Abstract
Species-specific immunity is induced when an effector protein from a specific pathogen strain is perceived by a cognate resistance (R) protein in the plant. In Arabidopsis, the R protein HRT, which confers resistance to turnip crinkle virus (TCV), is activated upon recognition of the TCV coat-protein (CP), a potent suppressor of host RNA silencing. Recognition by HRT does not require RNA silencing suppressor function of CP and is not associated with the accumulation of TCV-specific small-RNA. However, several components of the host RNA silencing pathway participate in HRT-mediated defense against TCV. For example, the double-stranded RNA-binding protein (DRB) 4 interacts with the plasma membrane localized HRT, and is required for its stability. Intriguingly, TCV infection promotes the cytosolic accumulation of the otherwise primarily nuclear DRB4, and this in turn inhibits HRT-DRB4 interaction. These data together with differential localization of DRB4 in plants inoculated with avirulent and virulent viruses, suggests that sub-cellular compartmentalization of DRB4 plays an important role in activation of HRT.
Keywords: DRB4, RNA silencing, plant defense, resistance protein, turnip crinkle virus
Resistance (R) gene-mediated or species-specific immunity is induced when a strain-specific avirulence (avr) protein from the pathogen associates with a cognate plant R protein.1-3 In the absence of the R protein, plants can induce a basal defense response against pathogens, although this mode of defense fails to contain the pathogen in contrast to R gene-mediated resistance. Basal defense to viruses often involves RNA silencing,4-10 which is induced upon the formation of double stranded (ds) RNA that is processed to small (s) 20–30 nucleotide (nt) dsRNA with staggered ends. The ribonuclease III enzymes called Dicers, mediate the processing of dsRNA into small RNA. Viral pathogens in turn express suppressors that target host RNA silencing components.4-10 Plants in turn have evolved to recognize these viral silencing suppressors as avr factors via R proteins, the activation of which induces defense responses. For instance, the Arabidopsis R protein HRT [Hypersensitive response (HR) to Turnip crinkle virus (TCV)] is activated by TCV coat protein (CP),11,12 which is a potent RNA silencing suppressor.13,14 A direct interaction between HRT and CP has not been detectable but TCV-CP was found to directly interact with a NAC transcription activator-like protein, TIP (TCV interacting protein) and inhibit the nuclear localization of TIP.15 Thus, it was suggested that HRT-mediated signaling is activated when interaction with TCV-CP alters the cellular distribution of TIP. However, loss of TIP does not inhibit HRT-mediated resistance to TCV16 but does compromise basal resistance to TCV and Cucumber mosaic virus.16,17
Most Arabidopsis ecotypes lack the R gene HRT and are therefore susceptible to TCV.12,18-28 Only Di-17, a resistant line isolated from the Dijon (Di) ecotype contains HRT, and is resistant to TCV. Following TCV infection, Di-17 plants develop HR, induce defense gene expression such as pathogenesis related (PR)-1, and accumulate salicylic acid (SA).12,18,21-24 In contrast, plants lacking the dominant gene HRT do not develop HR after TCV infection, and allow systemic spread of the virus that is associated with a crinkled leaf and drooping bolt appearance.12,18-28 Resistance to TCV is dependent upon the SA pathway18,19 as well as blue-light photoreceptors.21-23 Among various components of the SA pathway that regulate HRT-mediated resistance to TCV, enhanced disease susceptibility (EDS) 1, which interacts with HRT, is required for potentiation of CP-triggered HR.24 HRT also interacts with several other proteins including CRT1 (Compromised for Recognition of TCV).25-27 Unlike EDS1, CRT1 is not associated with HRT activation, rather is required for HRT stability.28
The activation of HRT does not require silencing suppressor function of CP.28 This is based on the fact that Di-17 plants inoculated with TCV mutants carrying the R130T (CPB) or R137H (CPC) mutations in CP, which impair RNA silencing suppressor activity of CP,14 can elicit normal HR and PR-1 expression.28 In contrast, the R8A mutant of TCV CP, which is a functional RNA silencing suppressor, is unable to induce normal HR on Di-17 plants. Although RNA silencing suppressor activity of CP does not interface with R-mediated signaling, a number of host RNA silencing components are intricately involved in HRT-mediated resistance signaling. These include RNA dependent RNA polymerase (RDR) 6, dicer-like (DCL) 4 and its interacting partner double-stranded RNA-binding protein (DRB) 4. Although these results suggest the possibility that host RNA silencing pathway interfaces with R-mediated signaling, a number of observations discount such a scenario. For example, viral specific small RNAs accumulate only in plants that do not contain HRT or are unable to activate HRT. Additionally, DRB4 and DCL4 regulate HRT levels, whereas RDR6 affects viral RNA replication. These results suggest that suppression of viral replication upon HRT activation may be efficient enough to not warrant the activation of RNA silencing. Thus, it appears that at least some of these RNA silencing components may fulfill novel functions in HRT-mediated signaling. For example, a loss-of-function mutation in DRB4 reduces HRT to levels that are unable to contain the virus. Furthermore, the HRT drb4 plants are also unable to contain the HR lesions formed in response to TCV inoculation and consequently HR spreads to cover the entire leaf.28 Likewise, a mutation in cry2, crt1 or phot2 resulted in degradation of HRT22,28 and these plants showed spreading HR phenotype and increased replication of TCV (Fig. 1). Thus, CRY2, PHOT2, CRT1, and DRB4 govern HRT stability and of these only DRB4 and CRT1 interact with HRT.
