Abstract
Most of the somatic embryogenesis (SE) process requires the presence, either before or during the embryogenic process, of at least one exogenous auxin. This exogenous auxin induces the presence of endogenous auxins, which appears to be essential for SE induction. We found that during the preincubation period of SE in Coffea canephora, there is an important increase in both free and conjugated indole-3-acetic acid (IAA), as well as indole-3-butyric acid. This increase is accompanied by an increase in the expression of YUCCA (CcYUC), TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (CcTAA1), and GRETCHEN HAGEN 3 (GH3) genes. On the other hand, most of the IAA compounds decreased during the induction of SE. The results presented in this research suggest that a balance between free IAA and its amide conjugates is necessary to allow the expression of SE-related genes.
Keywords: Auxins, GH3, growth regulators, indole-3-acetic acid, somatic embryogenesis
Introduction
Somatic embryogenesis (SE) is a powerful tool for basic studies of the embryogenic process. In addition, when this process is used in combination with cellular and molecular techniques, as well as traditional agricultural techniques, it provides an important mechanism for clonal propagation of crops.
Most of the SE process requires the presence, either before or during the embryogenic process, of at least one auxin. Auxins play an important role during the induction of SE; this is true not just for exogenous auxins, but also for endogenous ones.1 The endogenous auxins appear to be essential, since the use of antiauxins such as p–chlorophenoxyisobutyric acid and inhibitors of their transport during SE induction can produce abnormal embryos.2,3 The rapid SE induction in Medicago falcata is correlated with high indole–3–acetic acid (IAA) and low abscisic acid levels in the initial explants.4 The addition of exogenous auxins stimulates the accumulation of endogenous IAA in Daucus carota.1 During the indirect or direct SE of Acca sellowiana, a short transient pulse of IAA is observed during the third day of culture.5 In Dactylis glomerata, the SE response is correlated with the endogenous auxin content.6 It appears that the increase of endogenous IAA is essential and is closely associated with the mechanism of SE induction.
The increase in the content of endogenous auxins in response to an exogenous auxin was discovered by Andreae and Good.7 The treatment of excised Pisum sativum roots with IAA produces a small increase in the internal pool of IAA, as well as in the concentration of the conjugated N-(indolyl-3-acetyl)-D/l-aspartic acid (IAA-Asp).8 IAA-Asp has also been found in elongating segments of Phaseolus vulgaris incubated with IAA under light.8 It is not only IAA that produces an increase in auxin conjugates: it has been found that the incubation of P. sativum epicotyls with the synthetic auxin 1-naphthaleneacetic acid (NAA) produces an increase in the naphthyl acetyl aspartic acid.9 Despite the requirement for exogenous auxin supplementation for sustained growth, cultured plant cells produce substantial amounts of endogenous IAA.2,10 However, 2,4-dichlorophenoxyacetic acid (2,4-D) and NAA have minor effects on endogenous IAA concentrations in D. carota suspension cultures; NAA was found mostly in the conjugated form.11
Different tissues of D. carota, such as the petiole and hypocotyls, as well as the proliferation of suspension cultures, require exposure from one to several days to 2,4-D in order to induce SE.1,2,12 The addition of 2,4-D and the auxin inhibitor p–chlorophenoxyisobutyric acid modify the endogenous level of IAA and the anatomical composition of early somatic embryos of Abies alba (European silver fir).13 Other plants, such as Coffea canephora,14 Medicago spp.,15,16 and Manihot esculenta Crantz17 also require the addition of an auxin to induce SE.
The increase in IAA conjugates is related to the presence of enzymes with acyl-amidosynthetase activity; they are codified by genes belonging to the GRETCHEN HAGEN 3 (GH3) family.18,19 This family was originally identified in Glycine max as responsive to the presence of auxins,20 and since then, these genes have been identified in different plant species.21 The numbers of members differ between species, from 19 for Arabidopsis thaliana18 to 6 for Carica papaya.21 This family has been divided into 3 groups, according to the substrate that is used by the enzyme.22,23 Group I belongs to genes that code for enzymes that use jasmonic acid as the substrate, Group II comprises enzymes active on IAA, and Group III corresponds to enzymes active on neither of these compounds.
The first report on SE in C. canephora was published by Startsky24 and in C. arabica by Sondal and Sharp.25 Thereafter, several protocols have been described. These can be one step26,27 or a sequential series of steps on different culture media.25,28,29 The induction of SE has been achieved using explants such as plagiotropic and orthotropic buds,24 leaf explants,25,30 integument,31 and anthers and perisperm.32,33 However, the use of leaf tissue explants is the most common, due to the large number of explants achievable and this tissue’s availability throughout the year. Nevertheless, the time required for SE induction from different Coffea species, described in the first protocol, takes between 8 mo and a year.
The culture medium used for induction of coffee SE contains a mixture of auxins and cytokinins. However, SE can be induced using only cytokinins,26,27 because auxins have an inhibitory effect.34 Moreover, Hatanaka et al.35 also showed that using an inhibitor of ethylene synthesis (Co2+, Ag+) affected SE in coffee, suggesting a possible regulatory role for ethylene in SE of this species. Meanwhile, AgNO3 improves embryo production in 5 genotypes of C. canephora, whereas high doses of it has a negative effect.36 Salicylic acid, at low concentrations, also has been found to have a positive effect on the quality and quantity of C. arabica embryos obtained.37 Another compound that has been found to improve SE response in C. canephora as well as C. arabica is the triacontanol (a primary alcohol of 30 carbons).38
In our laboratory, we use young leaves from in vitro-grown C. canephora seedlings, pre-conditioned with NAA and kinetin (KIN) for 2 wk. SE is induced in Yasuda’s medium supplemented with 6-benzyladenine (BA) 5 μM and incubated in the dark. We have shortened the embryogenic response time in SE of C. canephora to 5 wk.14 Since an exogenous auxin is not added during the induction of SE, this system is excellent to study the internal auxins during the induction of SE in C. canephora.
Materials and Methods
Plant materials and growth conditions
Plantlets of C. canephora were obtained from somatic embryos as previously reported14 and cultured in Murashige and Skoog39 (MS; PhytoTechnology Laboratories, M524) medium, supplemented with 29.6 μM thiamine-HCl (Sigma, T3902–25G), 550 μM myo-inositol (Sigma, I5125–500G), 0.15 μM cysteine (Sigma, C-8277), 87.64 mM sucrose (Sigma, S5391) and 0.25% (w/v) gelrite (Sigma, G1910), pH 5.8 and cultured at 25 ± 2 °C under a 16/8h (light/darkness) photoperiod (150 μmol m–2 s–1) (Fig. S1).
Somatic embryogenesis induction
After 40 wk, when the plantlets had 6 pairs of leaves, they were pre-conditioned for 14 d in MS medium supplemented with 0.54 μM NAA (Sigma, N-1145) and 2.32 μM KIN (Sigma, K0753) under the same growth conditions described above. At the end of the preconditioning treatment, the leaves were cut into segments of 0.25 cm2, and 5 explants were incubated in liquid medium as previously described14 in the presence of 5 μM BA (PhytoTechnology Laboratories, B800) and cultured at 25 ± 2 °C in dark conditions at 55 rpm.
