Abstract
Carbon signaling can override carbon supply in the regulation of growth. At least some of this regulation is imparted by the sugar signal trehalose 6-phosphate (T6P) through the protein kinase, SnRK1. This signaling pathway regulates biosynthetic processes involved in growth under optimal growing conditions. Recently, using a seedling system we showed that under sub-optimal conditions, such as cold, carbon signaling by T6P/ SnRK1 enables recovery of growth following relief of the stress. The T6P/ SnRK1 mechanism thus could be selected as a means of improving low temperature tolerance. High-throughput automated Fv/Fm measurements provide a potential means to screen for T6P/ SnRK1, and here we confirm through measurements of Fv/Fm in rosettes that T6P promotes low temperature tolerance and recovery during cold to warm transfer. Further, to better understand the coordination between sugars, trehalose pathway, and temperature-dependent growth, we examine the interrelationship between sugars, trehalose phosphate synthase (TPS), and trehalose phosphate phosphatase (TPP) gene expression and T6P content in seedlings. Sucrose, particularly when fed exogenously, correlated well with TPS1 and TPPB gene expression, suggesting that these enzymes are involved in maintaining carbon flux through the pathway in relation to sucrose supply. However, when sucrose accumulated to higher levels under low temperature and low N, TPS1 and TPPB expression were less directly related to sucrose; other factors may also contribute to regulation of TPS1 and TPPB expression under these conditions. TPPA expression was not related to sucrose content and all genes were not well correlated with endogenous glucose. Our work has implications for understanding acclimation to sink-limited growth conditions such as low temperature and for screening cold-tolerant genotypes with altered T6P/ SnRK1 signaling.
Keywords: Arabidopsis thaliana, trehalose 6-phosphate, sucrose, SnRK1, growth, cold, Fv/Fm, high-throughput screening
The regulation of plant growth is a complex, multifaceted process. Plants assimilate carbon in photosynthesis and utilize this in growth as environmental conditions permit. With the exception of the light environment, sink processes that utilize assimilate are more sensitive to environmental perturbations than photosynthesis. It is clear that sugars not only provide carbon and energy for growth but also determine their own fate through regulation of genes involved in the utilization of sugar in growth. We have already shown the necessary involvement of the trehalose 6-phosphate (T6P)/ SnRK1 mechanism in regulation of growth in response to sucrose.1 This system can be perturbed through misregulation by trehalose feeding to elevate T6P2 and through transgenic modification of trehalose biosynthesis genes3 and overexpression of SnRK1.2 More recently, we showed that the T6P/ SnRK1 signaling mechanism is not only necessary for seedling growth under optimal conditions but is also necessary for recovery from stress. This was manifest in the growth spurt that follows relief of low temperature through priming gene expression by T6P/ SnRK1 during cold for subsequent growth recovery following transfer to warm.4
Given our previous findings in the cold, T6P/ SnRK1 is potentially a mechanism that could be selected for cold tolerance. A challenge is to find a means of rapid screening for this in lines with different T6P contents and SnRK1 activities. Chlorophyll fluorescence is a biological indicator of primary productivity and can be used as a proxy of plant stress. A ratio of variable to maximal fluorescence (Fv/Fm) can be calculated that approximates the potential quantum yield of photosystem II.5 We wished to determine if growth regulation by T6P after recovery from low temperature was also manifest in Fv/Fm. We assess this using an automated plant phenotyping platform.6 Further, we wished to establish more clearly the interrelationship between sugars, cold, and trehalose pathway gene expression in understanding growth responses and to determine if sucrose accumulation under low temperature could account solely for the cold induction of T6P content.
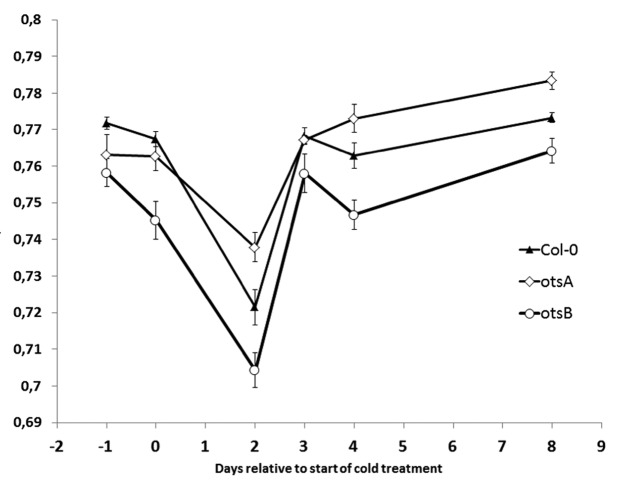
Decrease in Fv/Fm is more marked in otsB than in otsA following exposure to and recovery from cold
Figure 1 shows Fv/Fm in the warm (22 °C) and cold (10 °C) for 2 d and then after returning to 22 °C. Fv/Fm clearly decreases more in otsB than in otsA. otsB has lower T6P than otsA.1,3,6 Therefore the impaired growth recovery already observed4 is also manifest in decreased potential quantum yield, Fv/Fm. Therefore Fv/Fm could be used as part of a phenotyping platform as a rapid screen for cold tolerance in response to changes in T6P/ SnRK1 signaling.
