Abstract
Purpose
Chemotherapy-induced nausea and vomiting (CINV) have a negative impact on patients' quality of life and frequently pointed to as a major factor for treatment abandonment. Serotonin (5-HT3) receptor antagonist is considered as key treatment for CINV. Ramosetron and palonosetron are recently developed 5-HT3 receptor antagonists and known as more superior than other first-generation 5-HT3 receptor antagonists. The purpose of this study was to compare the efficacy of ramosetron and palonosetron and determine which drug is more effective for prevention of CINV.
Methods
Colorectal cancer patients treated with chemotherapy were enrolled consecutively. Patients were assigned to receive intravenous injection of ramosetron 0.3 mg or palonosetron 0.25 mg at 30 minutes before initiation of moderately emetogenic chemotherapy. Ramosetron group added oral administration of 0.1 mg ramosetron on the second and third days of chemotherapy. Efficacy parameter consisted of nausea and vomiting.
Results
Ninety-one patients received ramosetron and 89 patients received palonosetron. Presentation of vomiting and nausea symptoms was not significantly different between the two groups during acute (0-24 hours) and delayed period (after 24 hours).
Conclusion
The incidence of CINV between the ramosetron and the palonosetron group has not shown any difference during acute, delayed, and overall period.
Keywords: Vomiting, Ramosetron, Palonosetron, Adjuvant chemotherapy, Colorectal neoplasms
INTRODUCTION
Chemotherapy-induced nausea and vomiting (CINV) are the main adverse events during cancer management [1]. CINV has a negative impact on patients' quality of life and frequently a major cause factor for treatment abandonment [2]. Many neurotransmitters in the central nervous system and the afferent vagus nerve endings of the gastrointestinal tract are involved in CINV although the exact mechanisms of CINV are not fully understood [3,4]. However, among neurotransmitters, setotonin (5-hydroxytryptamine [5-HT3]) is considered to play a main role in initiating CINV. In the 1990s, the introduction of 5-HT3 receptor antagonists was attributed to improving control rates for CINV, and 5-HT3 receptor antagonists are now considered the standard treatment regimen for prevention of CINV [5].
CINV can be classified as acute and delayed phase. Acute CINV is defined as when symptoms initiate within the first 24 hours after the chemotherapy start and delayed CINV is defined as when they begin after the first 24 hours and may persist up to 1 week [6]. Although 5-HT3 receptor antagonists are effective for the prevention of CINV, a substantial portion of patients who received moderately or highly emetogenic chemotherapy still suffer from acute and delayed CINV [7]. Therefore, there is a need to use newly developed and clinically available agents to improve control rates for acute and delayed CINV. Among 5-HT3 receptor antagonists, ramosetron and palonosetron are recently developed agents. Ramosetron has been shown to have higher selectivity for serotonin than other first-generation 5-HT3 receptor antagonists in an animal study [8]. Moreover, in the comparative clinical studies, ramosetron had superior efficacy in the acute and delayed CINV than other first-generation 5-HT3 receptor antagonists [9,10]. Palonosetron is a second-generation 5-HT3 receptor antagonist. It has also been shown to have stronger binding affinity for the 5-HT3 receptor than other agents in the class and a prolonged plasma half-life of approximately 40 hours [11]. In the comparative phase III clinical trials, palonosetron had superior efficacy in acute and delayed CINV than other first-generation 5-HT3 receptor antagonists [12,13].
To our knowledge, there is no study that has evaluated the efficacy of ramosetron and palonosetron in the prevention of CINV, although many studies have compared different 5-HT3 receptor antagonists for the prevention of CINV. Therefore, we designed the present study to compare the efficacy of ramosetron and palonosetron to prevent CINV in patients who received combining 5-fluorouracil/leucovorin with irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) for adjuvant or neoadjuvant chemotherapy for colorectal cancer.
