google Scholar]
- 20.Olsnes S, Stirpe F, Sandvig K, Pihl A. Isolation and characterization of viscumin, a Toxic Lectin fromViscum album L. (Mistletoe) J Biol Chem. 1982;22:13263–70. [PubMed] [Google Scholar]
- 21.Gatehouse LN, Evans IM, Gatehouse JA, Croy RRD. Characterisation of a rape (Brassica napus L.) extensin gene encoding a polypeptide relatively rich in tyrosine. Plant Sci. 1990;71:223–31. doi: 10.1016/0168-9452(90)90012-D. [DOI] [Google Scholar]
- 22.Kumar MA, Timm DE, Neet KE, Owen WG, Peumans WJ, Rao AG. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J Biol Chem. 1993;268:25176–83. [PubMed] [Google Scholar]
- 23.Rao KV, Rathore KS, Hodges TK, Fu X, Stoger E, Sudhakar D, Williams S, Christou P, Bharathi M, Bown DP, et al. Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J. 1998;15:469–77. doi: 10.1046/j.1365-313X.1998.00226.x. [DOI] [PubMed] [Google Scholar]
- 24.Bohlool BB, Schmidt EL. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974;185:269–71. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- 25.LienerIE, SharonN, GoldsteinIJ. The lectins: properties, functions and Application in Biology and Medicine. Orlando, FL USA: Academic Press, 1986: 600. [Google Scholar]
- 26.Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume lectin structure. Biochim Biophys Acta. 1998;1383:9–36. doi: 10.1016/S0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 27.Sharma V, Surolia A. Analyses of carbohydrate recognition by legume lectins: size of the combining site loops and their primary specificity. J Mol Biol. 1997;267:433–45. doi: 10.1006/jmbi.1996.0863. [DOI] [PubMed] [Google Scholar]
- 28.McCubbin WD, Kay CM. Molecular weight studies on concanavalin A. Biochem Biophys Res Commun. 1971;44:101–9. doi: 10.1016/S0006-291X(71)80164-X. [DOI] [PubMed] [Google Scholar]
- 29.Bouckaert J, Loris R, Poortmans F, Wyns L. The crystal structures of Man(a1-3)Man(a1-O)Me and Man(a1-6)Man(a1-O)Me in complex with concanavalin A. J Biol Chem. 1999;271:16144–50. [Google Scholar]
- 30.Kumar MA, Timm DE, Neet KE, Owen WG, Peumans WJ, Rao AG. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J Biol Chem. 1993;268:25176–83. [PubMed] [Google Scholar]
- 31.Girbés T, de Torre C, Iglesias R, Ferreras JM, Mendez E. RIP for viruses. Nature. 1996;379:777–8. doi: 10.1038/379777b00. [DOI] [Google Scholar]
- 32.Battelli MG, Barbieri L, Bolognesi A, Buonamici L, Valbonesi P, Polito L, Van Damme EJ, Peumans WJ, Stirpe F. Ribosome-inactivating lectins with polynucleotide:adenosine glycosidase activity. FEBS Lett. 1997;408:355–9. doi: 10.1016/S0014-5793(97)00463-8. [DOI] [PubMed] [Google Scholar]
- 33.Maciel EVM, Araújo-Filho VS, Nakazawa M, Gomes YM, Coelho LCBB, Correia MTS. Mitogenic activity of Cratylia mollis lectin on human lymphocytes. Biologicals. 2004;32:57–60. doi: 10.1016/j.biologicals.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Yan Q, Jiang Z, Yang S, Deng W, Han L. A novel homodimeric lectin from Astragalus mongholicus with antifungal activity. Arch Biochem Biophys. 2005;442:72–81. doi: 10.1016/j.abb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Kaur M, Singh K, Rup PJ, Saxena AK, Khan RH, Ashraf MT, Kamboj SS, Singh J. A tuber lectin from Arisaema helleborifolium Schott with anti-insect activity against melon fruit fly, Bactrocera cucurbitae (Coquillett) and anti-cancer effect on human cancer cell lines. Arch Biochem Biophys. 2005;445:156–65. doi: 10.1016/j.abb.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Santos AFS, Luz LA, Argolo ACC, Teixeira JA, Paiva PMG, Coelho LCBB. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009;44:504–8. doi: 10.1016/j.procbio.2009.01.002. [DOI] [Google Scholar]