Figure 1. Fv/Fm changes during cold treatment in plant lines with modified T6P content. Plants were grown in 12 h light, 22 °C, 70% humidity, transferred for 2 d to 10 °C, then measured after 4 h recovery at 22 °C. Fv/Fm was measured with GROWSCREEN-Fluoro.8 n = 6–20 plants. Error bars indicate standard error.
Regulation of trehalose pathway gene expression by sucrose
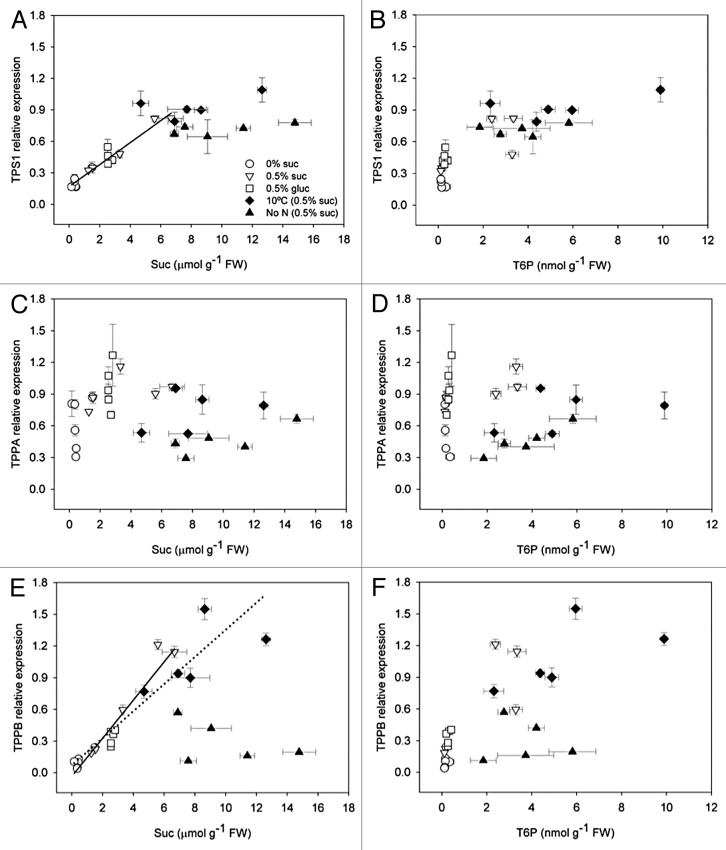
Given the involvement of T6P in growth response to temperature changes, we wished to establish more about how low temperature regulates trehalose pathway gene expression and whether this could be entirely regulated by sucrose or whether there were other potential low temperature inputs regulating trehalose pathway gene expression and T6P content. This understanding is important for determining molecular genetic approaches to modifying the trehalose pathway for cold tolerance. The only TPS enzyme unequivocally known to synthesize T6P is TPS1, so we examined the interrelationship between TPS1 expression and endogenous sucrose levels. We also analyzed the interaction between sucrose and TPPA and TPPB expression. These genes encode enzymes known to be catalytically active in the trehalose pathway.7 Our data show a good interrelationship between sucrose and TPS1 expression when sugars were fed exogenously, but not when sucrose content was altered by low temperature or by low N (Fig. 2A). It may be that TPS1 transcript only increases up to a certain level of sucrose or that other factors under low temperature and low N also regulate TPS1 expression. The interrelationship between TPS1 expression and T6P was very steep up to a level of 2.5 nmol T6P g–1 FW (Fig. 2B). Beyond this T6P was not related to TPS1 expression. TPPA expression did not show any relationship to sucrose or T6P content (Fig. 2C and D). However, TPPB expression correlated well with sucrose both when fed exogenously and when altered by low temperature but not when altered by low N (Fig. 2E). There was a weak correlation between TPPB expression and T6P content for all treatments except low N (Fig. 2F). It is possible there is general upregulation of trehalose pathway gene expression by sucrose at the level of TPS1 and TPPB to catalyze flux of carbon through the pathway in relation to sucrose availability. However, other factors may also regulate the gene expression, particularly under low N and also under low temperature. TPPA expression is likely regulated by factors other than sucrose. Expression of these genes was not well related to endogenous glucose (not presented). Further research will be required to elucidate all the factors that regulate trehalose pathway gene expression and regulation of T6P content when sucrose accumulates under different conditions.