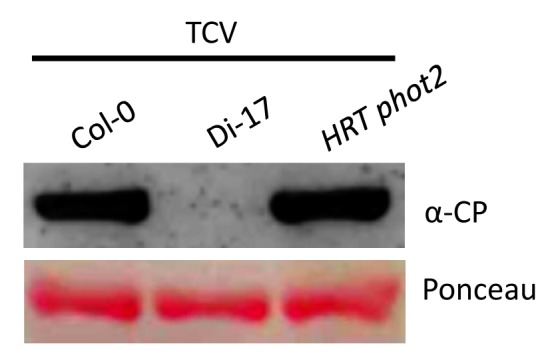
Figure 1. The HRT phot2 plants support increased replication of virus. Western blot showing relative CP levels in TCV infected Col-0 (susceptible), Di-17 (resistance) and HRT phot2 plants. Leaves were sampled at 3 dpi. Ponceau-S staining of the western blot was used as the loading control. This experiment was repeated twice with similar results.
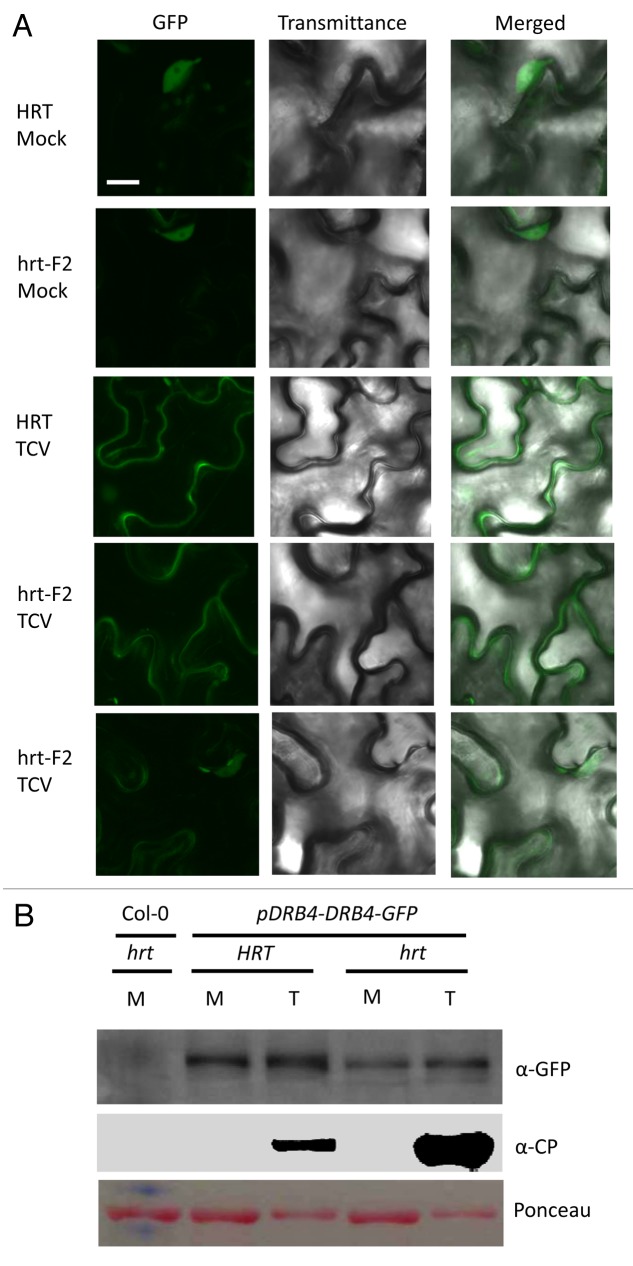
Even though the majority of DRB4 is present in the nucleus (Fig. 2A), only extranuclear pool of DRB4 interacts with HRT, which is consistent with the plasma membrane specific localization of HRT.22 Interestingly, inoculation with avirulent TCV (wild-type or CPB/CPC mutants) triggers an increase in the cytosolic pool of DRB4 within 24 h of inoculation and prior to any visible or microscopic HR formation. Depletion of the nuclear pool of DRB4 in TCV infected plants suggests that viral infection relocalizes nuclear DRB4 to the cytosol. Interestingly, Di-17 plants inoculated with the virulent virus R8A retain DRB4 in the nucleus, suggesting that localization of DRB4 may be important for resistance signaling. To determine if TCV-mediated relocalization of DRB4 in TCV inoculated plants occurred specifically in HRT-containing plants, we evaluated DRB4-GFP levels and localization in plants lacking HRT. These plants were obtained by crossing Di-17 DRB4-GFP (HRT/HRT) with Col-0 (hrt/hrt) ecotype and the F2’s segregating for HRT or hrt were inoculated with TCV and analyzed for the localization and levels of DRB4-GFP. As seen previously for wild-type TCV or R8A inoculated Di-17 plants,28 TCV infection increased DRB4-GFP levels in both HRT and hrt plants (Fig. 2). However, confocal analysis of hrt plants showed that a significant number of the infected cells contained DRB4-GFP in the nucleus, however DRB4-GFP was completely relocated to the cytosol of ~10% of of the infected cells (Fig. 2A). DRB4 also relocalizes to the cytoplasm in response to Turnip yellow mosaic virus (TYMV) infection, suggesting that a low level of subcellular redistribution of DRB4 also occurs