Abstract
Low oxygen stress imposed by floods creates a strong selection force shaping plant ecosystems in flood-prone areas. Plants inhabiting these environments adopt various adaptations and survival strategies to cope with increasing water depths. Two Rorippa species, R. sylvestris and R. amphibia that grow in naturally flooded areas, have high submergence tolerance achieved by the so-called quiescence and escape strategies, respectively. In order to dissect the molecular mechanisms involved in these strategies, we investigated submergence-induced changes in gene expression in flooded roots of Rorippa species. There was a higher induction of glycolysis and fermentation genes and faster carbohydrate reduction in R. amphibia, indicating a higher demand for energy potentially leading to faster mortality by starvation. Moreover, R. sylvestris showed induction of genes improving submergence tolerance, potentially enhancing survival in prolonged floods. Additionally, we compared transcript profiles of these 2 tolerant species to relatively intolerant Arabidopsis and found that only Rorippa species induced various inorganic pyrophosphate dependent genes, alternatives to ATP demanding pathways, thereby conserving energy, and potentially explaining the difference in flooding survival between Rorippa and Arabidopsis.
Keywords: Arabidopsis, Rorippa, fermentation, flooding tolerance, hypoxia, root, submergence
Flooding has detrimental effects on plants as a result of decreased underwater gas diffusion leading to rapid depletion of oxygen and a halt in aerobic respiration.1,2 A common plant response to low oxygen is increased glycolysis and a switch to anaerobic metabolism for supplying the plant with the necessary ATP, which help them to survive short periods of submergence. However, this leads to a faster consumption of carbohydrates since this ATP gain is much lower than that obtained via aerobic respiration.3 If submergence is prolonged, mortality is inevitable in this energy crisis. Thus flooding acts as a strong selection force on plant communities inhabiting flood-prone areas and as a result shapes typical adaptations in flood-exposed plant species.4,5 Understanding how these adaptations facilitate survival in submergence tolerant plants is very valuable for crop improvements as well as for management programs in flood-prone areas. We studied the responses of 2 submergence tolerant Rorippa species that inhabit naturally flooded sites and adopt different strategies to overcome lethal effects of the associated low oxygen stress.6 The oxygen content in the roots and shoots of flooded plants can be very different. Submerged shoots can often maintain close to normoxic levels of oxygen, especially when light levels are sufficient. In contrast, roots surrounded by an oxygen depleted soil often experience severe hypoxia. We performed global transcriptome profiling, carbohydrate, and metabolite analyses on the roots of Rorippa plants subjected to 24 h of complete submergence. Additionally, we compared the submergence responses in the roots of the relatively flood intolerant Rorippa relative, Arabidopsis, to potentially identify candidate genes or processes that could explain the highly effective adaptive mechanisms evolved in Rorippa.