- 37.Boyd WC, Shapleigh E. Specific Precipitating Activity of Plant Agglutinins (Lectins) Science. 1954;119:419. doi: 10.1126/science.119.3091.419. [DOI] [PubMed] [Google Scholar]
- 38.Etzler ME, Kabat EA. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970;9:869–77. doi: 10.1021/bi00806a022. [DOI] [PubMed] [Google Scholar]
- 39.Stojanovic D, Hughes RC, Feizi T, Childs RA. Interactions of a mammalian beta-galactoside-binding lectin with hamster fibroblasts. J Cell Biochem. 1983;21:119–27. doi: 10.1002/jcb.240210203. [DOI] [PubMed] [Google Scholar]
- 40.Utarabhand P, Akkayanont P. Purification of lectin from Parkia Javanica beans. Phytochemistry. 1995;38:281–4. doi: 10.1016/0031-9422(94)00550-D. [DOI] [Google Scholar]
- 41.Sengupta S, Singh S, Sengupta LK, Bisen PS. Phytolectins: natural molecules with immense biotechnological potential. Indian J Exp Biol. 1997;35:103–10. [PubMed] [Google Scholar]
- 42.LisH, SharonN, BoyerPD, DawidIB, MeisterA, eds. Lectin as molecules and as tools. In: Ann Rev Biochem 1986; 55:35-67. [DOI] [PubMed] [Google Scholar]
- 43.Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–6. [PubMed] [Google Scholar]
- 44.Falasca A, Franceschi C, Rossi CA, Stirpe F. Mitogenic and haemagglutinating properties of a lectinpurified from Hura crepitans seeds. Biochim Biophys Acta. 1980;632:95–105. doi: 10.1016/0304-4165(80)90252-4. [DOI] [PubMed] [Google Scholar]
- 45.Kolberg J, Sletten K. Purification and properties of a mitogenic lectin from Lathyrus sativus seeds. Biochim Biophys Acta. 1982;704:26–30. doi: 10.1016/0167-4838(82)90127-3. [DOI] [PubMed] [Google Scholar]
- 46.OlsnesS, PihlA. Toxic lectin and related protein. In: Cohen P and Van Heyningen S, eds. Molecular action of toxin and Viruses. New York, NY USA: Elsevier Scientific publishing Co, 1982:51:105. [Google Scholar]
- 47.Green DR, Eardley DD, Kimura A, Murphy DB, Yamauchi K, Gershon RK. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur J Immunol. 1981;11:973–80. doi: 10.1002/eji.1830111205. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RJ, Simpson S, Van Epps DE, Chenoweth DE. Wheat germ agglutinin inhibits the C5a receptor interaction: implications for receptor microheterogeneity and ligand binding site. J Leukoc Biol. 1992;52:3–10. doi: 10.1002/jlb.52.1.3. [DOI] [PubMed] [Google Scholar]
- 49.LienerIE, SharonN, GoldsteinIJ. The lectins: properties, functions and Application in Biology and Medicine. Orlando, FL USA: Academic Press, 1986; 660. [Google Scholar]
- 50.Wiley RG, Blessing WW, Reis DJ. Suicide transport: destruction of neurons by retrograde transport of ricin, abrin, and modeccin. Science. 1982;216:889–90. doi: 10.1126/science.6177039. [DOI] [PubMed] [Google Scholar]
- 51.Lin JY, Tserng KY, Chen CC, Lin LT, Tung TC. Abrin and ricin: new anti-tumour substances. Nature. 1970;227:292–3. doi: 10.1038/227292a0. [DOI] [PubMed] [Google Scholar]
- 52.Fodstad O, Pihl A. Synergistic effect of adriamycin and ricin on L1210 leukemic cells in mice. Cancer Res. 1980;40:3735–9. [PubMed] [Google Scholar]
- 53.Shoham J, Inbar M, Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970;227:1244–6. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- 54.Eckhardt AE, Malone BN, Goldstein IJ. Inhibition of Ehrlich ascites tumor cell growth by Griffonia simplicifolia I lectin in vivo. Cancer Res. 1982;42:2977–9. [PubMed] [Google Scholar]