Auxins and auxin conjugates extraction
One hundred mg of plantlet tissue were collected from the beginning of preincubation (days 14, 9, and 4 before induction) to the induction day (day zero). Samples were also collected at 30 and 60 min; and 1, 3, 5, 7, 14, and 21 d after the SE induction. The collected tissue was frozen and stored until its use. All the analyses were performed with 3 biological replicates from at least 2 independent experiments.
The frozen tissue was ground with liquid nitrogen and mixed with 1 ml of acid water (pH 2.8). The mixture was transferred to an assay tube with an additional 1 ml of acid water. This mixture was agitated during 1 min, with 1 ml of a solution of butylated hydroxytoluene (Acros Organics, 112992500) in ethyl acetate (1 mg ml−1; CTR Scientific, CTR 00184). Then, 5 ml of ethyl acetate were added and agitated for another min. Three ml of the organic phase were taken and evaporated with nitrogen gas. The dry sample was suspended in 1 ml of the HPLC mobile phase system (60% acetonitrile; J. T. Baker, 9017–03: 40% water containing 0.5% acetic acid; CTR Scientific, 00500) and filtered through a Millipore filter (0.22 μM).
To determine the efficiency of the extraction of IAA and its conjugates, we added a known amount of the compounds previously listed to a tissue sample. The efficiency of extraction was in all cases more than 99% (data not shown).
High-Performance Liquid Chromatography (HPLC)
Twenty μl of extract, as previously described, were injected into an HPLC system. Compounds in the tissue extracts were chromatographed by isocratic elution with a flow rate of 0.6 ml min–1 on a 250 mm × 4.6 mm reverse phase C18 column (Phenomenex). The chromatographic system (Agilent Technologies 1200) consisted of a quaternary system of pumps (Agilent Technologies G1311A) connected to an automated sample injector (Agilent Technologies G1329A). The injected samples were detected with a fluorescent detector (Agilent Technologies G1321A) using an emission wavelength of 280 nm and an excitation wavelength of 340 nm.
The retention times and height peaks of IAA (Fluka, 45533), indol-3-butyric acid (IBA; Sigma, 57310), NAA, IAA conjugated with glutamic acid (IAA-Glu) and IAA conjugated with alanine (IAA-Ala) were used to determine their presence in the analyzed samples. The IAA conjugates were prepared as described below.
Gas Chromatography-Mass Spectrometry (GC-MS)
In order to confirm the identity of the peaks, we used a GS-MS system. GC-MS analysis was performed with a GC (model 68890N, Agilent Technologies) fitted with a DB-1701 capillary column (30 min × 0.25-mm i.d.; J&W Scientific) coupled to a mass selective detector (model 5975B, Agilent Technologies). The GC-MS was controlled by the Chemstation software (Agilent version). For all the analysis, the injector temperature was 220 °C and the helium carrier gas was set at a flow rate of 1.5 ml min–1. The temperature program for the GC oven started at 150 °C with a 3 min hold and increased 10 °C min–1 to 280 °C with an 11 min hold.
The calibration curves were made for each standard, both free and conjugate auxins, using the peak area of each compound to calculate the amount of each compound in the samples.
Synthesis of auxin conjugates
Indoleacetyl-l-alanine acid
For the synthesis of IAA-Ala, 3.36 mmol (300 mg) of l-alanine acid, 3.7 mmol (712 mg) of p-toluenesulfonic acid (PTSA), and 13.4 mmol (1.4 ml) of benzyl alcohol were stirred with 5.6 ml toluene until homogenization. The mixture was heated under reflux for 5 h in a nitrogen atmosphere. The solvent was evaporated and a mixture of cold benzene and ethyl ether (1:1) was added for the crystallization of the IAA-Asp. The yield of the product, the sulfonic salt of the l-alanine benzyl ester, was 36%.
In a round-bottomed flask were dissolved 4.3 mmol (1,500 mg) of the sulfonic salt of the L-alaninedibenzyl ester, previously synthesized, and 2.85 mmol (385 mg) of hydroxybenzotriazole in 15 ml of dry tetrahydrofuran (THF). The flask was introduced into an ice bath, and 3.42 nmol (705.6 mg) of N,N'-dicyclohexylcarbodiimide (DCC) was added and the reaction proceeded during 1 h at 0 °C. The mixture was kept at 0 °C for the addition of 2.85 mmol (500 mg) of IAA and 4.3 mmol (0.6 ml) of triethanolamine (TEA); the reaction was performed for 60 min. Then, the temperature was allowed to rise to room temperature, and the mixture was stirred for 12 h. The mixture was filtered through a sintered-glass funnel, using ethyl acetate to wash the product. The solvent was removed under reduced pressure and then the remaining material was absorbed in silica gel and purified through a silica gel column using a gradient of ethyl acetate/hexanes as eluent. The product, the IAA-Asp dibenzyl ester (772 mg), elutes at 30% of ethyl acetate/70% hexanes. The yield of the product was 80%.
In a round-bottomed flask, 1.49 mmol (500 mg) of the acid (indole-3-acetyl)-l-alanine was dissolved in 29 ml of ethyl acetate. 0.074 mmol (158.1 mg) of Pd/C 5% (w/v) was added, and the mixture was placed in a hydrogen atmosphere. The mixture was stirred for 5 h. The crude material was filtered through a celita bed; the solvent was eliminated and a solid beige compound, the IAA-Asp (286.6 mg), was obtained with a yield of 78%. The product was characterized by 1H NMR and GC-MS. The characteristics of the compound for the 1H NMR (300 MHz, DMSO) were: δ 10.82 (s, 1H), 8.22 (d, J = 7.1 Hz, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.16 (s, 1H), 7.09 – 6.98 (m, 1H), 6.97 – 6.87 (m, 1H), 4.32 – 4.06 (m, 1H), 3.50 (s, 2H), 1.36 – 1.16 (m, 3H).
Indole-3-acetyl-l-glutamic acid
For the synthesis of IAA-Glu, 1.6 mmol (300 mg) of hydrochloride l-glutamic acid, 3.6 mmol (862 mg) of PTSA, and 13.1 mmol (1.7 ml) of benzyl alcohol were stirred in 4 ml benzene until homogenization. The mixture was heated under reflux for 5 h. The solvent was evaporated and a mixture of benzene and ethyl ether (1:1) was added to crystallize the IAA-Glu. The yield of the product, the sulfonic salt of the L-glutamic dibenzyl ester, was quantitative.