Different strategies for different flooding regimes
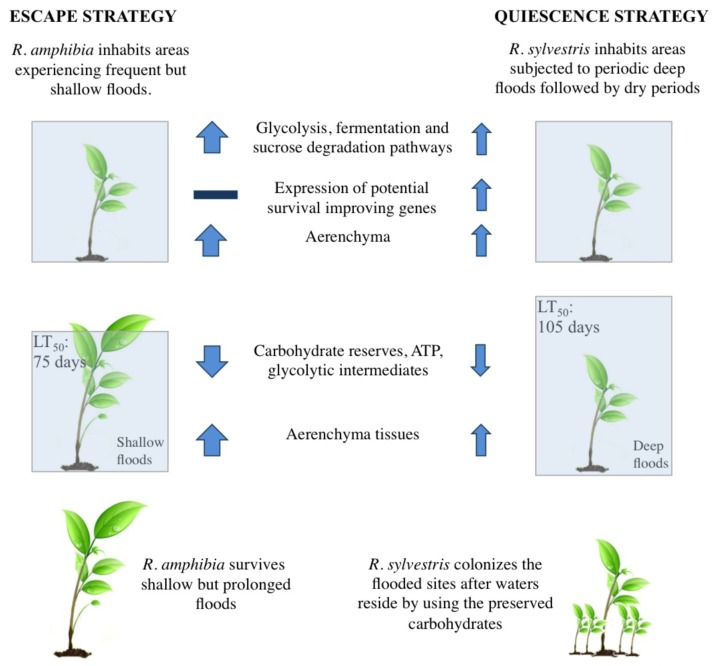
Rorippa amphibia and Rorippa sylvestris both grow in frequently flooded habitats, the former occurring in sites with more stable and shallow water tables and the latter with prolonged deep floods and summer dry-outs. Hence, R. sylvestris can survive much longer than R. amphibia under complete submergence and shows no mortality for up to 100 d while around 70% mortality is observed in R. amphibia under similar conditions.6,7 Adaptations evolved as a response to flooding gradients determine species distributions in flood prone environments.8 In accordance with the flooding regimes at their natural habitats, the survival difference in these Rorippa species can be explained by their contrasting strategies (Fig. 1). When completely submerged, R. sylvestris displays a quiescence strategy characterized by limited shoot growth while R. amphibia shows enhanced shoot elongation, typical for the escape strategy.
Figure 1. Submergence tolerance strategies in Rorippa. LT50 = Median lethal time (Number of days after which 50% of the population is dead due to stress). Arrows indicate submergence-induced increase (up) or decrease (down). Thickness of arrows indicates the magnitude of the effect.
The escape strategy, typified by R. amphibia, requires a considerable investment of carbohydrate and energy reserves in the submerged environment where photosynthesis and respiration is already restricted. Higher levels of glycolysis and fermentation related gene expression and reduction in ATP levels after 24 h of submergence in R. amphibia roots suggest that there is a higher demand for energy, possibly to supply for the aboveground growth. This coupled with higher induction of SUCROSE SYNTHASE and INVERTASE pathways in R. amphibia roots leads to reduction of soluble carbohydrates at earlier phases of submergence6 and later to a severe reduction in root starch reserves.7 Root carbohydrates are most likely channeled to the elongating shoot as evidenced by increased aboveground biomass in R. amphibia after 2 wk of submergence at the expense of root biomass.9
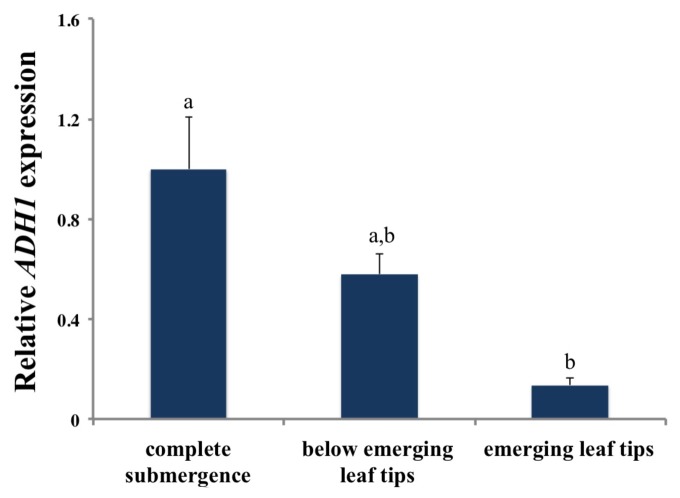
Although energetically expensive, the escape strategy pays off when the shoot outgrows shallow floodwaters. In R. amphibia, emergence of the shoot tip leads to an obvious increase in plant biomass.7 This is likely due to the improved aeration of the plant via the emergent shoot tip that is aerenchyma rich (high porosity in R. amphibia petioles). We also showed that when air contact was established after 3 d of submergence, ADH1 was downregulated dramatically within 2 h in the shoot tips above water, and to a lower extent in the submerged parts of these R. amphibia plants (Fig. 2). This rapid change indicates a fast recovery from low oxygen stress and that oxygen can be efficiently transported via aerenchyma tissues to submerged parts of the plant indicating the vital role of aerenchyma in the escape strategy. The quiescent R. sylvestris having much lower petiole porosity does not benefit from partial shoot emergence as much as R. amphibia, both in terms of biomass accumulation7 and reduction in ADH1 expression.