- 55.Lis H, Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- 56.Green DR, Eardley DD, Kimura A, Murphy DB, Yamauchi K, Gershon RK. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur J Immunol. 1981;11:973–80. doi: 10.1002/eji.1830111205. [DOI] [PubMed] [Google Scholar]
- 57.Parker WL, Martz E. Lectin-induced nonlethal adhesions between cytolytic T lymphocytes and antigenically unrecognizable tumor cells and nonspecific “triggering” of cytolysis. J Immunol. 1980;124:25–35. [PubMed] [Google Scholar]
- 58.Bonavida B, Katz J. Studies on the induction and expression of T cell-mediated immunity. XV. Role of non-MHC papain-sensitive target structures and Lyt-2 antigens in allogeneic and xenogeneic lectin-dependent cellular cytotoxicity (LDCC) J Immunol. 1985;135:1616–23. [PubMed] [Google Scholar]
- 59.Kurisu M, Yamazaki M, Mizuno D. Induction of macrophage-mediated tumor lysis by the lectin wheat germ agglutinin. Cancer Res. 1980;40:3798–803. [PubMed] [Google Scholar]
- 60.Maddox DE, Shibata S, Goldstein IJ. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982;79:166–70. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckhardt AE, Goldstein IJ. Occurrence of alpha-D-galactosyl-containing glycoproteins on Ehrlich tumor cell membranes. Biochemistry. 1983;22:5280–9. doi: 10.1021/bi00292a006. [DOI] [PubMed] [Google Scholar]
- 62.Sharon N. 2nd Datta lecture: Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987;7:1–13. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- 63.GailyJA, EdelmanGM. Biopolym. Symp. 1961; 1:367 [Google Scholar]
- 64.Broekaert WF, Mariën W, Terras FRG, De Bolle MFC, Proost P, Van Damme J, Dillen L, Claeys M, Rees SB, Vanderleyden J, et al. Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry. 1992;31:4308–14. doi: 10.1021/bi00132a023. [DOI] [PubMed] [Google Scholar]
- 65.Sá RA, Gomes FS, Napoleao TH, Santos NDL, Melo CML, Gusmao NB, Coelho LCBB, Paiva PMG, et al. Antibacterial and antifungal activities of Myracrodruon urundeuva heartwood. Wood Sci Technol. 2009;43:85–95. doi: 10.1007/s00226-008-0220-7. [DOI] [Google Scholar]
- 66.Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313X.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- 67.Balzarini J, Schols D, Neyts J, Van Damme E, Peumans W, De Clercq E. α-(1-3)- and α-(1-6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991;35:410–6. doi: 10.1128/AAC.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller WE, Renneisen K, Kreuter MH, Schröder HC, Winkler I. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type I to H9 cells and human lymphocytes in vitro. J Acquir Immune Defic Syndr. 1988;1:453–8. [PubMed] [Google Scholar]
- 69.Hansen JES, Nielsen CM, Nielsen C, Heegaard P, Mathiesen LR, Nielsen JO. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS. 1989;3:635–41. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Keyaerts E, Vijgen L, Pannecouque C, Van Damme EJM, Peumans WJ, Egberink H, Balzarini J, Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–87. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swanson MD, Winter HC, Goldstein IJ, Markovitz DM. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem. 2010;285:8646–55. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang EF, Lin P, Wong JH, Tsao SW, Ng TB. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J Agric Food Chem. 2010;58:2221–9. doi: 10.1021/jf903964u. [DOI] [PubMed] [Google Scholar]