In a round-bottomed flask, 0.85 mmol (427.7 mg) of the sulfonic salt of the L-glutamic dibenzyl ester was dissolved. This material was previously synthesized with 0.57 mmol (77.2 mg) of hydroxybenzotriazole in 3 ml of THF. The round-bottomed flask was introduced into an ice bath, and 0.68 nmol (141 mg) of DCC was added. The reaction proceeded for one hour at 0 °C. The mixture was kept at 0 °C for the addition of 0.57 mmol (100 mg) of IAA and 0.86 mmol (0.12 ml) of TEA; the reaction continued for 15 min, and then the temperature was raised to that of the environment and the mixture was stirred by 12 h. The mixture was filtered through a sintered-glass funnel, using ethyl acetate to wash the product. The organic layer was washed with HCl 10% (2 × 10 ml), K2CO3 5% (w/v) (2 × 10 ml), and brine (3 × 10 ml). The organic phase was dried with anhydrous MgSO4 and filtered, and the solvent was removed under reduced pressure. The remaining material was adsorbed on silica gel and purified through a silica gel column using a gradient of ethyl acetate/hexanes as eluent. The yield of the product, the IAA-gludibenzyl ester, was 66%.
In a round-bottomed flask, 0.206 mmol (100 mg) of IAA-gludibenzyl ester was dissolved in 4.1 ml of ethyl acetate. Then, 0.0103 mmol (21.9 mg) of Pd/C 5% (w/v) was added, and the mixture was placed under a hydrogen atmosphere. The mixture was stirred for 5 h at room temperature. The product of the reaction was purified in a celita bed; the solvent was eliminated and a beige solid compound, the IAA-Glu (28.4 mg), was obtained with a yield of 48%. The product was characterized by 1H NMR and GC-MS. The characteristics of the compound for the 1H NMR (300 MHz, DMSO) were δ 10.82 (s, 1H), 8.23 (d, J = 7.9 Hz, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.15 (d, J = 1.9 Hz, 1H), 7.09 – 6.97 (m, 1H), 6.97 – 6.86 (m, 1H), 4.18 (td, J = 7.9, 4.8 Hz, 1H), 3.52 (s, 2H), 2.28 – 2.20 (t, 2H), 2.00 – 1.86 (m, 2H), 1.83 – 1.69 (m, 2H).
Sequence analysis and primers design
To design the primers to amplify the biosynthesis and conjugation auxins of related genes in C. canephora, the selection of nucleotide sequences was performed using the following databases: TAIR, http://www.arabidopsis.org/; Sol Genomic Network, http://solgenomics.net/; and NCBI, http://www.ncbi.nlm.nih.gov/. Alignment of nucleotides in multiple sequences was performed using the software BioEdit sequence alignment Editor 5.0.640 and the CLUSTAL W 1.82 software.41 The design of primers for GH3 genes, GH3.1, GH3.6, GH3.17, and TAA1, YUCCA1, and YUCCA3 was performed using Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi), and then they were analyzed using the online program Oligo Analyzer 3.1 (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/Default.aspx). The primers used are aligned on conserved regions or domain features of the encoded proteins by the above-mentioned genes. Primers generated for qPCR and RT-PCR are listed in Table S1.
The PCR products were cloned in the pGEM-T vector (Promega, USA) using competent Escherichia coli INVαDH10b cells according to kit instructions and sequencing. The sequences for GH3.1 (U625024), GH3.6 (U615082), and GH3.17 (U613253) are in the Sol Genomics network bank. The new sequences reported in this research have been deposited in the Gen-Bank database [accession numbers KF605330 (CcYUC1), KF605331 (CcYUC3), and KF605332 (CcTAA1)].
RNA extraction
Total RNA was extracted from 0.1 g sample tissue (between 10 and 12 circle explants) using the BRLTrizol reagent (Invitrogen) and repurified with the QiagenRNeasy Mini Kit following the manufacturer’s instructions. Reverse transcriptase reactions were performed in a 20 μl volume containing 2 μg of total RNA and 200 units of the M-MLV Reverse Transcriptase (Invitrogen), following the manufacturer’s instructions. cDNA templates for both PCR and qRT-PCR amplification were prepared from 3 individual samples for each condition.
qPCR analysis
Each reaction contained 50 ng of cDNA template, 10 μM of each primer, and 1 × EXPRESS SYBR® GreenERTMqPCRSuperMix Universal (11784–200-Invitrogen). Real-time PCR assays were performed in a StepOneTM Real-Time PCR System (Applied Biosystems) under the following conditions: for GH3.1 and TAA1 94 °C for 5 min, followed by 20 cycles of 94 °C for 1 min, 51.3 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min; GH3.6, GH3.17, and YUC3 94 °C for 5 min, followed by 20 cycles of 94 °C for 1 min, 55.6 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min; ACTIN and YUC1 94 °C for 5 min, followed by 20 cycles of 94 °C for 1 min, 63 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min. Transcript levels of GH3.1, GH3.6, GH3.17, TAA1, YUC1, and YUC3 in the samples were normalized to the level of ACTIN and the data was expressed as the relative expression level. The specificity of the PCR product amplifications was determined by a melting curve analysis. Data obtained from real-time PCR were used to calculate the relative quantification of the target gene expression and compared with the expression of the ACTIN using the 2-ΔΔct method.42 Statistical analysis of the differences between the mean values was performed using the Tukey test, and the differences were considered to be significant at P < 0.05.
RT-PCR analysis
The cDNA for the RT-PCR was prepared as mentioned earlier. For each studied gene, the PCR reaction mixture contained Platinum Taq polymerase (1.25 U, Invitrogen), 10 mM dNTPs, and 10 μM each primer (listed in Table S1) in 25-μl volume. The conditions of the reaction were as follows: for GH3.1 and TAA1 94 °C for 5 min, followed by 25 cycles of 94 °C for 1 min, 51.3 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min; GH3.6, GH3.17, and YUC3 94 °C for 5 min, followed by 25 cycles of 94 °C for 1 min, 55.6 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min; ACTIN and YUC1 94 °C for 5 min, followed by 25 cycles of 94 °C for 1 min, 63 °C for 1.30 min, 72 °C for 1 min, and a final cycle of 72 °C for 5 min. The PCR products were electrophoresed in a 1.5% agarose gel and stained with GelRed (Biotium, 41003) and the images were acquired using the Gel Doc™ XR + System (BIO-RAD). Each RT-PCR was conducted with 3 biological replicates.
In order to confirm that each primer leads to the determination of each specific gene, we cloned the purified PCR product of each gene and sequenced it. The sequence for each gene matched each expected gene (data not shown).
Statistical analysis
The data were processed and analyzed using an analysis of variance (ANOVA) program. The significance grade between the mean values was determined using the Tukey test. Differences were considered to be significant at P ≤ 0.05. Data were analyzed by Origin 8 (Data Analysis and Graphing Software).
Results
Auxins play an important role during the induction of SE. This is true not only for the exogenous auxins but also for the endogenous auxins.1 However, information regarding the kinetics of the auxins and their conjugates during the induction of SE is very limited. The induction of SE from explants of C. canephora depends on the pretreatment of the plantlets with NAA and KIN for 14 d before the explants are placed under SE induction conditions.14 SE, as previously described, is very efficient (Fig. 1). The first globular embryogenic structures appear after 21 d in the induction medium. After 56 d, the number of embryogenic structures reached an average of 150 ± 20.

