Abstract
The phytohormone abscisic acid (ABA) plays a crucial role in numerous aspects of plant growth and environmental stress responses. Endogenous ABA levels are regulated by a balance between its biosynthetic and catabolic activities. This balance may occur at multiple levels and includes the expression of genes involved in these processes. ABA UDP-glucosyltransferase (UGT), the major player in the ABA conjugation pathway, has been shown to have a marginal effect on free ABA levels. However, recent studies provide new insight into the importance of the ABA conjugation pathway in contributing to the control of ABA homeostasis. Gain-of-function and loss-of-function mutant analyses have revealed that UGT71B6, an ABA UGT, and its 2 closely related homologs, UGT71B7 and UGT71B8, play a crucial role in ABA homeostasis and in adaptation to various abiotic stresses.
Keywords: ABA UDP-glucosyltransferases, abiotic stress, ABA homeostasis, ABA catabolism, coordination of ABA metabolic pathways
Introduction
The phytohormone abscisic acid (ABA) plays crucial roles in plant growth and development, and stress responses.1,2 Cellular ABA levels fluctuate constantly to adjust plants to changing environmental and physiological conditions.3,4 The level of the hormone is under the control of complex regulatory processes involving biosynthesis, catabolism, and transport.5-7
Since the discovery of ABA in the late 1960s, the ABA de novo synthesis pathway was extensively studied by isolating various biosynthetic mutants at every step in the biosynthesis pathway in Arabidopsis, maize, and tomato.8,9 All of the steps in the de novo ABA biosynthetic pathway occur in plastids, with the exception of the last 2 steps that occur in the cytosol.10 Another pathway for ABA synthesis is the simple one-step hydrolysis of glucose-conjugated ABA (ABA-GE) to ABA by 2 β-glucosidases, AtBG1 and AtBG2, which localize to the ER and vacuole, respectively.11,12
In addition to ABA biosynthesis, catabolism is a major process for controlling cellular ABA levels. ABA catabolism is composed of 2 pathways, hydroxylation and glucose conjugation. Hydroxylation is performed by 4 members of the cytochrome P450 family, CYP707A1 to CYP707A4, which hydroxylate ABA at the 8′ position to produce unstable 8′-hydroxyl ABA.6 In the conjugation pathway, ABA is conjugated with glucose by ABA UDP-glucosyltransferase (UGT) to produce ABA-GE, which is a storage or transport form of ABA and accumulates in the vacuole and apoplast.13 Although extensive work has been conducted on the hydroxylation pathway, little is known about the conjugation pathway. In particular, despite the fact that the conjugation pathway can inactivate ABA and thereby lower the cellular ABA level, its contribution in the homeostasis regulation of cellular ABA levels has been less clear.
In this review, we focus on the recent findings which improve our understanding of the role of UGT71B6 and its 2 homologs in ABA homeostasis.
The molecular function of UGT71B6 and its 2 homologs in ABA homeostasis
UGT71B6 has ABA -glucosyltransferase activity that can conjugate ABA with glucose to produce ABA-GE.14 However, its involvement in regulation of the cellular ABA level has not been fully understood. The loss-of-function mutant of UGT71B6 does not show any noticeable phenotype, and UGT71B6 overexpression has a marginal effect on reducing ABA content.14 In Arabidopsis, UGTs represent a superfamily containing more than 100 homologs.15 Thus, it is possible that multiple genes encoding UGTs glucosylate ABA, and mutation in one of these genes does not produce any phenotype because of functional redundancy. Consistent with this idea is that the amino acid sequence alignment of members in UGT sub-group E reveals 2 additional homologs (UGT71B7 and UGT71B8) that show more than 90% amino acid sequence similarity to UGT71B6. Indeed, UGT71B6 and its 2 closely related homologs, UGT71B7 and UGT71B8, are able to modulate the cellular ABA levels.16 There are also several lines of supporting evidence. First, ectopic expression of 3 UGTs (UGT71B6, UGT71B7, and UGT71B8) in protoplasts inhibited the ABA-induced expression of the firefly luciferase reporter gene (LUC) driven by ABA-responsive RD29A promoter, RD29Ap. Second, UGT RNAi transgenic plants which had lower levels of the UGT transcripts displayed multiple growth defective phenotypes, such as smaller rosette leaves, shorter roots and pale green leaves and also a 2-fold increase in the cellular ABA levels. Moreover, UGT RNAi transgenic plants showed hypersensitivity to exogenous ABA during germination and post-germination growth, and displayed enhanced resistance to osmotic and dehydration stresses. Thus, suppression of 3 UGTs leads to a defect in ABA-mediated responses to abiotic stresses and disturbs ABA homeostasis.
