Abstract
Toxic epidermal necrolysis (TEN) is a rare but serious dermatological emergency characterised by diffuse exfoliation of the skin and mucous membranes due to immune mediated destruction of the epidermis which can lead to sepsis and respiratory distress. Trimethoprim-sulfamethoxazole is a widely used antibiotic which can rarely lead to TEN. Early diagnosis and aggressive medical care is essential for the reduction of high morbidity and mortality associated with this disease. We present a case of successfully recovered TEN due to trimethoprim-sulfamethoxazole in a 62 -year-old woman.
Background
Toxic epidermal necrolysis (TEN) is a rare dermatological condition presenting with characteristic erythematous maculopapular rashes progressing into diffuse exfoliation of skin along with constitutional symptoms and internal organ involvement.1 TEN usually represents a drug-induced, idiosyncratic reaction, but may result from a variety of infections including HIV, Myocoplasma pneumoniae, or may have an unknown aetiology.2 3 The incidence is about 0.4–1.2 cases/million person-years, but the mortality can be as high as 30–40%.4 5
A variety of drugs can cause TEN; most commonly implicated ones are non-steroidal anti-inflammatory drugs, sulfonamides and anticonvulsants.3 In a series of 87 cases of TEN, trimethoprim-sulfamethoxazole (TMP-SMX) was the alleged drug in about 18% of the cases.6 TMP-SMX is a widely used antibiotic and is often recommended as the first-line agent for many infections including urinary tract infections and Pneumocystis carinii pneumonia. Being a commonly prescribed drug, it is important that physicians are aware of this dangerous but potentially treatable adverse effect. Early recognition and treatment of this condition can alter the progression of the disease and save the life of a patient.
In this report, we present a case of a 62-year-old woman who developed TEN without any prodromal symptoms following second dose of oral TMP-SMX. Our case is unique from two aspects, absence of a prodromal symptom and occurrence following the second-dose administration within 24 h.
Case presentation
A 62-year-old woman presented to the emergency department with diffuse erythematous rashes over her body following the second dose of oral TMP-SMX prescribed for uncomplicated lower urinary tract infection. The lesion started as painful erythematous macules, progressed into blisters followed by diffuse exfoliation of the skin involving bilateral lower extremities, back, abdomen and both forearms. The symptoms developed within a few hours. Medical history was significant for hypertension, dyslipidemia, coronary artery disease and coronary artery bypass graft. There was no known history of malignancy. Her daily medications included simvastatin, clopidogrel, aspirin, lisinopril and metoprolol. Patient denied any known drug allergy. She also denied taking any herbal medications.
At presentation patient was afebrile, tachycardic with a pulse rate of 102/min and a blood pressure of 110/76 mm Hg. Physical examination was remarkable for multiple areas of redness, blisters and extensive detachment of skin involving 45% body surface area. Face and oral cavities were spared. Nikolsky sign was positive.
Investigations
Laboratory investigations were significant for a white cell count of 6100/cm3 with normal differentials, haemoglobin of 13.5 g/dL, and a platelet count of 210 000/cm3. Serum electrolytes, urea, creatinine, liver profile, prothrombin time, partial thromboplastic time and International Normalised Ratio were within normal limits. A punch skin biopsy was obtained. Blood, urine and wound cultures sent. Chest X-ray was unremarkable.
Differential diagnosis
Differential diagnosis of multiple blisters with diffuse exfoliation of skin includes TEN/Stevens Johnson Syndrome (SJS), Staphylococcal Scarlet Skin Syndrome (SSSS), Toxic Shock Syndrome (TSS) and bullous disorders of skin including pemphigus and bullous pemphigoid. Usually, patients with TSS and SSSS are febrile and have an elevated white cell count which was not seen in the patient. A diagnosis of TEN was later made in our patient based on the findings of the skin biopsy which was typical for TEN. Pemphigus and pemphigoid could be ruled out based on negative immunofluorescence on skin biopsy.
Treatment
The patient was admitted to the burn unit. TMP-SMX was stopped immediately. The patient was managed with intravenous fluids, prophylactic antibiotics and proper wound care. Electrolytes were replaced as needed. She was monitored continuously for haemodynamic stability. Review of skin biopsy revealed full-thickness epidermal necrosis with subepidermal blister formation and the presence of perivesicular lymphocytes, as well as dermal inflammation (figure 1). Immunofluoroscence test was negative for basilar deposits. This was consistent with TEN.
Figure 1.