Figure 1. Induction of the somatic embryogenesis in C. canephora. The SE process was induced as has been reported previously.14 Each picture was taken after 7, 14, 21, 35, 42, and 56 d after SE induction. Plantlets were cultivated in vitro in MS medium, and supplemented with 0.54 μM NAA and 2.32 μM KIN for 2 wk before the induction of SE. After the preconditioning period, the explants were incubated in liquid Yasuda medium14 supplemented with 5 μM BA.
First, we established that under the HPLC running conditions, the retention times for the standards of IAA, IBA, and NAA were 5.143, 6.278, and 7.51 min, respectively (Fig. S3). The retention times for the IAA-Glu and IAA-Asp standards were 3.909 and 4.352 min, respectively. The minimum detected amount of free or conjugated auxins was 0.25 pmoles. This amount is in the range previously reported by Castillo et al.43 using a HPLC technique.
Using 100 mg of leaf tissue (between 10 and 12 circle explants), the area under the peak (5.018 min, IAA) was 13.92. Since auxins oxidize very rapidly, we used 1 mg l–1 of 2, 6-di-t-butyl-4-methylphenol (BHT) in order to prevent this.44 We also determined that the amount of tissue used was in the range of sensitivity of the method. In order to determinate the best amount of material, we used 100, 300, and 500 mg of foliar tissue from the C. canephora seedlings cultivated in vitro. This amount of tissue is in the range reported by other authors.45 The areas under the peak of each of these amounts were 73.35, 282.8, and 341.73, respectively. The correlation coefficient of the graph was 0.97, suggesting that a positive correlation between the size of the sample and the amount of IAA exists.
Previously, we used GC-MS as described in Materials and Methods, in order to identify the different auxins present in the tissues. We identified 4 auxins and derivatives in the control tissues, IAA, IBA, IAA-Glu, and IAA-Ala and, as expected, NAA in the treated samples. It is possible that there are other auxin derivatives in the tissues, but they are under the detection level of the method. The fragmentation pattern for pure IAA, after electron impact ionization, was the same as that previously reported.46-48 The expected quinolinium ion with an m/z of 130, as well as the other expected fragments, were present in IAA (Fig. S4) and its IAA-Glu conjugate (Fig. S5).
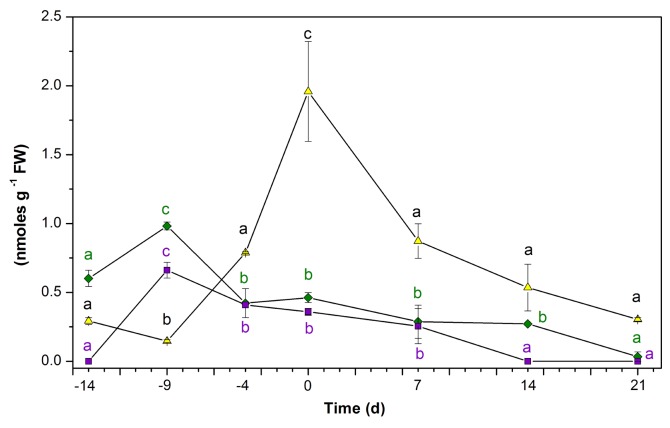
The measurement of the auxins NAA, IAA, and IBA, found in the plant after incubation, yielded very interesting results (Fig. 2). The endogenous concentration of NAA reached 0.359 nmoles g–1 FW after 14 d in the preconditioned culture medium. The IBA was present in the explants before incubation in the preconditioned medium (0.601 nmoles g–1 FW); this concentration decreased to 0.462 nmoles g–1 FW after 14 d of preconditioning. On the other hand, the initial IAA amount (0.292 nmoles g–1 FW) increased to 1.958 nmoles g–1 FW by the end of the preincubation period (Fig. 2). When the explants were transferred to the SE induction medium, all 3 free auxins decreased. Both NAA and IBA decreased to amounts under the detection limit. However, IAA decreased very sharply, to 0.303 nmoles g–1 FW; this amount of IAA is more than 6 times less than at the beginning of SE induction (Fig. 2).
Figure 2. Endogenous auxin content before and during the induction of somatic embryogenesis in C. canephora. Endogenous IAA (▲), IBA (◆), NAA (■) content. One hundred mg of tissue was collected from the beginning of the preincubation of the plantlets (days –14, –9, and –4) to the induction day (day zero). Samples were also collected 7, 14, and 21 d after the induction of SE. Samples were analyzed as described in Materials and Methods. All the analyses were performed with 3 biological replicates from at least 2 independent experiments. Error bars represent the standard error (n = 3). Different letters and symbols represent the statistical significance of mean differences between each determination at a given time according to the Tukey test (P ≤ 0.01).
Most plant tissues contain auxins, mainly IAA. However, during the past 15 y, the body of evidence suggests that most of the auxins inside the tissues are conjugated.49,50
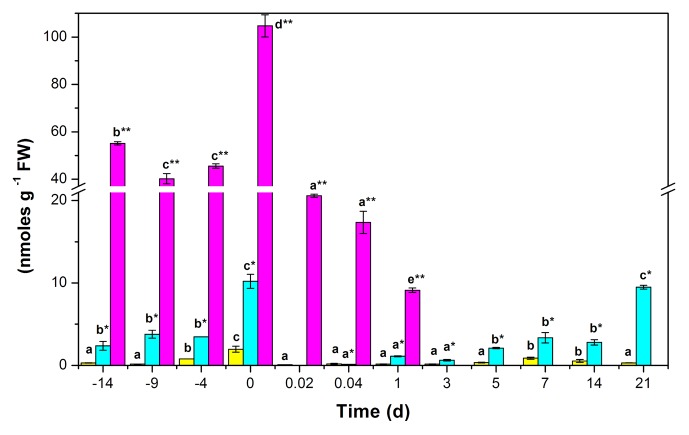
We found 2 IAA amide conjugates, IAA-Ala and IAA-Glu. The amount of these conjugates in the leaf tissues of C. canephora before pretreatment was 2.36 and 55.13 nmoles g–1 FW, respectively. These levels remained steady during the first 10 d of pretreatment. However, at the end of the incubation period (14 d), there was a noticeable increase in the auxins in the tissue. The total amount of free auxins and their conjugates reached the amount of 116.88 nmoles g–1 FW. This is an increase of almost 50% in relation to the initial amount of these compounds. Meanwhile, the free auxin increased more than 6.5 times its initial concentration (Fig. 3). This amount of free IAA accounts for 1.95 nmoles g–1 FW, while the content of the conjugates reached 10.20 and 104.72 nmoles g–1 FW for IAA-Ala and IAA-Glu, respectively.