METHODS
Consecutive patients who received FOLFOX or FOLFIRI chemotherapy for colorectal cancer were enrolled in this study. Patients were recruited at six tertiary referral hospitals in Korea. This study was approved by the Institutional Review Board of participating hospitals and written informed consent was obtained from each patient. Inclusion criteria were as follows: (1) histologically proven adenocarcinoma of colon and rectum, (2) age more than 19 years, (3) Eastern Cooperative Oncology Group performance status ≤2, (4) No history of previous chemotherapy, immunotherapy, or radiotherapy, (5) adequate bone marrow function (neutrophil counts ≥1.5 × 109/L, hematocrit ≥30%, and platelets ≥100 × 109/L), liver function (total bilirubin ≤1.5 × the upper limit of normal), and renal function (serum creatinine ≤1.25 × the upper limit of normal). Exclusion criteria included other malignancy, severe cardiac or pulmonary disease, radiotherapy, and other medications (antiemetics, steroids, or psychoactive medication) within 24 hours.
Chemotherapy consisted of a regimen of oxaliplatin 85 mg/m2 (FOLFOX regimen) or irinotecan 180 mg/m2 (FOLFIRI regimen) administered intravenously over 30-90 minutes, leucovorin 400/m2 (200 mg/m2 if L form was used) infused over 2 hours, 5-fluorouracil 400 mg/m2 intravenous (i.v.) bolus, and 5-fluorouracil 2,400 mg/m2 continously infused for 46 hours on day 1.
During the first cycle of chemotherapy, all patients had prophylactic 5-HT3 antagonists. Patients were assigned to receive intravenous injection of ramosetron 0.3 mg plus dexamethasone 10 mg (ramosetron group) or palonosetron 0.25 mg i.v. bolus plus dexamethasone 10 mg (palonosetron group) at 30 minutes before chemotherapy on day 1. Oral administration of ramosetron 0.1 mg was given to patients on day 2 and 3 in the ramosetron group, however, no more antiemetics was administered in the palonosetron group except on day 1. Additional rescue antiemetic drugs were permitted if patients complained of symptoms associated with CINV after chemotherapy. Response to antiemetic medication was assessed for three consecutive days during the treatment. Follow-up check was performed at 5 to 7 days via telephone contact. Acute CINV was defined as occurring within 24 hours after initiation of chemotherapy and delayed CINV was defined as occurring on day 2 through 5 to 7 days after chemotherapy infusion. The maximum grade of anorexia, nausea, and vomiting was assessed by the Common Toxicity Criteria [14].
Sample size was caluculated by Siz program ver. 1.0 (Cytel Inc., Cambridge, MA, USA). Allowing an a error of 5% and a b error of 20%, it was estimated that 82 patients were distributed into two groups based on the assumption of a responder rate of 70% in the palonosetron group, and ramosetron group would be required to show 15% difference. Assuming a 10% drop out rate, 91 patients per group needed to be enrolled. Chi-square or Fisher exact test was used for statistical analysis. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
One hundred eighty-two patients were recruited between September 2010 and October 2011. Of the 182 patients, two did not receive study medication. Therefore, a total of 180 patients were evaluable in this study. Ramosetron group had 91 patients and palonosetron group had 89 patients. The distribution of patients by gender, age, performance status, body surface area, and history of alcohol consumption was similar between the two groups. However, patients with advanced stage of disease (P < 0.001) and FOLFIRI chemotherapy (P = 0.01) were more frequent in the palonosetron group. As shown in Table 1, demographic variables except stage and chemotherapy regimen were relatively well matched between the two groups. But, palonosetron group had a greater proportion of stage IV disease and FOLFIRI chemotherapy regimen.
Table 1.