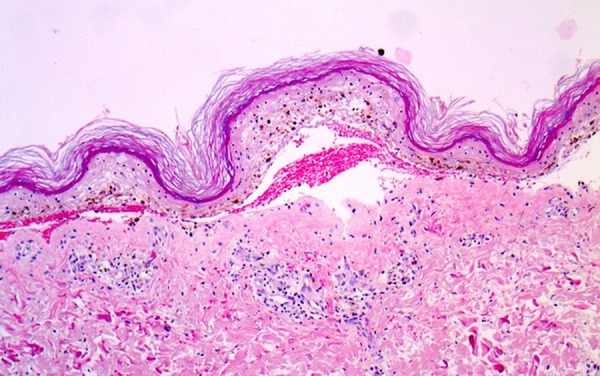
Skin biopsy revealed full-thickness epidermal necrosis with subepidermal blister formation and the presence of perivesicular lymphocytes, as well as dermal inflammation.
Outcome and follow-up
Hospital course was complicated by cellulitis of the right leg secondary to Pseudomonas aeruginosa which responded to intravenous meropenem. She also had central venous catheter-related sepsis due to Acinetobacter baumanni sensitive only to tobramycin. There was an episode of acute respiratory distress syndrome, for which she required ventilatory support for few days. Subsequently, she was extubated and did not require respiratory support afterwards. Her hospital course was further complicated by acute kidney injury and diastolic congestive heart failure which responded to conservative treatment. The patient had remarkable regeneration of skin over subsequent few weeks and she was discharged home in a stable condition after 1 month by which time her skin had healed remarkably.
Discussion
TEN is a rare but severe and potentially fatal dermatological condition characterised by erythematous macules, epidermal detachment, vesicular and bullous lesions involving the skin and the mucous membranes.7 It is part of a spectrum of dermatological conditions involving three variants as per the body surface area (BSA) involved; SJS <10% BSA, SJS-TEN overlap syndrome 10–30% BSA, and TEN >30% BSA.8
Drug induced TEN accounts for upto 80–95% of cases of TEN.3 More than 200 different commonly used medications have been associated with TEN including antibiotics, anticonvulsants, non-steroidal anti-inflammatory drugs, allopurinol and corticosteroids. Of these medications, sulfonamides are known to have the highest association with TEN accounting for upto 30% of the drug-induced cases.9 10 The molecular mechanism by which sulfonamide and other group of medications induce TEN is unknown. TEN is thought to stem from an immunological process involving cytotoxic T cells, natural killer cells and the production of a cytotoxic protein called granulosyn which is aimed at the destruction of the keratinocyte skin resulting in diffuse separation of epidermis from dermis.11 12
Patients with TEN typically present with a prodrome of fever, malaise, headache, sore throat and arthralgias.3 13 The initial skin lesions consist of diffuse erythema with pain, followed by maculopapular lesions progressing to full thickness sloughing of the epidermis and necrosis with diffuse exfoliation. Mucosal involvement like eyes, mouth and vagina are very common. Nikolsky sign is characteristic.3 Loss of protective barrier from denuded skin and damaged mucosa predispose to dehydration and infection leading to sepsis, respiratory distress and shock.7 The diagnosis of TEN is made clinically based on history of drug exposure, prodrome of flu-like illness and sloughing necrotic skin lesions involving more than 30% BSA. It is further supported by histological findings, which includes full-thickness epidermal necrosis, separation of epidermis dermal–epidermal junction, subepithelial bullae with moderate infiltration of the upper dermis by mononuclear cells.14
We made a diagnosis of TEN in our case based on history of drug exposure with a typical clinical manifestation of erythema, blistering and detachment of skin involving >30% of BSA, supported by typical histological findings. TMP-SMX is a well known cause of TEN.6 There was no history of any other new drug or herbal ingestion and the rest of her medications were not reported to cause SJS or TEN. Furthermore, she had used her regular medications for many years without severe skin disease. There was no history of malignancy. The Naranjo probability score was 7, suggesting probable drug reaction.15 Also, the patient developed the reaction after the second dose of antibiotic. In terms of dose relatedness, timing and patient susceptibility (DoTS) classification, this adverse reaction could be classified as Do, hypersusceptibility; T, intermediate; S, not understood.16 However, we did not confirm the reaction with dechallenge/rechallenge testing. Treatment of TEN is similar to burns and, and is largely supportive. Early diagnosis and prompt withdrawal of the causative agent is the key in management and has been shown to improve outcome. Garcia-Doval et al17 showed that mortality rates can decrease from 26% to 5% when causative drugs with short half-lives are withdrawn early. The patients should be treated in burn unit or intensive care unit with meticulous wound care, intravenous fluids, nutritional support, electrolyte balance and active surveillance of sepsis.18 The use of prophylactic antibiotics is not recommended.19 The role of immunosuppressive therapy is poorly defined despite the fact that immunological basis of the TEN is well established.20
Our case had several unique features. TEN usually occurs between 7 days and 8 weeks after drug ingestion, with a mean time of onset ranging from 6 days to 2 weeks.13 Our patient developed TEN after second dose of oral antibiotic within 24 h. TEN may occur within a few hours on re-exposure of the drug.10 Although our patient denied any past allergic reactions to drugs, it is possible that she had a minor allergic reaction to TMP-SMX that was unnoticed. Lipozencic et al2 reported TMP-SMX induced TEN in an 86-year-old man within the first 24 h of drug administration. The patient had a history of allergic reaction to TMP-SMX in the past.2 Guillame et al6 in his series, reported two cases of drug-induced TEN that manifested within 48 h of administration of noramidopyrine and carbamazepine, respectively.21 Arora and Venubabu et al22 reported TMP-SMX induced TEN in a patient with P.carinii pneumonia that developed 48 h after the first dose.22
Severe skin manifestation in the absence of prodromal symptoms is extremely rare in TEN, but is a distinct possibility, which is illustrated in this case. Despite very high mortality rate associated with TEN, the patient had complete resolution of the symptoms with remarkable skin regeneration, which highlights the importance of early diagnosis, appropriate intervention, meticulous wound care and active surveillance of complications to prevent morbidity and mortality.