Figure 3. Endogenous IAA and IAA conjugate content, before and during the induction of somatic embryogenesis in C. canephora. Endogenous free IAA (yellow bars), IAA-Ala (blue bars), IAA-Glu (purple bars). One hundred mg of tissue were collected from the beginning of the preincubation of the plantlets (days –14, –9, and –4) to the induction day (day zero). Samples were also collected 0.02, 0.041, 1, 3, 5, 7, 14, and 21 d after the induction of SE. Samples were analyzed as described in Materials and Methods. All the analyses were performed with 3 biological replicates from at least 2 independent experiments. Error bars represent the standard error (n = 3). Different letters in the bars represent the statistical significance of mean differences between each determination at a given time according to the Tukey test (P ≤ 0.01).
The amount of the IAA and its conjugates decreased by a factor of 5.7 during the first 30 min of the incubation of the explants in the SE medium. The amount of free IAA decreased by a factor of 26.4, and the conjugated IAA-Ala decreased below the level of detection, while the IAA-Glu decreased by a factor of 5. One day after the induction of the SE, the amount of IAA-Glu had decreased to 9.126 nmoles g–1 FW and, after that, it decreased to undetectable levels until the end of the study (Fig. 3). The fate of IAA-Ala was very different. This compound decreased sharply during the first 30 min of incubation in the SE induction medium, and then increased during the following days to 9.48 nmoles g–1 FW after 21 d. The IAA conjugates reached a maximum of 98.3% after the explants were put into the induction medium. Then, the conjugates decreased to 79.6% after 3 d from the beginning of SE induction. Twenty-one days after the induction of SE, just when the first embryogenic structures were beginning to appear, the total concentration of conjugates was back to 97% of the total auxin.
The increase in free IAA after 14 d of preincubation of the explants (Fig. 2) suggests 2 possibilities: 1) that de novo synthesis of the compound is occurring, or 2) that the IAA comes from the hydrolysis of previously synthesized IAA conjugates. However, the increase in IAA-Ala suggests that the IAA is synthesized de novo. To explore this possibility, we analyzed the expression of 3 genes involved in IAA biosynthesis.
IAA is biosynthesized through 5 different pathways, according to Mano.51 One is through the biosynthesis of indole-3-pyruvic acid (IPA). This reaction is catalyzed by the tryptophan aminotransferase (TAA1; TRYPTOPHAN AMINOTRANSFERASE of ARABIDOPSIS 1), and the IPA is then biosynthesized into IAA by the product of a family of genes, the flavinmonooxygenase-like enzymes (YUC).51 We used quantitative PCR to measure the expression of the TAA1, YUC1, and YUC3 genes during the preincubation and induction of SE.
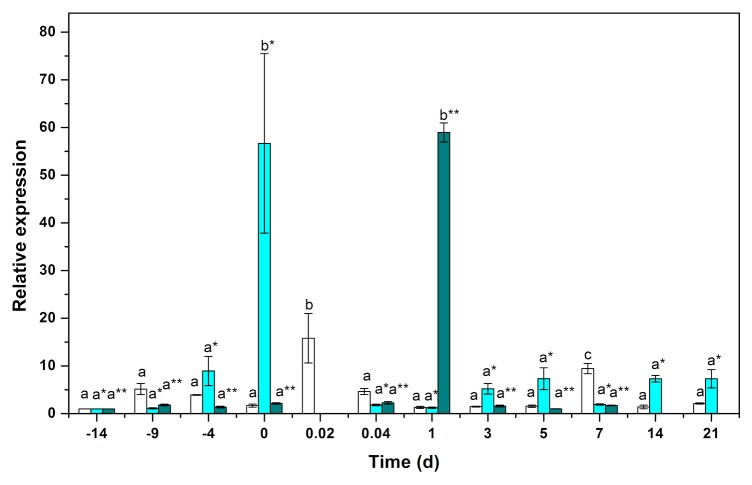
The gene expression of CcTAA1 increased during the first 10 d of preincubation, reached a maximum 30 min after the transfer of the explants to the SE induction medium, and then decreased for the next 6 d. Seven days after the induction of SE, and coinciding with an increase in free IAA, the expression of CcTAA1 increased to 9.5 times the initial level of expression (Fig. 4). After this increase, CcTAA1 decreased and stayed around the same level of expression that it had in the plants before preincubation.
Figure 4. qPCR analysis for the expression of the genes CcTAA1 (void bars), CcYUC1 (clear blue bars), and CcYUC3 (green bars). One hundred mg of tissue was collected from the beginning of the preincubation of the plantlets (days –14, –9, and –4) to the induction day (day zero). Samples were also collected 0.02, 0.041, 1, 3, 5, 7, 14, and 21 d after the induction of SE. Samples were analyzed as described in Materials and Methods. All the analyses were performed with 3 biological replicates from at least 2 independent experiments. Error bars represent the standard error (n = 3). Different letters in symbols represent the statistical significance of mean differences between each determination at a given time according to the Tukey test (P ≤ 0.01).
The expression of the CcYUC1 gene increased during the pretreatment period, reaching a maximum of 56 times the initial level at the end of 14 d. In contrast, the gene CcYUC3 only doubled during the same period. However, during the induction of SE, the expression of the CcYUC3 gene increased 58-fold after 1 d in the SE induction medium and then decreased to undetectable levels after 14 d. On the other hand, the expression of the gene CcYUC1 increased 7-fold by day 5 after the induction of SE, and it was still active, at the same level, 21 d after the induction (Fig. 4).
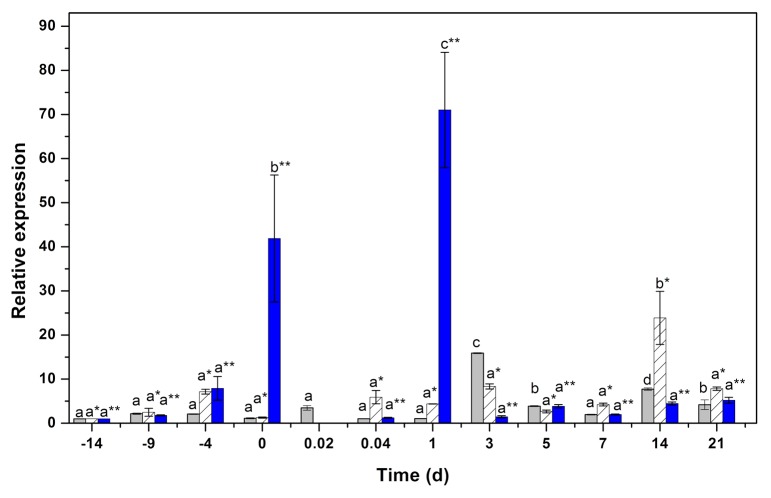
The increase in the IAA conjugates involves the activity of the products of the GH3 genes.18,19 The gene expression of GH3.17 increased more than 40 times at the end of the preincubation period (Fig. 5). During the first 60 min of the SE induction, the activity of these genes decreased. One day after the induction of SE, the expression of GH3.17 increased by a factor of 70. After this increase, GH3.17 decreased and stayed around the same level of expression that it had in the plants before preincubation. In contrast, GH3.1 increased to 15 times its initial amount after 3 d in the induction medium (Fig. 5) and then remained low until the end of the experiment. The gene GH3.6 increased around 10 d after the induction of the SE, decreased during the next 11 d, and had significant increase 2 wk after the induction of SE (Fig. 5).