Demographic characteristics
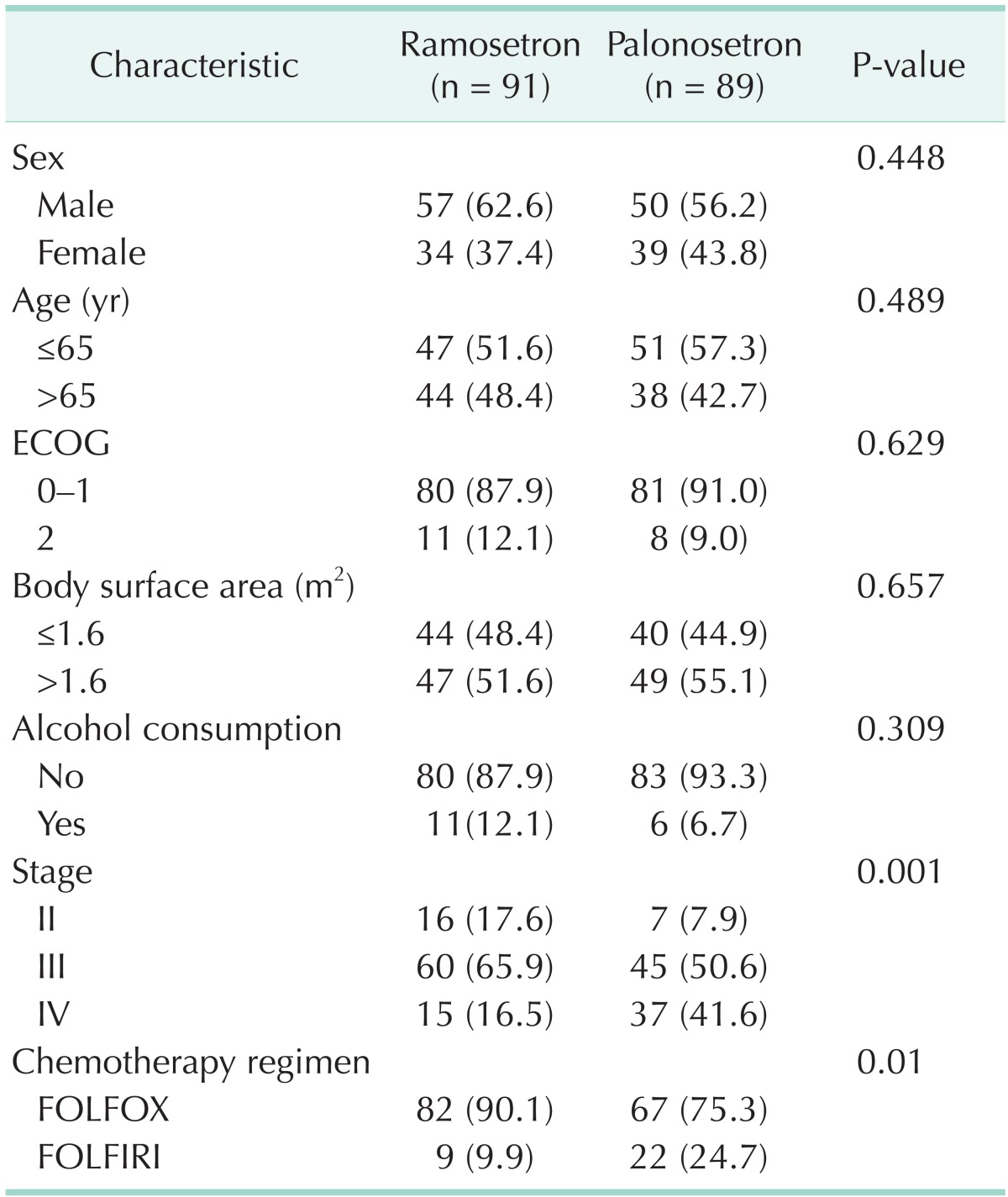
Values are presented as number (%).
ECOG, Eastern Cooperative Oncology Group; FOLFOX, 5-fluorouracil/leucovorin with oxaliplatin; FOLFIRI, 5-fluorouracil/leucovorin with irinotecan.
Vomiting symptoms occurred in 2.2% of patients during the acute period and 6.6% of patients during the delayed period in the ramosetron group. Similarly, vomiting was noted in 4.5% of patients during the acute period and in 10.1% of patients during the delayed period in the palonosetron group. Nausea symptoms occurred in 9.9% of patients during the acute period and 36.3% during the delayed period in the ramosetron group. Similarly, nausea was noted in 11.2% of patients during the acute period and in 42.7% of patients during the delayed period in the palonosetron group. Therefore, the incidence of CINV was not statistically different during acute, delayed, and overall period between the two groups (Table 2).