- 73.Zhao JK, Wang HX, Ng TB. Purification and characterization of a novel lectin from the toxic wild mushroom Inocybe umbrinella. Toxicon. 2009;53:360–6. doi: 10.1016/j.toxicon.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Sequeira L, Gaard G, DeZoeten G. Interaction of bacteria and host cell walls: its relation to mechanisms of induced resistance. Physiol Plant Pathol. 1977;10:43–50. doi: 10.1016/0048-4059(77)90006-6. [DOI] [Google Scholar]
- 75.Peumans WJ, Van Damme EJM. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–52. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.BroekaertWF, PeumansWJ. Lectin release from seeds of Datura stramonium and interference of the Datura strumonium lectin with bacterial motility. In Bog-Hansen TC, Van Driessche E, eds. Lectins, Biology, Biochemistry, Clinical Biochemistry. Berlin, Germany: Walter de Gruyter, 1986;57-65. [Google Scholar]
- 77.Ayouba A, Causse H, Van Damme EJM, Peumans WJ, Cambillau C, Rougé P. Interactions of plant lectins with the components of the bacterial cell wall peptidoglycan. Biochem Syst Eco1. 1994;22:153–159. doi: 10.1016/0305-1978(94)90005-1. [DOI] [Google Scholar]
- 78.Dodson JM, Lenkowski PW, Jr., Eubanks AC, Jackson TFHG, Napodano J, Lyerly DM, Lockhart LA, Mann BJ, Petri WA., Jr. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J Infect Dis. 1999;179:460–6. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- 79.Pusztai A, Grant G, Spencer RJ, Duguid TJ, Brown DS, Ewen SWB, Peumans WJ, Van Damme EJ, Bardocz S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J Appl Bacteriol. 1993;75:360–8. doi: 10.1111/j.1365-2672.1993.tb02788.x. [DOI] [PubMed] [Google Scholar]
- 80.Grant G, Bardocz S, Ewen SW, Brown DS, Duguid TJ, Pusztai A, Avichezer D, Sudakevitz D, Belz A, Garber NC, et al. Purified Pseudomonas aeruginosa PA-I lectin induces gut growth when orally ingested by rats. FEMS Immunol Med Microbiol. 1995;11:191–5. doi: 10.1111/j.1574-695X.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 81.Marschal P, Herrmann J, Leffler H, Barondes SH, Cooper DNW. Sequence and specificity of a soluble lactose-binding lectin from Xenopus laevis skin. J Biol Chem. 1992;267:12942–9. [PubMed] [Google Scholar]
- 82.Sánchez Riera A, Daud A, Gallo A, Genta S, Aybar M, Sánchez S. Antibacterial activity of lactose-binding lectins from Bufo arenarum skin. Biocell. 2003;27:37–46. [PubMed] [Google Scholar]
- 83.Pusztai A, Bardocz S. Biological effects of plantlectins on the gastrointestinal tract: metabolic consequences and applications. Trends Glycosci Glycotechnol. 1996;8:149–65. doi: 10.4052/tigg.8.149. [DOI] [Google Scholar]
- 84.Huesing JE, Murdock LL, Shade RE. Rice and stiiging nettle lectins: insecticidal activity similar to wheat germ agglutinin. Phytochemistry. 1991;30:3565–8. doi: 10.1016/0031-9422(91)80066-A. a. [DOI] [Google Scholar]
- 85.Macedo ML, das Graças Machado Freire M, da Silva MB, Coelho LC. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae) Comp Biochem Physiol A Mol Integr Physiol. 2007;146:486–98. doi: 10.1016/j.cbpa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Farías G, Brutti O, Grau R, Di Leo Lira P, Retta D, et al. Morphological, yielding and quality descriptors of four clones of Origanum spp. (Lamiaceae) from the argentine littoral region Germplasm bank. Ind Crops Prod. 2010;32:472–80. doi: 10.1016/j.indcrop.2010.06.019. [DOI] [Google Scholar]
- 87.Marban-Mendoza N, Jeyaprakash A, Jansson HB, Damon RA, Zuckerman BM. Control of Root-knot Nematodes on Tomato by Lectins. J Nematol. 1987;19:331–5. [PMC free article] [PubMed] [Google Scholar]
- 88.GatehouseAMR, PowellKS, PeumansWJ, Van DammeEJM, GatehouseJA. Insecticidal properties of plant lectins: their potential in plant protection. In: Pusztai A, Bardocz S, eds. Lectins: Biomedical Perspectives Taylor and Francis, London: 1995,331:35-58. [Google Scholar]