Figure 2. Interrelationship between trehalose pathway gene expression, sucrose, and T6P in response to sugar feeding and sink-limiting treatments that cause sugars to accumulate. (A) TPS1 (At1g78580) and sucrose (correlation for sugar feeding treatments, white symbols only, Pearson’s r = 0.972; P < 0.001); (B) TPS1 and T6P; (C) TPPA (At5g51460) and sucrose (no correlation); (D) TPPA and T6P (no correlation); (E) TPPB (At1g78090) and sucrose (correlation for sugar feeding treatments, white symbols only, solid line, Pearson’s r = 0.960; P < 0.001 and correlation for sugar feeding together with 10 °C treatment, dotted line, Pearson’s r = 0.921; P < 0.001); (F) TPPB and T6P. Horizontal error bars are the SD of 3 independent sucrose or T6P samples, and the vertical error bars are the SD of 3 independent qRT-PCR samples.
Conclusion
The trehalose pathway is an important regulator of gene expression in relation to growth in plants. We show that T6P relates sucrose supply to growth under optimal and suboptimal conditions including low temperature through regulation of gene expression. At low temperature Fv/Fm reflects the growth response of plants with altered T6P/ SnRK1 signaling. This could be used as a rapid screen for T6P/ SnRK1-induced changes in cold tolerance. In terms of understanding T6P homeostasis the regulation of T6P synthesis can be explained through regulation of TPS1 gene expression by sucrose. However, under low temperature and low N this interrelationship becomes nonlinear suggesting a threshold level of sucrose for TPS1 transcript or that other factors also regulate TPS1 gene expression under low temperature and low N. Therefore, while sucrose, T6P, and T6P/ SnRK1-dependent gene expression correlate well, changes in TPS1 expression can be explained only in part by changes in sucrose; other factors as indicators of sink activity or stress may also regulate TPS1 expression and T6P content at other control points under low temperature and low N. Our work has implications for the improvement of adaption of crops to and recovery from sink-limited growing conditions such as low temperature.
Acknowledgments
Rothamsted Research receives strategic funding from the Biotechnology and Biological Sciences Research Council (BBSRC). Nunes C acknowledges funding for her PhD studentship (SFRH/BD/44918/2008) from Fundação para a Ciência e Tecnologia, Portugal. Part of this research was enabled by the Transnational Access capacities of the European Plant Phenotyping Network (EPPN, grant agreement no. 284443) funded by the FP7 Research Infrastructures Programme of the European Union (Paul M and Schluepmann H). We thank S Braun, T Brehm, B Bleise, K Heinz, S Kleinen and A Putz (JPPC) for support with the phenotyping experiment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009;149:1860–71. doi: 10.1104/pp.108.133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delatte TL, Selman MHJ, Schluepmann H, Somsen GW, Smeekens SC, de Jong GJ. Determination of trehalose-6-phosphate in Arabidopsis seedlings by successive extractions followed by anion exchange chromatography-mass spectrometry. Anal Biochem. 2009;389:12–7. doi: 10.1016/j.ab.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2003;100:6849–54. doi: 10.1073/pnas.1132018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunes C, O’Hara LE, Primavesi LF, Delatte TL, Schluepmann H, Somsen GW, Silva AB, Fevereiro PS, Wingler A, Paul MJ. The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol. 2013;162:1720–32. doi: 10.1104/pp.113.220657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilger W, Schreiber U, Bock M. Determination of the quantum efficiency of photosystem II and of non photochemical quenching of chlorophyll fluroescecen in the field. Oecologia. 1995;102:425–32. doi: 10.1007/BF00341354. [DOI] [PubMed] [Google Scholar]
- 6.Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ, Schluepmann H. Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol. 2012;158:1241–51. doi: 10.1104/pp.111.191908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandesteene L, López-Galvis L, Vanneste K, Feil R, Maere S, Lammens W, Rolland F, Lunn JE, Avonce N, Beeckman T, et al. Expansive evolution of the trehalose-6-phosphate phosphatase gene family in Arabidopsis. Plant Physiol. 2012;160:884–96. doi: 10.1104/pp.112.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen M, Gilmer F, Biskup B, Nagel KA, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, et al. Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Funct Plant Biol. 2009;36:902–14. doi: 10.1071/FP09095. [DOI] [PubMed] [Google Scholar]