Table 2.
Percentage of patients with symptoms after chemotherapy infusion
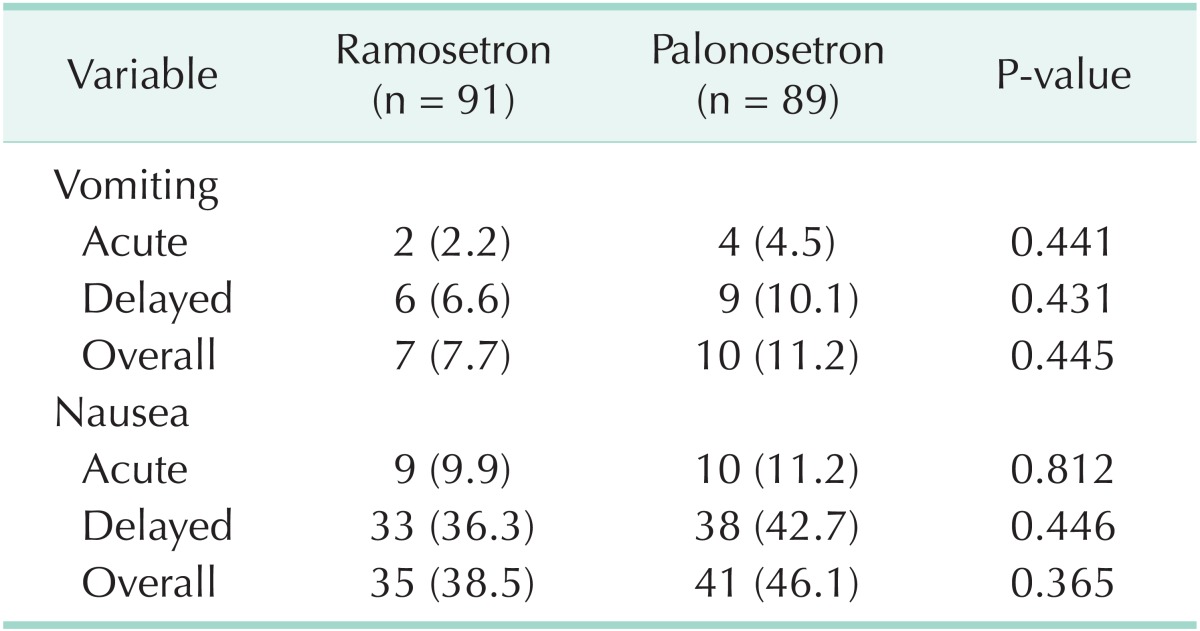
Values are presented as number (%).
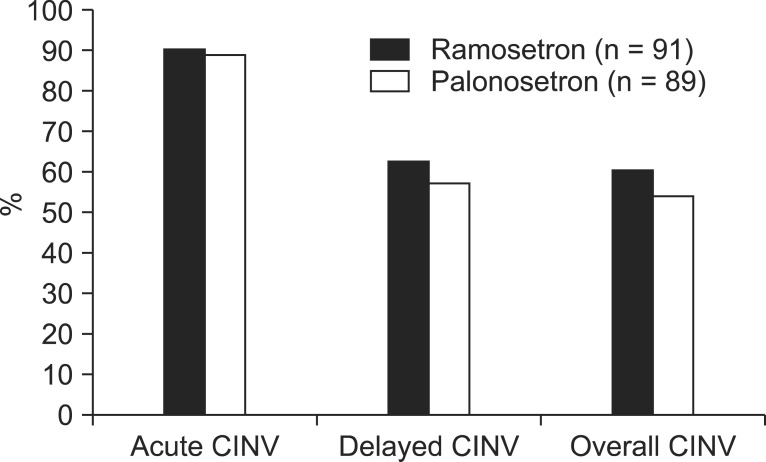
Percentages of patients with no emetic episode during acute, delayed, and overall time period after administration of chemotherapy regimen are shown in Fig. 1. The complete response rate in the two groups during the acute period was 90.1% and 88.8%, respectively (P = 0.812), while 9.9% in ramosetron group and 11.2% in palonosetron group were classified as failures. Similarly, for delayed and overall period after administration of chemotherapy regimen, the percentages of patients with no nausea and no vomiting was not significantly different between the two groups (62.6% vs. 57.3%, P = 0.543, and 60.4% vs. 53.9%, P = 0.451, respectively), while 37.4% in ramosetron group and 42.7% in palonosetron group were classified as failures during the delayed period.