Figure 5. qPCR analysis for the expression of the genes GH3.1 (gray bars), GH3.6 (hatched bars) and GH3.17 (blue bars). One hundred mg of tissue was collected from the beginning of the preincubation of the plantlets (days –14, –9, and –4) to the induction day (day zero). Samples were collected also 0.02, 0.041, 1, 3, 5, 7, 14, and 21 d after the induction of SE. Samples were analyzed as described in Materials and Methods. All the analyses were performed with 3 biological replicates from at least 2 independent experiments. Error bars represent the standard error (n = 3). Different letters in symbols represent the statistical significance of mean differences between each determination at a given time according to the Tukey test (P ≤ 0.01).
Discussion
SE is a complex biological process. It requires the interaction of several factors, including the level of different plant growth regulators and the compartmentalization of these regulators, their transport, and the expression of different genes. In this context, we have developed an efficient SE protocol for C. canephora,14 which enabled us to use it to study the SE process.
The induction and progression of SE in C. canephora is completely dependent on 2 factors and is performed in 2 phases. The first phase requires exogenous auxin, whereas the second phase does not. Furthermore, the first phase involves the preincubation of the plants that are the source of the explants in NAA and KIN for 2 wk. The second phase is performed in the presence of BA during the induction of SE. There is no exogenous auxin requirement during the induction of SE. For several years, it has been known that the presence of an exogenous auxin can induce the endogenous biosynthesis of IAA.1,5,6,13 However, it is also possible that the exogenous auxin promotes the inhibition of IAA degradation by reversible conjugating reactions.52,53 Thus, the homeostasis of the auxins is critical for the induction of the SE process.
In our plant, there is an important increase of both free auxin and auxin amide conjugates during the preincubation process (Fig. 2). The increase in the concentration of NAA was expected, since this growth regulator is in the culture medium. However, the increase in the concentration of IAA and IBA was surprisingly high; it is possible that this increase is a consequence of other factors, such as biosynthesis, hydrolysis from previously synthesized conjugates, or transport from other tissues or organs, since plants closely regulate cellular levels of IAA through synthesis, inactivation, and transport.54 At the same time, most of the auxin is in the amide conjugate form and accounts for more than 99% of the total auxins. Similar results have been obtained for the SE of D. carota. The induction of SE in D. carota is performed by cultivating the suspension cultures in the presence of 2,4-D. Then the 2,4-D is eliminated and the suspension culture diluted. The D. carota SE is performed in the absence of any auxin in the SE induction medium. During the induction of SE, 94% of the auxins were found to be in the chemical form of esters or amide conjugates, and only 6% as free auxin.50 On the other hand, 2,4-D induces the tryptophan-dependent synthesis of IAA in D. carota suspension cultures during the induction of SE. In Medicago truncatula, the addition of 2,4-D to leaf protoplast-derived cells produces an increase in the IAA and IAA conjugate pools.16
The modification of IAA by conjugation is a general mechanism for regulation of its activity and stability.55 This modification is part of the plant’s homeostasis and it is very important to plant health. The IAA amino acid conjugates have biological activity in the cell and this activity depends on the identity of the bound amino acid.7,56-60 Among the different IAA-amino acid conjugates tested for biological activity, IAA-Ala is the most active,56 since it can be stored and reused later on.
The decrease in the endogenous concentration of IAA is essential for the development of somatic embryos. In D. carota, the level of IAA decreased 10-fold from that of the initial cell clusters,1 as well as in Abies alba somatic embryos.13 It has been suggested that in the continuous presence of auxin, carrot cell lines are able to develop into the globular stage, but not beyond.61 In addition, recently it has been suggested that the removal of exogenous auxin from the medium triggers both auxin polar transport and auxin gradient establishment for SE induction.62,63 Our results show a sharp decrease in both auxins and their amide conjugates during the first 60 min of the induction of SE (Fig. 3). We observed that the first globular structures appear after the level of free IAA decreases to the same level found in the original plant before incubation (Figs. 1 and3; 0.3 nmoles g–1 FW). These data suggest that the transition from an undifferentiated stage to the globular stage requires the expression of new genes that are synthesized only when endogenous auxin is removed. However, the mechanisms lying beneath the initiation of SE are poorly understood.
A remaining question is whether the dramatic increase in endogenous IAA produced by the presence of NAA is synthesized de novo or originates in IAA conjugates stored inside the cell. In this study, we showed that during the preincubation of the explants there was a 56-fold increase in the expression of CcYUC1 (Fig. 4). This gene encodes a key enzyme (flavinmonooxygenase-like enzyme) in auxin biosynthesis.51 Our results suggest that at least part of the increase in the IAA content could be due to biosynthesis. Recently, Bo et al.62 have shown that local auxin biosynthesis is required for SE initiation in Arabidopsis thaliana and is at least partially mediated by the basal level of ethylene during SE initiation, suggesting that the ethylene response is important for SE induction.
We chose to study the gene expression of 3 genes of the GH3 family, GH3.1, GH3.6, and GH3.17, members of the group II of the GH3 family, which use IAA as a substrate. We noted a significant important increase in the transcript of GH3.17 at the end of the preincubation period (Fig. 5). This increment could explain the big boost of IAA conjugates detected at the same time (Fig. 3). During day 3, there is an important increase in the expression of GH3.1, when the rise in IAA-Ala concentration initiated.
The presence of the exogenous auxin increases both the free IAA and the IAA amide conjugates during the preincubation period. At least part of the increase of the auxin content is due to de novo synthesis. This increment in the endogenous auxin is indispensable to changing the genetic program of the cells and preparing them for the second phase. Until now, very little was known about how the exogenous auxin used in the first phase interacts with the endogenous auxin-mediated homeostasis of the explant used to induce SE during the induction phase.
During the past few years, our understanding of the role of auxin homeostasis during the induction of SE has significantly improved. However, there are still many gaps in our knowledge of this process. It can be concluded that the induction of SE from explants of C. canephora depends upon both endogenous and exogenous auxins. Our results suggest that a balance among free IAA and its amide conjugates is necessary to allow the expression of the embryogenic potential. The next step will be to understand how the homeostasis of the auxins modified the genetic program of the cell to produce a new plant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant received from the National Council for Science and Technology (CONACyT; 157014 to VMLV). Ayil-Gutiérrez B was supported by a scholarship (204929) from CONACyT. The authors thank Fabiola Escalante and Geovanny Nic-Can for their technical assistance. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Ayil-Gutiérrez B performed the cellular, molecular, and analytical studies. Galaz-Ávalos RM performed part of the analytical studies. Peña-Cabrera E synthesized and characterized the IAA conjugates. Loyola-Vargas VM conceived of the study, participated in its design and coordination, and drafted the manuscript. All authors read and approved the final manuscript.