Figure 2. Relative ADH1 expression in completely submerged shoots, submerged shoots below emerging leaves and emerging leaf tips of R. amphibia. Data points represent means ± standard errors (n = 4). ANOVA Tukey test HSD results are indicated with letters at P < 0.05. Submergence started 4 h after the onset of the photoperiod and experiments were continued in 9/15 h day/night regime. Gene expression was measured in 2 sets of plants: 1) completely submerged for 74 h, and 2) plants that established air contact for 2 h after 72 h of complete submergence (emerging leaf tips and below emerging leaf tips). 18S was used as an internal reference gene. Primer sequences for ADH1 and 18S rRNA genes were same as in Akman et al. 2012.
Establishing aerial contact not only supplies oxygen but also the carbon dioxide necessary for underwater photosynthesis, which is an important factor affecting submergence tolerance.10,11 Floods are usually coupled with turbid waters, thus light intensity decreases with water depth.12 Elongating new leaves of submerged R. amphibia show higher specific leaf area (data not shown) in order to increase underwater gas diffusion and potentially to capture more light as the photosynthetic tissues get close to the surface and hence higher intensities of light. Moreover, underwater shoot elongation might also be a plastic response enhanced by light availability and carbohydrate levels, since submergence in darkness obscures the elongation differences between R. amphibia and R. sylvestris (data not shown).
R. sylvestris inhabits sites with shorter and deeper floods, where an escape strategy would not be useful. This species displays a more conservative use of ATP and carbohydrates and the accumulation of glycolytic intermediates all pointing toward a restriction of energetically expensive processes and economization of reserves typical of the quiescence strategy. This conservative strategy might enable fast colonization of previously flooded sites after waters reside.13 However, this requires adaptation to submergence and associated stresses for longer periods and genes expressed at higher levels specifically in R. sylvestris might potentially explain the difference in survival between the 2 Rorippa species. These include CATALASE1, an antioxidant gene, and a WRKY transcription factor (AtWRKY75), member of a gene family recently shown to be important in submergence tolerance in Arabidopsis.14 The F1 hybrid of these Rorippa species,7 displays an escape strategy similar to R. amphibia. However, the higher growth did not lead to a lower survival like that of R. amphibia, but was rather intermediate. We also studied the root transcript profiles of the hybrid and found that although the hybrid is phenotypically more similar to R. amphibia than to R. sylvestris based on survival strategies, this conclusion does not hold when gene expression patterns are considered (data not shown). It is highly likely that expression of the above-mentioned potential survival improving genes of R. sylvestris also facilitate survival in the hybrid, despite the higher growth rate.
PPi-dependent alternative pathways: potential tolerance associated genes enhancing survival in Rorippa
The significant relatedness of Rorippa and Arabidopsis allows a more direct comparison of their submergence responses and reveals potential mechanisms leading to the survival difference between these 2 closely related genera. There is a significant variation in submergence tolerance even in naturally non-flooded Arabidopsis accessions.15 However, even the most tolerant accession, C24, has a lethal median time of 20 d, much lower than 75 d for R. amphibia and 105 d for R. sylvestris. This substantial difference could be as a result of various mechanisms that evolved in Rorippa as a consequence of inhabiting flood-prone environments. One of these mechanisms is proposed to be the conservation of ATP via utilization of PPi-dependent pathways.16,17 A preference for these alternative PPi-dependent over ATP consuming pathways in Rorippa would conserve ATP pools and might also contribute to the higher tolerance in these 2 species compared with their relative Arabidopsis.