Fig. 1.
Proportion of patients with no emetic episodes during the acute, delayed and overall time period (n = 180). CINV, chemotherapy-induced nausea and vomiting.
DISCUSSION
Nausea and vomiting is one of the most frequently observed complications during cancer therapy [1,2]. Emetic response in CINV is very complex and triggered by afferent inputs that arrive from the chemoreceptor trigger zone, pharynx, gastrointestinal tract, and cerebral cortex. Various neurotransmitters and receptor systems such as histaminergic, cholinergic, dopaminergic, neurokininergic and serotonergic mediate these signals. However, a final common pathway for emesis has not yet been identified. Therefore, no single antiemetic agent can be expected to show complete remission from CINV [15]. Among available antiemetic regimens, 5-HT3 receptor antagonists have been considered the main agent for prevention of CINV [5]. The American Society of Clinical Oncology and National Comprehensive Cancer Network recommend the use of antiemetics before initiation of chemotherapy. Oxaliplatin and irinotecan are categorized as moderate risk group for CINV, and prophylaxis with the 5-HT3 receptor antagonist is recommended [15,16]. In addition to chemotherapeutic agent, age, sex, prior chemotherapy, and alcohol consumption can also affect the occurrence of CINV [17].
Ramosetron is a relatively recently developed 5-HT3 antagonist that has a longer and more effective agent compared with previously developed 5-HT3 antagonists [9,10]. Furthermore, the pharmaceutical effect of ramosetron can last up to 48 hours [18]. These superiorities of ramosetron may be attributed to the longer half life and higher receptor affinity of ramosetron [8]. Palonosetron is the latest 5-HT3 receptor antagonist. Palonosetron has been regarded as the first of a second generation of 5-HT3 receptor antagonists because of its unique properties. It has been shown to have the longest elimination half-life with 40 hours and avid receptor binding affinity compared with other 5-HT3 antagonists [11]. Palonosetron is differentiated from other 5-HT3 receptor antagonists by internalization of the 5-HT3 receptor and decrease in the function of the receptor [19]. This fact led to the hypothesis that palonosetron would have stronger and longer antiemetic effects compared with ramosetron. In our study, there were no statistical differences in prevention of CINV between ramosetron and palonosetron group. This result may be attributed to the addition of oral intake of ramosetron 0.1 mg on the second and third day of chemotherapy as per the manufacturer's instruction. Oral addition of ramosetron may enhance the duration of antiemetic effect although previous comparative studies of palonosetron with ondansetron, granisetron, and dolansetron have showed superior antiemetic effects of palonosetron [20]. A prolonged duration of antiemetics could be valuable because CINV can persist for several days after completion of chemotherapy.
The proportion of no emetic episodes in this study was similar to those reported previously with palonosetron 0.25 mg i.v. bolus. The proportion of no emetic episodes ranges from 63% to 81% for acute CINV, 54% to 74% for delayed CINV, and 46% to 69.3% for overall CINV in other similarly designed phase III trials of palonosetron for prevention of moderate emetogenic risk of chemotherapy [12,13]. The consistency of these rates with those reported in the current study (89.4% for acute CINV, 60% for delayed CINV, and 57.2% for overall CINV) highlights the validity of this study.