during the virulent response.29 However, unlike in hrt plants, the majority of HRT cells (~90%) showed relocalization of DRB4, which in turn was associated with activation of HRT. Notably, increased cytosolic accumulation of DRB4 in the TCV infected Di-17 plants resulted in decreased interaction between HRT and DRB4 rather than promoting it.28 Furthermore, transient expression of CP along with HRT and DRB4 in Nicotiana benthamiana completely abolished the HRT-DRB4 interaction, but not the HRT-CRT1 or HRT-EDS1 interactions.28 Together, these results suggest that CP-mediated activation of HRT is associated with reduced interaction between HRT and DRB4 proteins (Fig. 3). Whether CP dissociates existing HRT-DRB4 complex or prevents association between newly formed HRT and DRB4 proteins remain unknown at this stage. In this regard, it is interesting to note that at least some portion of CP associates with DRB4. Whether the CP-DRB4 interaction contributes to HRT activation needs further investigation. The fact that DRB4 also interacts with the P6 protein of Cauliflower mosaic virus,30 suggests that it might be a common target for many viral pathogens. DRB4 protein also associates with viral RNA and has been suggested to participate in repression of viral RNA translation.29 Thus, several possibilities exist with regards to how DRB4 might function in viral resistance. DRB4 is also required for R-mediated resistance against bacterial pathogens,28 suggesting a more universal role in pathogen resistance. Moreover, as with HRT, a mutation in DRB4 also reduces levels of the R proteins RPS2 and RPM1,28 which specify resistance against Pseudomonas syringae expressing avrRpt2 or avrRpm1, respectively. This suggests that DRB4 functions as a common regulator of R-mediated defense against multiple pathogens and that its role in R-mediated signaling is likely different from the role that it plays in RNA silencing pathway.28 Future work should help elucidate how DRB4 functions in the activation of HRT and other R proteins and the biological significance of pathogen-induced relocalization of DRB4.