Glossary
Abbreviations:
- 2,4-D
2,4-dichlorophenoxyacetic acid
- BA
6-benzyladenine
- BHT
2,6-di-t-butyl-4-methylphenol
- GH3
Gretchen Hagen 3
- KIN
kinetin
- IAA
indole-3-acetic acid
- IAA-Ala
indole-3-acety-L-alanine acid
- IAA-Glu
indole-3-acetyl-L-glutamic acid
- NAA
1-naphthaleneacetic acid
- SE
somatic embryogenesis
References
- 1.Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD. Regulation of indole-3-acetic Acid biosynthetic pathways in carrot cell cultures. Plant Physiol. 1992;100:1346–53. doi: 10.1104/pp.100.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimura T, Komamine A. Involvement, of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z Pflanzenphysiol. 1979;95:13–9. doi: 10.1104/pp.64.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiavone FM, Cooke TJ. Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Cell Differ. 1987;21:53–62. doi: 10.1016/0045-6039(87)90448-9. [DOI] [PubMed] [Google Scholar]
- 4.Ivanova A, Velcheva M, Denchev P, Atanassov A, Van Onckelen HA. Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant. 1994;92:85–9. doi: 10.1111/j.1399-3054.1994.tb06658.x. [DOI] [Google Scholar]
- 5.Pescador R, Kerbauy GB, Melo Ferreira W, Purgatto E, Suzuki RM, Guerra MP. A hormonal misunderstanding in Acca sellowiana embryogenesis: levels of zygotic embryogenesis do not match those of somatic embryogenesis. Plant Growth Regul. 2012;68:67–76. doi: 10.1007/s10725-012-9694-2. [DOI] [Google Scholar]
- 6.Wenck AR, Conger BV, Trigiano RN, Sams CE. Inhibition of somatic embryogenesis in orchardgrass by endogenous cytokinins. Plant Physiol. 1988;88:990–2. doi: 10.1104/pp.88.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreae WA, Good NE. The formation of indoleacetylaspartic acid in pea seedlings. Plant Physiol. 1955;30:380–2. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PJ. The fate of exogenously applied indoleacetic acid in light grown stems. Physiol Plant. 1972;27:262–70. doi: 10.1111/j.1399-3054.1972.tb03612.x. [DOI] [Google Scholar]
- 9.Zenk MH. Aufnahme und Stoffwechsel von α-Naphthyl-Essigsäure durch Erbsenepicotyle. Planta. 1962;58:75–94. doi: 10.1007/BF01938896. [DOI] [Google Scholar]
- 10.Moloney MM, Hall JF, Robinson GM, Elliott MC. Auxin requirements of sycamore cells in suspension culture. Plant Physiol. 1983;71:927–31. doi: 10.1104/pp.71.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribnicky DM, Ilic N, Cohen JD, Cooke TJ. The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism. The implications for carrot somatic embryogenesis. Plant Physiol. 1996;112:549–58. doi: 10.1104/pp.112.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez VM, Bangerth F. Endogenous hormone levels in explants and in embryogenic and non-embryogenic cultures of carrot. Physiol Plant. 2001;111:389–95. doi: 10.1034/j.1399-3054.2001.1110317.x. [DOI] [PubMed] [Google Scholar]
- 13.Vondrakova Z, Eliásová K, Fischerová L, Vágner M. The role of auxins in somatic embryogenesis of Abies alba. Cen Eur J Biol. 2011;6:587–96. doi: 10.2478/s11535-011-0035-7. [DOI] [Google Scholar]
- 14.Quiroz-Figueroa FR, Monforte-González M, Galaz-Avalos RM, Loyola-Vargas VM. Direct somatic embryogenesis in Coffea canephora In: Loyola-Vargas VM, Vázquez-Flota FA, eds. Plant cell culture protocols. Totowa, NJ: Humana Press, 2006: 111-7 [DOI] [PubMed] [Google Scholar]
- 15.Bögre L, Stefanov I, Abrahám M, Somogyi I, Dudits D. Differences in responses to 2,4-dichlorophenoxy acetic acid (2,4-D) treatment between embryogenic and non-embryogenic lines of alfalfa. In: Nijkamp HJJ, Van der Plas LHW, Van Aartrijk J, eds. Progress in plant cellular and molecular biology. The Netherlands: Kluwer Academic Publishers, 1990: 427-36 [Google Scholar]
- 16.Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudits D, Fehér A. The Role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002;129:1807–19. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossin CB, Rey MEC. Effect of explant source and auxins on somatic embryogenesis of selected cassava (Manihot esculenta Crantz) cultivars. S Afr J Bot. 2011;77:59–65. doi: 10.1016/j.sajb.2010.05.007. [DOI] [Google Scholar]
- 18.Wang H. Tian Ce, Duan J, Wu K. Research progresses on GH3s, one family of primary auxin-responsive genes. Plant Growth Regul. 2008;56:225–32. doi: 10.1007/s10725-008-9313-4. [DOI] [Google Scholar]
- 19.Chen Q, Westfall CS, Hicks LM, Wang S, Jez JM. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J Biol Chem. 2010;285:29780–6. doi: 10.1074/jbc.M110.146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen G, Kleinschmidt A, Guilfoyle T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta. 1984;162:147–53. doi: 10.1007/BF00410211. [DOI] [PubMed] [Google Scholar]
- 21.Okrent RA, Wildermuth MC. Evolutionary history of the GH3 family of acyl adenylases in rosids. Plant Mol Biol. 2011;76:489–505. doi: 10.1007/s11103-011-9776-y. [DOI] [PubMed] [Google Scholar]
- 22.Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–15. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terol J, Domingo C, Talón M. The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene. 2006;371:279–90. doi: 10.1016/j.gene.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Staritsky G. Embryoid formation in callus tissues of coffee. Acta Bot Need. 1970;19:509–14. [Google Scholar]
- 25.Söndahl MR, Sharp WR. High frequency induction of somatic embryos in cultured leaf explants of Coffea arabica L. Z Pflanzenphysiol. 1977;81:395–408. [Google Scholar]
- 26.Dublin P. Embryogenèse somatique directe sur fragments de feuilles de caféier Arabusta. Cafe, Cacao, The (Paris) 1981;25:237–42. [Google Scholar]
- 27.Yasuda T, Fujii Y, Yamaguchi T. Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol. 1985;26:595–7. [Google Scholar]
- 28.Dublin P. Techniques de reproduction végétative in vitro et amélioration génétique chez les caféiers cultivés. Cafe, Cacao, The (Paris) 1984;XXVIII:231–44. [Google Scholar]
- 29.Zamarripa CA, Ducos JP, Tessereau H, Bollon H, Eskes AB, Pétiard V. Devéloppement d'un procédé de multiplication en masse du caféier par embryogenèse somatique en milieu liquide. Paris: 14è Colloque Scientifique Internationale sur le Café, Association Scientifique Internationale du Café, 1991: 392-402 [Google Scholar]
- 30.Quiroz-Figueroa FR, Fuentes-Cerda CFJ, Rojas-Herrera R, Loyola-Vargas VM. Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep. 2002;20:1141–9. doi: 10.1007/s00299-002-0464-x. [DOI] [Google Scholar]