The limitation of our study deserves mention. A selection bias probably existed because the proportion of patients with stage IV and FOLFIRI chemotherapy is higher in the palonosetron than in the ramosetron group. In the beginning of investigation, each group was not randomly assigned. Consequently, patients enrolled for chemotherapy had heterogenous grouping. This bias may influence emetic symptoms. However, there were no statistical differences in patient characteristics between the ramosetron and palonosetron group in terms of age, gender, performance status, body surface area, and history of alcohol consumption.
In conclusion, our study demonstrated that a single i.v. dose of palonosetron resulted prevention of acute and delayed CINV following FOLFOX or FOLFIRI chemotherapy, which is known as a moderate emetogenic agent. Moreover, the incidence of CINV between the ramosetron and palonosetron group did not show a difference during all time intervals. Thus, single dose of palonosetron and oral administration of ramosetron following i.v. bolus ramosetron would be a significant and important antiemetic regimen.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, et al. On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol. 1996;7:189–195. doi: 10.1093/oxfordjournals.annonc.a010548. [DOI] [PubMed] [Google Scholar]
- 2.Almeida EP, Gutierrez MG, Adami NP. Monitoring and evaluation of side effects of chemotherapy in patients with colon cancer. Rev Lat Am Enfermagem. 2004;12:760–766. doi: 10.1590/s0104-11692004000500009. [DOI] [PubMed] [Google Scholar]
- 3.Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 4.Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol. 1992;59:221–226. doi: 10.1254/jjp.59.221. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh PJ. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest. 2000;18:163–173. doi: 10.3109/07357900009038248. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005;6:93–102. doi: 10.1016/S1470-2045(05)01735-3. [DOI] [PubMed] [Google Scholar]
- 7.Kaizer L, Warr D, Hoskins P, Latreille J, Lofters W, Yau J, et al. Effect of schedule and maintenance on the antiemetic efficacy of ondansetron combined with dexamethasone in acute and delayed nausea and emesis in patients receiving moderately emetogenic chemotherapy: a phase III trial by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12:1050–1057. doi: 10.1200/JCO.1994.12.5.1050. [DOI] [PubMed] [Google Scholar]
- 8.Miyata K, Yamano M, Kamato T, Akuzawa S. Effect of serotonin (5-HT)3-receptor antagonists YM060, YM114 (KAE-393), ondansetron and granisetron on 5-HT4 receptors and gastric emptying in rodents. Jpn J Pharmacol. 1995;69:205–214. doi: 10.1254/jjp.69.205. [DOI] [PubMed] [Google Scholar]
- 9.Kang YK, Park YH, Ryoo BY, Bang YJ, Cho KS, Shin DB, et al. Ramosetron for the prevention of cisplatin-induced acute emesis: a prospective randomized comparison with granisetron. J Int Med Res. 2002;30:220–229. doi: 10.1177/147323000203000302. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, et al. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: A prospective randomized controlled study. Chemotherapy. 2007;53:44–50. doi: 10.1159/000098418. [DOI] [PubMed] [Google Scholar]
- 11.Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in U.S. and Japanese healthy subjects. J Clin Pharmacol. 2004;44:520–531. doi: 10.1177/0091270004264641. [DOI] [PubMed] [Google Scholar]
- 12.Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–1577. doi: 10.1093/annonc/mdg417. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473–2482. doi: 10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer Therapy Evaluation Program: CTCAE v4.0 Open Comment Period [Internet] Bethesda: National Cancer Institute; [cited 2014 Oct 13]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 15.Ettinger DS, Armstrong DK, Barbour S, Berger MJ, Bierman PJ, Bradbury B, et al. Antiemesis. J Natl Compr Canc Netw. 2012;10:456–485. doi: 10.6004/jnccn.2012.0047. [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006;14:354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 18.Fujii Y, Tanaka H, Ito M. Ramosetron compared with granisetron for the prevention of vomiting following strabismus surgery in children. Br J Ophthalmol. 2001;85:670–672. doi: 10.1136/bjo.85.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626:193–199. doi: 10.1016/j.ejphar.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer. 2011;19:823–832. doi: 10.1007/s00520-010-0908-8. [DOI] [PubMed] [Google Scholar]