Learning points.
Toxic epidermal necrolysis (TEN) is a life-threatening adverse drug reaction that can occur secondary to use of a variety of drugs including trimethoprim-sulfamethoxazole.
Diagnosis of TEN is mainly clinical with the history of drug exposure, prodromal symptoms and characteristic sloughing skin lesions.
Early diagnosis and prompt withdrawal of causative agents can significantly reduce the mortality rate.
Antibiotics are most often associated with drug-induced forms of TEN. Judicious use of antibiotics is crucial in preventing such adverse events.
Footnotes
Contributors: JPR and TP came up with the idea of the project. JPR and SG wrote the first draft of the manuscript. VRB edited the final version of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med 2011;39:1521–32 [DOI] [PubMed] [Google Scholar]
- 2.Lipozencic J, Milavec-Puretic V, Kotrulja L, et al. Toxic epidermal necrolysis due to cotrimoxazole. J Eur Acad Dermatol Venereol 2002;16:182–3 [DOI] [PubMed] [Google Scholar]
- 3.See S, Mumford JM. Trimethoprim/sulfamethoxazole-induced toxic epidermal necrolysis. Ann Pharmacother 2001;35:694–7 [DOI] [PubMed] [Google Scholar]
- 4.Lissia M, Mulas P, Bulla A, et al. Toxic epidermal necrolysis (Lyell's disease). Burns 2010;36:152–63 [DOI] [PubMed] [Google Scholar]
- 5.Wolkenstein P, Revuz J. Toxic epidermal necrolysis. Dermatol Clin 2000;18:485–95, ix [DOI] [PubMed] [Google Scholar]
- 6.Guillaume JC, Roujeau JC, Revuz J, et al. The culprit drugs in 87 cases of toxic epidermal necrolysis (Lyell's syndrome). Arch Dermatol 1987;123:1166–70 [PubMed] [Google Scholar]
- 7.Langlois MR, Derk F, Belczyk R, et al. Trimethoprim-sulfamethoxazole-induced Stevens-Johnson syndrome: a case report. J Am Podiatr Med Assoc 2010;100:299–303 [DOI] [PubMed] [Google Scholar]
- 8.Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92–6 [PubMed] [Google Scholar]
- 9.Wolkenstein PE, Roujeau JC, Revuz J. Drug-induced toxic epidermal necrolysis. Clin Dermatol 1998;16:399–408 [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;333:1600–7 [DOI] [PubMed] [Google Scholar]
- 11.Abe R, Yoshioka N, Murata J, et al. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann Intern Med 2009;151:514–15 [DOI] [PubMed] [Google Scholar]
- 12.Saha K, Gupta AK. Toxic epidermal necrolysis: current concepts in pathogenesis and treatment. Indian J Dermatol Venereol Leprol 2000;66:10–17 [PubMed] [Google Scholar]
- 13.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol 2013;69:173.e1–13 [DOI] [PubMed] [Google Scholar]
- 14.Rzany B, Hering O, Mockenhaupt M, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 1996;135:6–11 [PubMed] [Google Scholar]
- 15.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45 [DOI] [PubMed] [Google Scholar]
- 16.Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ 2003;327:1222–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Doval I, LeCleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000;136:323–7 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013;69:187 e1–16 [DOI] [PubMed] [Google Scholar]
- 19.Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol 2012;66:995–1003 [DOI] [PubMed] [Google Scholar]
- 20.Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol 2012;167:424–32 [DOI] [PubMed] [Google Scholar]
- 21.Guillaume J, Roujeau J, Revuz J, et al. The culprit drugs in 87 cases of toxic epidermal necrolysis (lyells syndrome). Arch Dermatol 1987;123:1166–70 [PubMed] [Google Scholar]
- 22.Arora VK, Venubabu K. Cotrimoxazole induced toxic epidermal necrolysis in a suspected case of Pneumocystis carinii pneumonia with human immuno deficiency virus infection. Indian J Chest Dis Allied Sci 1998;40:125–9 [PubMed] [Google Scholar]