Figure 2. TCV inoculation increases the cytosolic pool of DRB4. (A) Confocal micrographs showing localization of DRB4-GFP in mock- or TCV-infected Arabidopsis plants. The leaves were analyzed at 3 dpi. The experiment was repeated twice (4 replicates per experiment) with similar results. Scale bars, 10 µM. (B) western blot showing DRB4-GFP and CP levels in mock (M)- and TCV (T)-infected transgenic plants expressing DRB4-GFP via self-promoter. This experiment was repeated twice with similar results.
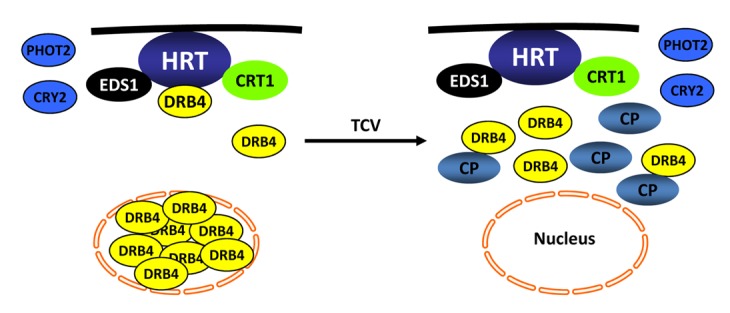
Figure 3. A sketch of components involved in HRT-mediated resistance signaling against TCV. HRT is present on the plasma membrane (shown by black line) and interacts with EDS1, CRT1, and DRB4 proteins. Of these, DRB4 is primarily present in the nucleus, EDS1 is both nuclear and cytoplasmic, whereas CRT1 is on endomembranes. TCV-induced resistance response is initiated in the presence of TCV-CP. Upon recognition of TCV-CP, majority of the DRB4 relocalizes to cytoplasm, where a small pool of DRB4 interacts with CP. The presence of CP also prevents HRT-DRB4 complex formation but has no effect on HRT-EDS1 and HRT-CRT1 interactions. EDS1 potentiates HRT-mediated cell death response and acts redundantly with salicylic acid to regulate HR to TCV. Mutations in DRB4, CRT1, and the blue-light photoreceptors CRY2 and PHOT2, reduce HRT level. Consequently the mutant plants are unable to regulate HR or replication of TCV. No interaction has been detected between CRY2/PHOT2 and HRT, suggesting that these proteins stabilize HRT by inhibiting a negative regulator that mediates degradation of HRT.
Experimental Procedures
Plant growth conditions, genetic analysis and generation of transgenic plants
Plants were grown in MTPS 144 Conviron (Winnipeg, MB, Canada) walk-in-chambers at 22 °C, 65% relative humidity and 14 h photoperiod. The photon flux density of the day period was 106.9 µmoles m–2 s–1 and was measured using a digital light meter (Phytotronic Inc., Earth city, MO). The cross between Di-17::DRB4-GFP and Col-0 plants were performed by emasculating the flowers of the Col-0 genotype and pollinating with the pollen from the Di-17::DRB4-GFP. F2 plants containing the GFP transgene were scored using PCR analysis. The genotype at the HRT locus was determined using CAPS analysis as described earlier.12,18,22,24,28
TCV infections
Transcripts synthesized in vitro from a cloned cDNA of TCV using T7 RNA polymerase were used for viral infections. For inoculations, the viral transcript was suspended at a concentration of 0.05 µg/ µL in inoculation buffer, and the inoculation was performed as described earlier.19 After viral inoculations, the plants were transferred to a Conviron MTR30 reach-in chamber maintained at 22 °C, 65% relative humidity and 14 h photoperiod. HR was determined visually three-to-four days post-inoculation (dpi). Resistance and susceptibility was scored at 14 to 21 dpi and confirmed by northern gel blot analysis. Susceptible plants showed stunted growth, crinkling of leaves and drooping of the bolts.
Protein extraction and Immunoblot analysis
Proteins were extracted in buffer containing 50 mM TRIS-HCl, pH 7.5, 10% glycerol, 150 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 5 mM DTT, and 1X protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Protein concentration was measured by the Bio-RAD protein assay (Bio-Rad, CA). For Ponceau-S staining, PVDF membranes were incubated in Ponceau-S solution (40% methanol (v/v), 15% acetic acid (v/v), 0.25% Ponceau-S). The membranes were destained using deionized water. Proteins (30–50 µg) were fractionated on a 7–10% SDS-PAGE gel and subjected to immunoblot analysis using α-CP or α-GFP antibody. Immunoblots were developed using alkaline-phosphatase-based color detection.