- 31.Lanaud C. Production of Coffea canephora plantlets by somatic embryogenesis obtained by in vitro culture of ovules. Cafe, Cacao, The (Paris) 1981;XXV:231–6. [Google Scholar]
- 32.Sreenath HL, Shanta HM, Babu KH, Naidu MM. Somatic embryogenesis from integument (perisperm) cultures of coffee. Plant Cell Rep. 1995;14:670–3. doi: 10.1007/BF00232736. [DOI] [PubMed] [Google Scholar]
- 33.Ascanio ECE, Arcía MM. Haploids from anther culture in Coffea arabica L. International Congress of Plant Tissue Culture, Tropical Species. Bogotá, Colombia, 1987: 68 [Google Scholar]
- 34.Hatanaka T, Arakawa O, Yasuda T, Uchida N, Yamaguchi T. Effect of plant growth regulators on somatic embryogenesis in leaf cultures of Coffea canephora. Plant Cell Rep. 1991;10:179–82. doi: 10.1007/BF00234290. [DOI] [PubMed] [Google Scholar]
- 35.Hatanaka T, Sawabe E, Azuma T, Uchida N, Yasuda T. The role of ethylene in somatic embryogenesis from leaf disks of Coffea canephora. Plant Sci. 1995;107:199–204. doi: 10.1016/0168-9452(95)04103-2. [DOI] [Google Scholar]
- 36.Fuentes SRL, Calheiros MBP, Manetti J, Vieira LGE. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult. 2000;60:5–13. doi: 10.1023/A:1006474324652. [DOI] [Google Scholar]
- 37.Quiroz-Figueroa FR, Méndez-Zeel M, Larqué-Saavedra A, Loyola-Vargas VM. Picomolar concentrations of salycilates induce cellular growth and enhance somatic embryogenesis in Coffea arabica tissue culture. Plant Cell Rep. 2001;20:679–84. doi: 10.1007/s002990100386. [DOI] [Google Scholar]
- 38.Giridhar P, Indu EP, Ravishankar GA, Chandrasekar A. Influence of triacontanol on somatic embryogenesis in Coffea arabica L. and Coffea canephora P. ex Fr. In Vitro Cell Dev Biol Plant. 2004;40:200–3. doi: 10.1079/IVP2003519. [DOI] [Google Scholar]
- 39.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 40.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser. 1999;41:95–8. [Google Scholar]
- 41.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Castillo G, Altuna B, Michelena G, Sánchez-Bravo J, Acosta M. Cuantificación del contenido de ácido indolacético (AIA) en un caldo de fermentación microbiana. Ann Biol. 2005;27:137–42. [Google Scholar]
- 44.Yamaguchi H, Tanaka H, Hasegawa M, Tokuda M, Asami T, Suzuki Y. Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012;196:586–95. doi: 10.1111/j.1469-8137.2012.04264.x. [DOI] [PubMed] [Google Scholar]
- 45.Vine JH, Noiton D, Plummer JA, Baleriola-Lucas C, Mullins MG. Simultaneous quantitation of indole 3-acetic Acid and abscisic Acid in small samples of plant tissue by gas chromatography/mass spectrometry/selected ion monitoring. Plant Physiol. 1987;85:419–22. doi: 10.1104/pp.85.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam YY, Epstein E, Normanly J. Characterization of auxin conjugates in Arabidopsis. Low steady-state levels of indole-3-acetyl-aspartate, indole-3-acetyl-glutamate, and indole-3-acetyl-glucose. Plant Physiol. 2000;123:589–96. doi: 10.1104/pp.123.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR. A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J. 2003;35:405–17. doi: 10.1046/j.1365-313X.2003.01800.x. [DOI] [PubMed] [Google Scholar]
- 48.Catalá C, Östin A, Chamarro J, Sandberg G, Crozier A. Metabolism of indole-3-acetic acid by pericarp discs from Immature and mature tomato (Lycopersicon esculentum Mill) Plant Physiol. 1992;100:1457–63. doi: 10.1104/pp.100.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jourdain I, Lelu MA, Label P. Hormonal changes during growth of somatic embryogenic masses in hybrid larch. Plant Physiol Biochem. 1997;35:741–9. [Google Scholar]
- 50.Ceccarellil N, Monding A, Curadi M, Lorenzi R, Schiavo FL. Auxin metabolism and transport in an embryogenic cell line of Daucus carota L. J Plant Physiol. 2000;157:17–23. doi: 10.1016/S0176-1617(00)80130-3. [DOI] [Google Scholar]
- 51.Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot. 2012;63:2853–72. doi: 10.1093/jxb/ers091. [DOI] [PubMed] [Google Scholar]
- 52.Cohen JD, Bandursky RS. Chemistry and physiology of the bound auxins. Annu Rev Plant Physiol. 1982;33:403–30. doi: 10.1146/annurev.pp.33.060182.002155. [DOI] [Google Scholar]
- 53.Michalczuk L, Cooke TJ, Cohen JD. Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry. 1992;31:1097–103. doi: 10.1016/0031-9422(92)80241-6. [DOI] [Google Scholar]
- 54.Strader LC, Bartel B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol Plant. 2011;4:477–86. doi: 10.1093/mp/ssr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westfall CS, Zubieta C, Herrmann J, Kapp U, Nanao MH, Jez JM. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science. 2012;336:1708–11. doi: 10.1126/science.1221863. [DOI] [PubMed] [Google Scholar]
- 56.Bialek K, Meudt WJ, Cohen JD. Indole-3-acetic acid (IAA) and IAA conjugates applied to bean stem sections. Plant Physiol. 1983;73:130–4. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feung CS, Hamilton RH, Mumma RO. Metabolism of indole-3-acetic acid. Plant Physiol. 1977;59:91–3. doi: 10.1104/pp.59.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hangarter RP, Peterson MD, Good NE. Biological activities of indoleacetylamino acids and their use as auxins in tissue culture. Plant Physiol. 1980;65:761–7. doi: 10.1104/pp.65.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Purves WK, Hollenberg SM. Metabolism of exogenous indoleacetic acid to its amide conjugates in Cucumis sativus L. Plant Physiol. 1982;70:283–6. doi: 10.1104/pp.70.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hangarter RP, Good NE. Evidence that IAA conjugates are slow-release sources of free IAA in plant tissues. Plant Physiol. 1981;68:1424–7. doi: 10.1104/pp.68.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borkird C, Choi JH, Sung ZR. Effect of 2,4-dichlorophenoxyacetic Acid on the expression of embryogenic program in carrot. Plant Physiol. 1986;81:1143–6. doi: 10.1104/pp.81.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai B, Su YH, Yuan J, Zhang XS. Induction of somatic embryos in Arabidopsis requires local YUCCA expression mediated by the down-regulation of ethylene biosynthesis. Mol Plant. 2013;6:1247–60. doi: 10.1093/mp/sss154. [DOI] [PubMed] [Google Scholar]
- 63.Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009;59:448–60. doi: 10.1111/j.1365-313X.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.