The study of wild species like Rorippa that have naturally evolved to survive in flood prone niches is important to understand the mechanisms mediating distinct flooding survival strategies. Exploring not only molecular and physiological changes but also the ecology of flood tolerant species and the mechanisms of adaptation to their habitats serves as a strong potential for understanding and improving plant reactions to increasing water depths.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Armstrong W. Aeration in Higher Plants. In: Advances in Botanical Research. Woolhouse HW, ed. Academic Press, 1980:225-332. [Google Scholar]
- 2.Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytol. 2006;170:213–26. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 3.Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–39. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- 4.Blom CWPM. Adaptations to flooding stress: From plant community to molecule. Plant Biol. 1999;1:261–73. doi: 10.1111/j.1438-8677.1999.tb00252.x. [DOI] [Google Scholar]
- 5.Van Eck WHJM, Van De Steeg HM, Blom CWPM, De Kroon H. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos. 2004;107:393–405. doi: 10.1111/j.0030-1299.2004.13083.x. [DOI] [Google Scholar]
- 6.Sasidharan R, Mustroph A, Boonman A, Akman M, Ammerlaan AMH, Breit T, Schranz ME, Voesenek LACJ, van Tienderen PH. Root transcript profiling of two Rorippa species reveals gene clusters associated with extreme submergence tolerance. Plant Physiol. 2013;163:1277–92. doi: 10.1104/pp.113.222588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akman M, Bhikharie AV, McLean EH, Boonman A, Visser EJ, Schranz ME, van Tienderen PH. Wait or escape? Contrasting submergence tolerance strategies of Rorippa amphibia, Rorippa sylvestris and their hybrid. Ann Bot. 2012;109:1263–76. doi: 10.1093/aob/mcs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voesenek LACJ, Rijnders JHGM, Peeters AJM, van de Steeg HM, de Kroon H. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology. 2004;•••:16–27. doi: 10.1890/02-740. [DOI] [Google Scholar]
- 9.Stift M, Luttikhuizen PC, Visser EJW, van Tienderen PH. Different flooding responses in Rorippa amphibia and Rorippa sylvestris, and their modes of expression in F1 hybrids. New Phytol. 2008;180:229–39. doi: 10.1111/j.1469-8137.2008.02547.x. [DOI] [PubMed] [Google Scholar]
- 10.Mommer L, de Kroon H, Pierik R, Bögemann GM, Visser EJW. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytol. 2005;167:197–206. doi: 10.1111/j.1469-8137.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- 11.Mommer L, Pons TL, Visser EJW. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J Exp Bot. 2006;57:283–90. doi: 10.1093/jxb/erj015. [DOI] [PubMed] [Google Scholar]
- 12.Parolin P. Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Ann Bot. 2009;103:359–76. doi: 10.1093/aob/mcn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsell B. Studies in the North-West European species of Rorippa s. str. Symb Bot Ups. 1968;19:1–221. [Google Scholar]
- 14.Hsu F-C, Chou M-Y, Chou S-J, Li Y-R, Peng H-P, Shih M-C. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell. 2013;25:2699–713. doi: 10.1105/tpc.113.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vashisht D, Hesselink A, Pierik R, Ammerlaan JMH, Bailey-Serres J, Visser EJW, Pedersen O, van Zanten M, Vreugdenhil D, Jamar DCL, et al. Natural variation of submergence tolerance among Arabidopsis thaliana accessions. New Phytol. 2011;190:299–310. doi: 10.1111/j.1469-8137.2010.03552.x. [DOI] [PubMed] [Google Scholar]
- 16.Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S. Low levels of pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to decreased vitality under oxygen deficiency. Ann Bot. 2005;96:717–26. doi: 10.1093/aob/mci223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Colmer TD, Millar AH. Does anoxia tolerance involve altering the energy currency towards PPi? Trends Plant Sci. 2008;13:221–7. doi: 10.1016/j.tplants.2008.02.007. [DOI] [PubMed] [Google Scholar]