Confocal microscopy
For confocal imaging, samples were scanned on an Olympus FV1000 microscope (Olympus America, Melvile, NY). The transgenic Arabidopsis plants expressing DRB4-GFP under its native promoter were inoculated with TCV and 72 h later, water-mounted sections of leaf tissue were examined by confocal microscopy using a water immersion PLAPO60XWLSM 2 (NA 1.0) objective on a FV1000 point-scanning/point-detection laser scanning confocal 3 microscope (Olympus) equipped with lasers spanning the spectral range of 405–633 nm. Transmittance and GFP overlay images (40X magnification) were acquired at a scan rate of 10 ms/pixel. Olympus FLUOVIEW 1.5 was used to control the microscope, image acquisition and the export of TIFF files.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from National Science Foundation (IOS #10641576) and Kentucky Science and Engineering Foundation (1214-RDE-009).
References
- 1.Martin GB, Bogdanove AJ, Sessa G. Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol. 2003;54:23–61. doi: 10.1146/annurev.arplant.54.031902.135035. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–14. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Kachroo P, Chandra-Shekara AC, Klessig DF. Plant signal transduction and defense against viral pathogens. Adv Virus Res. 2006;66:161–91. doi: 10.1016/S0065-3527(06)66004-1. [DOI] [PubMed] [Google Scholar]
- 4.Pumplin N, Voinnet O. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat Rev Microbiol. 2013;11:745–60. doi: 10.1038/nrmicro3120. [DOI] [PubMed] [Google Scholar]
- 5.Mlotshwa S, Pruss GJ, Vance V. Small RNAs in viral infection and host defense. Trends Plant Sci. 2008;13:375–82. doi: 10.1016/j.tplants.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Ding S-W, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–26. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voinnet O. Post-transcriptional RNA silencing in plant-microbe interactions: a touch of robustness and versatility. Curr Opin Plant Biol. 2008;11:464–70. doi: 10.1016/j.pbi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Moffett P. Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res. 2009;75:1–33. doi: 10.1016/S0065-3527(09)07501-0. [DOI] [PubMed] [Google Scholar]
- 9.Carr JP, Lewsey MG, Palukaitis P. Signaling in induced resistance. Adv Virus Res. 2010;76:57–121. doi: 10.1016/S0065-3527(10)76003-6. [DOI] [PubMed] [Google Scholar]
- 10.Ding S-W. RNA-based antiviral immunity. Nat Rev Immunol. 2010;10:632–44. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, DelGrosso L, Yigit E, Dempsey DA, Klessig DF, Wobbe KK. The amino terminus of the coat protein of Turnip crinkle virus is the AVR factor recognized by resistant arabidopsis. Mol Plant Microbe Interact. 2000;13:1015–8. doi: 10.1094/MPMI.2000.13.9.1015. [DOI] [PubMed] [Google Scholar]
- 12.Cooley MB, Pathirana S, Wu HJ, Kachroo P, Klessig DF. Members of the Arabidopsis HRT/RPP8 family of resistance genes confer resistance to both viral and oomycete pathogens. Plant Cell. 2000;12:663–76. doi: 10.1105/tpc.12.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi CW, Qu F, Ren T, Ye X, Morris TJ. RNA silencing-suppressor function of Turnip crinkle virus coat protein cannot be attributed to its interaction with the Arabidopsis protein TIP. J Gen Virol. 2004;85:3415–20. doi: 10.1099/vir.0.80326-0. [DOI] [PubMed] [Google Scholar]
- 14.Cao M, Ye X, Willie K, Lin J, Zhang X, Redinbaugh MG, Simon AE, Morris TJ, Qu F. The capsid protein of Turnip crinkle virus overcomes two separate defense barriers to facilitate systemic movement of the virus in Arabidopsis. J Virol. 2010;84:7793–802. doi: 10.1128/JVI.02643-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren T, Qu F, Morris TJ. The nuclear localization of the Arabidopsis transcription factor TIP is blocked by its interaction with the coat protein of Turnip crinkle virus. Virology. 2005;331:316–24. doi: 10.1016/j.virol.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Jeong RD, Chandra-Shekara AC, Kachroo A, Klessig DF, Kachroo P. HRT-mediated hypersensitive response and resistance to Turnip crinkle virus in Arabidopsis does not require the function of TIP, the presumed guardee protein. Mol Plant Microbe Interact. 2008;21:1316–24. doi: 10.1094/MPMI-21-10-1316. [DOI] [PubMed] [Google Scholar]