The glucose-conjugated form of ABA is biologically inactive.17 Therefore, it is reasonable that overexpression of UGT71B6 that can glucosylate ABA to ABA-GE causes typical ABA-deficient phenotypes. However, UGT71B6-overexpressing plants produced essentially no observable ABA-deficient phenotype. Recent work shows that in addition to the de novo ABA biosynthesis, ABA can also be produced by 2 β-glucosidases, AtBG1 and AtBG2, through the hydrolysis of ABA-GE, the product of ABA glucosyltransferase.11,12 When UGT71B6 was introduced into the atbg1 mutant plants, UGT71B6:GFP/atbg1 plants showed more significant ABA-deficient phenotypes than UGT71B6:GFP-overexpressing transgenic plants or atbg1 mutant plants, suggesting that in UGT-overexpressing plants the UGT71B6-mediated reduction of ABA levels was compensated by ABA generated through AtBG1- and/or AtBG2-mediated ABA-GE hydrolysis, which results in no change in overall ABA levels. As a long-distance transport form of stress hormone,18 ABA-GE also provides a source of ABA via subsequent hydrolysis, indicating that a close connection exists between the conjugation of glucose to ABA by UGT71B6 and the hydrolysis of ABA-GE to ABA by AtBG1 and/or AtBG2. Therefore, UGTs, AtBG1/2, and long distance transport together play crucial roles in the homeostasis of cellular ABA levels (Fig. 1).
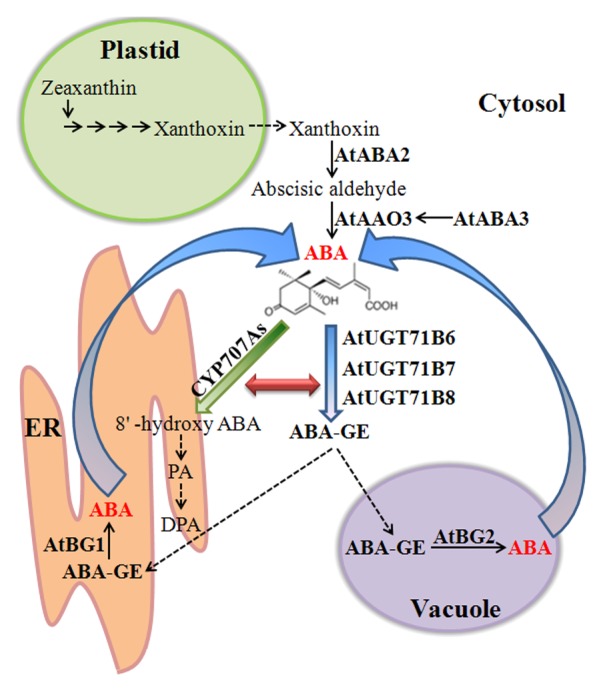
Figure 1. Coordination of ABA biosynthesis and catabolism in ABA homeostasis in Arabidopsis. The steps of ABA de novo biosynthesis from zeaxanthin are shown. Hydrolysis of ABA-GE is shown with the corresponding enzymes. In the catabolic pathways, ABA is inactivated by hydroxylation or glucose conjugation. Postulated pathways are shown in broken lines, and confirmed pathways are shown in solid lines. Arrows represent the activity of each enzyme. ER, endoplasmic reticulum; ABA-GE, ABA glucosyl ester; PA, phaseic acid; DPA, dihydrophaseic acid.
Coordination of biosynthesis and catabolism to maintain ABA homeostasis
Hydroxylation and conjugation are 2 ABA catabolic pathways. However, these 2 pathways may differ in their consequences for plant physiology. Hydroxylation of ABA by CYP707As produces 8′-hydroxyl ABA, which is then converted to phaseic acid by spontaneous isomerization, leading to an irreversible degradation of ABA. In contrast, conjugation of ABA with glucose produces ABA-GE, which can be converted back to ABA by the hydrolytic activity of AtBG1 and AtBG2. Thus, the conjugation pathway can be considered as part of the rapid ABA biosynthetic pathway through the activity of AtBG1 and AtBG2. This may underlie the difference in the phenotype between transgenic plants overexpressing UGT71B6 and CYP707A. Whereas UGT71B6 overexpression had a marginal effect on reducing ABA levels; overexpression of CYP707A3 effectively reduced the endogenous ABA levels and resulted in a remarkable ABA-deficient phenotype.19 Suppression of UGTs induced the expression of CYP707A1 to CYP707A4; in parallel, overexpression of UGT71B6 suppressed CYP707As. These results suggest that the catabolic pathways are finely coordinated by a certain regulatory circuit at the transcription level, and the loss of one pathway is compensated for by the transcription change of genes in other pathways (Fig. 1). A similar phenomenon was observed in the cyp707a1 cyp707a3 double mutants, which accumulated higher levels of ABA-GE than WT plants.20 Therefore, it is likely that active regulatory mechanisms are involved in coordinating the activities of the catabolic pathways for ABA homeostasis. Similarly, the multiple biosynthetic pathways are closely coordinated by a regulatory circuit. The loss of ABA production in the atbg1 mutant plants was compensated by the overexpression of NCED3 or AtBG2,12 indicating that the multiple biosynthetic pathways are coordinated to determine the endogenous ABA levels in plant cells.