- 89.Huesing JE, Shade RE, Chrispeels MJ, Murdock LL. Alpha-amylase inhibitor, not phytohemagglutinin, explains resistance of common bean seeds to cowpea weevil. Plant Physiol. 1991;96:993–6. doi: 10.1104/pp.96.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirkov TE, Wahlstrom JM, Hagiwara K, Finardi-Filho F, Kjemtrup S, Chrispeels MJ. Evolutionary relationships among proteins in the phytohemagglutinin-arcelin-alpha-amylase inhibitor family of the common bean and its relatives. Plant Mol Biol. 1994;26:1103–13. doi: 10.1007/BF00040692. [DOI] [PubMed] [Google Scholar]
- 91.Schroeder HE, Gollasch S, Moore A, Tabe LM, Craig S, Hardie DC, Chrispeels MJ, Spencer D, Higgins TJV. Bean a -amylase inhibitor confers resistance to the pea weevil (Bruchus pisorum) in transgenic peas (Pisum sativum L.) Plant Physiol. 1995;107:1233–9. doi: 10.1104/pp.107.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabre C, Causse H, Mourey L, Koninkx J, Rivière M, Hendriks H, Puzo G, Samama JP, Rougé P. Characterization and sugar-binding properties of arcelin-1, an insecticidal lectin-like protein isolated from kidney bean (Phaseolus vulgaris L. cv. RAZ-2) seeds. Biochem J. 1998;329:551–60. doi: 10.1042/bj3290551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maudlin I, Welburn SC. The role of lectins and trypanosome genotype in the maturation of midgut infections in Glossina morsitans. Trop Med Parasitol. 1988;39:56–8. [PubMed] [Google Scholar]
- 94.GatehouseAMR, PowellKS, PeumansWJ, Van DammeEJM, GatehouseJA. Insecticidal properties of plant lectins: their potential in plant protection. In: Pusztai A, Bardocz S, eds. Lectins: Biomedical Perspectives Taylor and Francis, London: 1995,331:35-58. [Google Scholar]
- 95.Felton GW, Bi JL, Summers CB,, Mueller AJ, Duffey SS. Potential role of lipoxygenases indefense against insect herbivory. J Chem Ecol. 1994;20:651–66. doi: 10.1007/BF02059605. [DOI] [PubMed] [Google Scholar]
- 96.Kaur M, Singh K, Rup PJ, Saxena AK, Khan RH, Ashraf MT, Kamboj SS, Singh J, Singh JA. A tuber lectin from Arisaema helleborifolium Schott with anti-insect activity against melon fruit fly, Bactrocera cucurbitae (Coquillett) and anti-cancer effect on human cancer cell lines. Arch Biochem Biophys. 2006;445:156–65. doi: 10.1016/j.abb.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 97.Ohizumi Y, Gaidamashvili M, Ohwada S, Matsuda K, Kominami J, Nakamura-Tsuruta S, Hirabayashi J, Naganuma T, Ogawa T, Muramoto K. Mannose-binding lectin from yam (Dioscorea batatas) tubers with insecticidal properties against Helicoverpa armigera (Lepidoptera: Noctuidae) J Agric Food Chem. 2009;57:2896–902. doi: 10.1021/jf8040269. [DOI] [PubMed] [Google Scholar]
- 98.Majumder P, Mondal HA, Das S. Insecticidal activity of Arum maculatum tuber lectin and its binding to the glycosylated insect gut receptors. J Agric Food Chem. 2005;53:6725–9. doi: 10.1021/jf051155z. [DOI] [PubMed] [Google Scholar]
- 99.Lagarda-Diaz I, Guzman-Partida AM, Urbano-Hernandez G, Ortega-Nieblas MM, Robles-Burgueño MR, Winzerling J, Vazquez-Moreno L. Insecticidal action of PF2 lectin from Olneya tesota (Palo Fierro) against Zabrotes subfasciatus larvae and midgut glycoconjugate binding. J Agric Food Chem. 2009;57:689–94. doi: 10.1021/jf802557m. [DOI] [PubMed] [Google Scholar]