- 17.Donze T, Qu F, Twigg P, Morris TJ. Turnip crinkle virus coat protein inhibits the basal immune response to virus invasion in Arabidopsis by binding to the NAC transcription factor TIP. Virology. 2014;449:207–14. doi: 10.1016/j.virol.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandra-Shekara AC, Navarre D, Kachroo A, Kang H-G, Klessig D, Kachroo P. Signaling requirements and role of salicylic acid in HRT- and rrt-mediated resistance to turnip crinkle virus in Arabidopsis. Plant J. 2004;40:647–59. doi: 10.1111/j.1365-313X.2004.02241.x. [DOI] [PubMed] [Google Scholar]
- 19.Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell. 2000;12:677–90. doi: 10.1105/tpc.12.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempsey DA, Pathirana MS, Wobbe KK, Klessig DF. Identification of an Arabidopsis locus required for resistance to turnip crinkle virus. Plant J. 1997;11:301–11. doi: 10.1046/j.1365-313X.1997.11020301.x. [DOI] [PubMed] [Google Scholar]
- 21.Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J. 2006;45:320–34. doi: 10.1111/j.1365-313X.2005.02618.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeong R-D, Chandra-Shekara AC, Barman SR, Navarre D, Klessig DF, Kachroo A, Kachroo P. Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2010;107:13538–43. doi: 10.1073/pnas.1004529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong R-D, Kachroo A, Kachroo P. Blue light photoreceptors are required for the stability and function of a resistance protein mediating viral defense in Arabidopsis. Plant Signal Behav. 2010;5:1504–9. doi: 10.4161/psb.5.11.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Jeong R-D, Venugopal SC, Lapchyk L, Navarre D, Kachroo A, Kachroo P. SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against turnip crinkle virus. PLoS Pathog. 2011;7:e1002318. doi: 10.1371/journal.ppat.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang HG, Kuhl JC, Kachroo P, Klessig DF. CRT1, an Arabidopsis ATPase that interacts with diverse resistance proteins and modulates disease resistance to turnip crinkle virus. Cell Host Microbe. 2008;3:48–57. doi: 10.1016/j.chom.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Kang HG, Oh CS, Sato M, Katagiri F, Glazebrook J, Takahashi H, Kachroo P, Martin GB, Klessig DF. Endosome-associated CRT1 functions early in resistance gene-mediated defense signaling in Arabidopsis and tobacco. Plant Cell. 2010;22:918–36. doi: 10.1105/tpc.109.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang H-G, Hyong WC, von Einem S, Manosalva P, Ehlers K, Liu P-P, Buxa SV, Moreau M, Mang H-G, Kachroo P, et al. CRT1 is a nuclear-translocated MORC endonuclease that participates in multiple levels of plant immunity. Nat Commun. 2012;3:1297. doi: 10.1038/ncomms2279. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S, Jeong R-D, Lim G-H, Yu K, Wang C, Chandra-Shekara AC, Navarre D, Klessig DF, Kachroo A, Kachroo P. Double-stranded RNA-binding protein 4 is required for resistance signaling against viral and bacterial pathogens. Cell Rep. 2013;4:1168–84. doi: 10.1016/j.celrep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Jakubiec A, Yang SW, Chua N-H. Arabidopsis DRB4 protein in antiviral defense against Turnip yellow mosaic virus infection. Plant J. 2012;69:14–25. doi: 10.1111/j.1365-313X.2011.04765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas G, Azevedo J, Moissiard G, Geldreich A, Himber C, Bureau M, Fukuhara T, Keller M, Voinnet O. Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J. 2008;27:2102–12. doi: 10.1038/emboj.2008.129. [DOI] [PMC free article] [PubMed] [Google Scholar]