Abstract
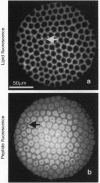
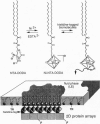
In molecular biology, the expression of fusion proteins is a very useful and well-established technique for the identification and one-step purification of gene products. Even a short fused sequence of five or six histidines enables proteins to bind to an immobilized metal ion chelate complex. By synthesis of a class of chelator lipids, we have transferred this approach to the concept of self-assembly. The specific interaction and lateral organization of a fluorescent fusion molecule containing a C-terminal oligohistidine sequence was studied by film balance techniques in combination with epifluorescence microscopy. Due to the phase behavior of the various lipid mixtures used, the chelator lipids can be laterally structured, generating two-dimensional arrays of histidine-tagged biomolecules. Because of the large variety of fusion proteins already available, this concept represents a powerful technique for orientation and organization of proteins at lipid interfaces with applications in biosensing, biofunctionalization of nanostructured interfaces, two-dimensional crystallization, and studies of lipid-anchored proteins.
Full text
PDF
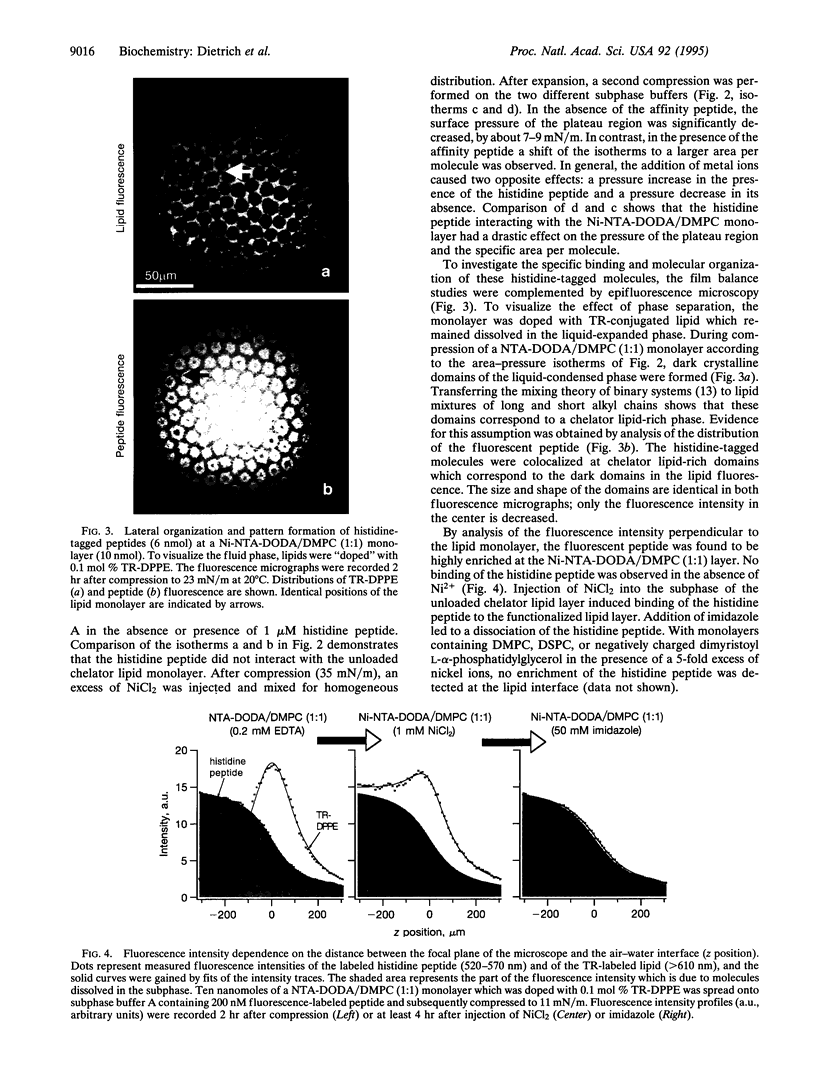
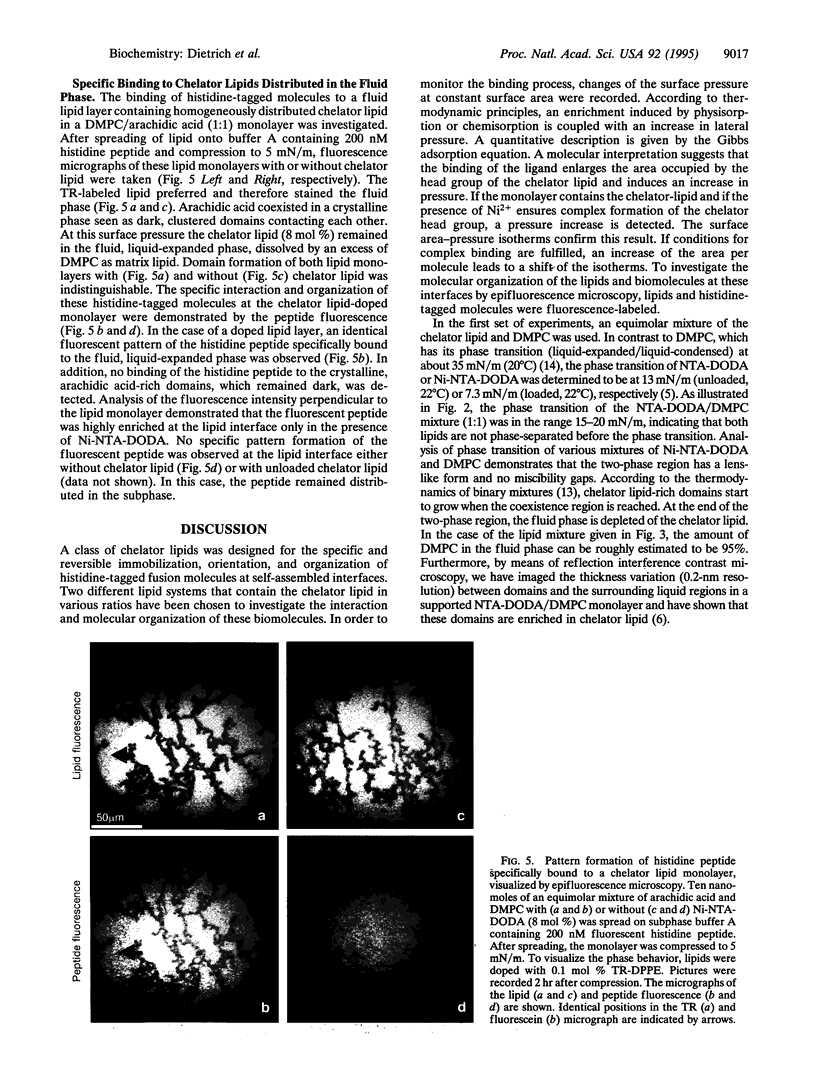
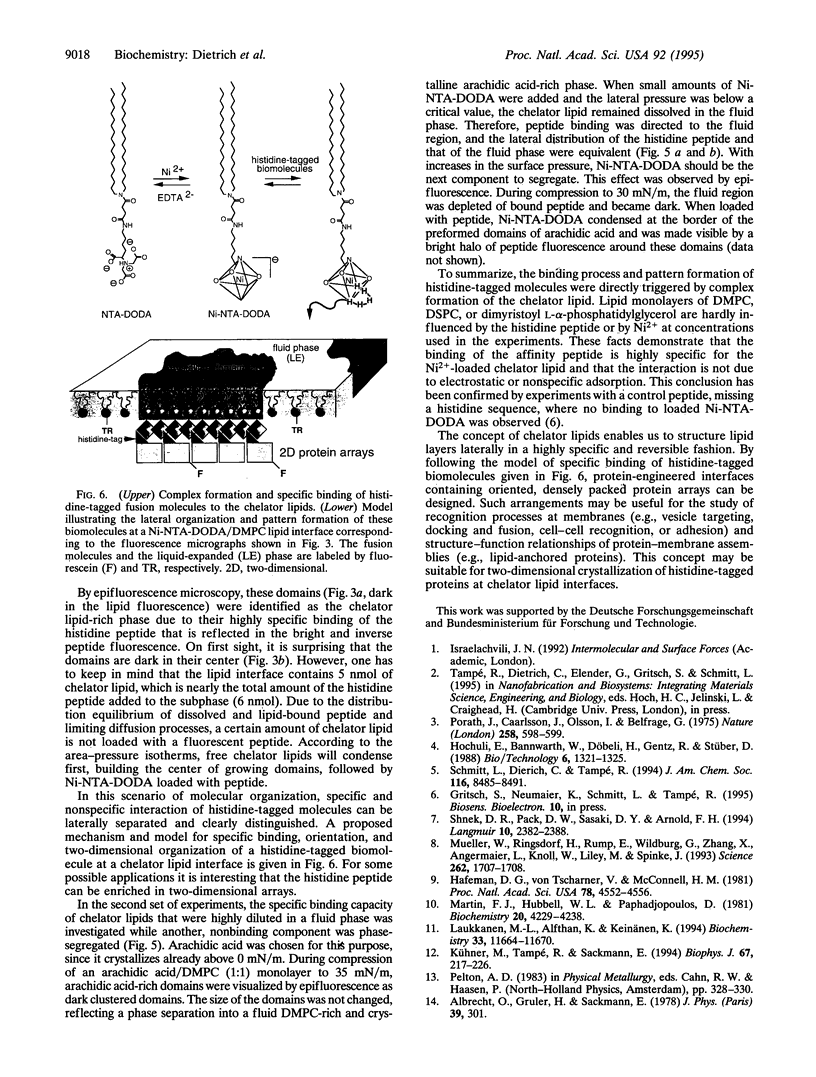
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hafeman D. G., von Tscharner V., McConnell H. M. Specific antibody-dependent interactions between macrophages and lipid haptens in planar lipid monolayers. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4552–4556. doi: 10.1073/pnas.78.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühner M., Tampé R., Sackmann E. Lipid mono- and bilayer supported on polymer films: composite polymer-lipid films on solid substrates. Biophys J. 1994 Jul;67(1):217–226. doi: 10.1016/S0006-3495(94)80472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen M. L., Alfthan K., Keinänen K. Functional immunoliposomes harboring a biosynthetically lipid-tagged single-chain antibody. Biochemistry. 1994 Sep 27;33(38):11664–11670. doi: 10.1021/bi00204a031. [DOI] [PubMed] [Google Scholar]
- Martin F. J., Hubbell W. L., Papahadjopoulos D. Immunospecific targeting of liposomes to cells: a novel and efficient method for covalent attachment of Fab' fragments via disulfide bonds. Biochemistry. 1981 Jul 7;20(14):4229–4238. doi: 10.1021/bi00517a043. [DOI] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]