- 100.Coelho MB, Marangoni S, Macedo ML. Insecticidal action of Annona coriacea lectin against the flour moth Anagasta kuehniella and the rice moth Corcyra cephalonica (Lepidoptera: Pyralidae) Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:406–14. doi: 10.1016/j.cbpc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Muñoz-Gotera RJ, Hernández-González EO, Mendoza-Hernández G, Contreras RG, Mújica A. Exocytosis of a 60 kDa protein (calreticulin) from activated hamster oocytes. Mol Reprod Dev. 2001;60:405–13. doi: 10.1002/mrd.1103. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki S, Kamakura M, Kusuda R. Isolation of birnavirus from Japanese pearl oyster Pinctada fucata. Fish Sci. 1999;64:343. [Google Scholar]
- 103.KleinRM, AveryJK, eds. Development, structure, and function of salivary glands. In: Oral development and histology. 3rd edition. New York, NY USA, Thieme, 2002:352-79. [Google Scholar]
- 104.Liener IE. From soybeans to lectins: a trail of research revisited. Carbohydr Res. 1991;213:1–5. doi: 10.1016/S0008-6215(00)90592-5. [DOI] [PubMed] [Google Scholar]
- 105.Xia L, Ng TB. A hemagglutinin with mitogenic activity from dark red kidney beans. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:213–6. doi: 10.1016/j.jchromb.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 106.Cheung AH, Wong JH, Ng TB. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine. 2009;16:594–600. doi: 10.1016/j.phymed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 107.Fang EF, Lin P, Wong JH, Tsao SW, Ng TB. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J Agric Food Chem. 2010;58:2221–9. doi: 10.1021/jf903964u. [DOI] [PubMed] [Google Scholar]
- 108.Oka Y, Ben-Daniel B, Cohen Y. Nematicidal activity of powder and extracts of Inula viscosa. Nematology. 2001;3:735–42. doi: 10.1163/156854101753625245. [DOI] [Google Scholar]
- 109.Yu LG, Andrews N, Weldon M, Gerasimenko OV, Campbell BJ, Singh R, Grierson I, Petersen OH, Rhodes JM. An N-terminal truncated form of Orp150 is a cytoplasmic ligand for the anti-proliferative mushroom Agaricus bisporus lectin and is required for nuclear localization sequence-dependent nuclear protein import. J Biol Chem. 2002;277:24538–45. doi: 10.1074/jbc.M203550200. [DOI] [PubMed] [Google Scholar]
- 110.Lin JY, Chou TB. Isolation and characterization of a lectin from edible mushroom, Volvariella volvacea. J Biochem. 1984;96:35–40. doi: 10.1093/oxfordjournals.jbchem.a134826. [DOI] [PubMed] [Google Scholar]
- 111.Zhao C, Sun H, Tong X, Qi Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem J. 2003;374:321–7. doi: 10.1042/BJ20030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Q, Yu LG, Campbell BJ, Milton JD, Rhodes JM. Identification of intact peanut lectin in peripheral venous blood. Lancet. 1998;352:1831–2. doi: 10.1016/S0140-6736(05)79894-9. [DOI] [PubMed] [Google Scholar]
- 113.Yu LG, Fernig DG, Smith JA, Milton JD, Rhodes JM. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993;53:4627–32. [PubMed] [Google Scholar]
- 114.Rattray EAS, Palmer R, Pusztai A. Toxicity of kidney beans (Phaseolus vulgaris L). to conventional and gnotobiotic rats. J Sci Food Agric. 1974;25:1035–40. doi: 10.1002/jsfa.2740250819. [DOI] [PubMed] [Google Scholar]
- 115.Jönsson T, Olsson S, Ahrén B, Bøg-Hansen TC, Dole A, Lindeberg S. Agrarian diet and diseases of affluence--do evolutionary novel dietary lectins cause leptin resistance? BMC Endocr Disord. 2005;5:10. doi: 10.1186/1472-6823-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pusztai A, Clarke EMW, King TP. The nutritional toxicity of Phaseolus vulgaris lectins. Proc Nutr Soc. 1979;38:115–20. doi: 10.1079/PNS19790015. [DOI] [PubMed] [Google Scholar]