Finally, the next question would be how the biosynthetic and the catabolic pathways of ABA are coordinated to maintain homeostasis of cellular ABA levels. The biosynthetic and catabolic pathways are localized to multiple types of organelles. All steps in the de novo biosynthetic pathway take place in plastids, except for the last 2 steps that occur in the cytosol. By contrast, the hydrolysis of ABA-GE to ABA by AtBG1 and AtBG2 takes places in the ER and vacuole, respectively. Regarding the catabolic pathways, the 3 UGTs localize to the cytosol, whereas CYP707As localize in the ER membrane.21 Therefore, the localization of all key enzymes involved in ABA metabolism in multiple organelles raises an intriguing possibility of a complicated regulatory network(s) involving multiple organelles to maintain the cellular ABA levels. For example, in plants, ABA-GE is stored in the vacuole and apoplastic space, whereas AtBG1 localizes to the ER. In response to abiotic stresses, ABA-GE should be imported in to the ER to rapidly meet the plant requirement for ABA. This pathway may be under a fine and precise control, as AtBG1 and its substrate are stored separately in the cell, and only brought together when plants need to increase ABA levels. Moreover, compared with the lengthy and complex de novo biosynthetic pathway, hydrolysis of ABA-GE to ABA is a one-step process. Thus, this pathway seems to be another elegant mechanism for plants to increase ABA levels robustly in a short time upon stresses.
The presence of active ABA pools in 3 different compartments raises the possibility that the local concentration of ABA, rather than the overall cellular ABA content, is critical for initiating ABA-mediated signaling in particular processes. Furthermore, ABA produced in different subcellular compartments may require different ABA receptors, which coincides with the fact that plant cells contain multiple types of ABA receptors in different subcellular compartments, including the cytosol, chloroplasts and plasma membrane.22-24 According to this hypothesis, it is possible that ABA receptors that perceive different pools of ABA can induce different physiological responses. Currently, the most prevailing model for ABA perception and signal transduction is based on the cytosol and nucleus-localized PYR/RCAR-type ABA receptors, which interact with protein phosphatase 2Cs and SNF1-related protein kinase 2 (SnRK2). However, exactly how ABA produced in the ER and vacuole initiates ABA-mediated signaling in plant cells is still unknown.
Conclusions and Perspectives
Based on the fact that processes involved in ABA metabolism are compartmentalized in multiple types of organelles, we propose that a complex and flexible regulatory mechanism is required to coordinate these multiple biosynthetic and catabolic pathways as well as transport to achieve the desired cellular ABA levels. Moreover, this compartmentalized ABA metabolism should also be incorporated into the ABA-mediated signaling circuit so as to adjust the plants in response to changing physiological and environmental conditions. A direction of future research would be to define the exact role of active ABA pools produced in different compartments and to determine how ABA produced in different compartments leads to ABA signaling and physiological responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by a grant from National Research Foundation (2013070270), the Ministry of Science, ICT, and Future Planning (Korea).
References
- 1.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol. 2000;3:217–23. doi: 10.1016/S1369-5266(00)80068-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ. 2002;25:195–210. doi: 10.1046/j.0016-8025.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Chernys JT, Zeevaart JA. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000;124:343–53. doi: 10.1104/pp.124.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler AJ, Krochko JE. Formation and breakdown of ABA. Trends Plant Sci. 1999;4:472–8. doi: 10.1016/S1360-1385(99)01497-1. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci U S A. 2010;107:2361–6. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell. 2002;14:2723–43. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996;15:2331–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002;7:41–8. doi: 10.1016/S1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell. 2006;126:1109–20. doi: 10.1016/j.cell.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, et al. A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell. 2012;24:2184–99. doi: 10.1105/tpc.112.095935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz KJ, Sauter A, Wichert K, Messdaghi D, Hartung W. Extracellular beta-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J Exp Bot. 2000;51:937–44. doi: 10.1093/jexbot/51.346.937. [DOI] [PubMed] [Google Scholar]
- 14.Priest DM, Ambrose SJ, Vaistij FE, Elias L, Higgins GS, Ross ARS, Abrams SR, Bowles DJ. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006;46:492–502. doi: 10.1111/j.1365-313X.2006.02701.x. [DOI] [PubMed] [Google Scholar]
- 15.Ross J, Li Y, Lim E, Bowles DJ. Higher plant glycosyltransferases. Genome Biol. 2001;2:S3004–, 6. doi: 10.1186/gb-2001-2-2-reviews3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong T, Xu ZY, Park Y, Kim DH, Lee Y, Hwang I. ABA UDP-glucosyltransferases play a crucial role in ABA homeostasis in Arabidopsis. Plant Physiol. 2014 doi: 10.1104/pp.114.239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeevaart JA. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983;71:477–81. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, Hartung W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. J Exp Bot. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- 19.Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006;46:171–82. doi: 10.1111/j.1365-313X.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto M, Kushiro T, Jikumaru Y, Abrams SR, Kamiya Y, Seki M, Nambara E. ABA 9′-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry. 2011;72:717–22. doi: 10.1016/j.phytochem.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Seo M, Koshiba T. Transport of ABA from the site of biosynthesis to the site of action. J Plant Res. 2011;124:501–7. doi: 10.1007/s10265-011-0411-4. [DOI] [PubMed] [Google Scholar]
- 22.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 2010;24:1695–708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci U S A. 2001;98:2053–8. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–48. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]


