Abstract
The IRON-REGULATED TRANSPORTER1 (IRT1) is the principal importer of soil iron in Arabidopsis thaliana. It has a complex intracellular trafficking behavior, including continuous cycling between plasma membrane and endosomes. SORTING NEXIN1 is required for the recycling of endosome-localized IRT1. In its absence, IRT1 is mistargeted for degradation, resulting in reduced plant iron-uptake efficiency. Consequently, IRT1 promoter activity gets limited to a specific portion of the root. We tested the influence of two hormones known to positively affect iron uptake on IRT1 spatial regulation. We found that ethylene treatment in wild-type background mimics the effects of the SNX-loss-of-function situation. Conversely, auxin splits the IRT1 expression zone and forces it toward the two extremities of the root. This shows that IRT1 expression along the root is modulated by ethylene-auxin interplay.
Keywords: iron uptake, sorting nexin, gene expression, ethylene, auxin, IRT1
Plants take up soil iron throughout their development. In Arabidopsis thaliana, which uses a three step reduction-based iron acquisition strategy,1 import of solubilized soil iron from the root apoplast is achieved by the bivalent metal transporter IRON-REGULATED TRANSPORTER1 (IRT1).2,3 As a transmembrane protein transporting iron across the plasma membrane (PM), the subcellular localization of IRT1 plays an important role in the regulation of its activity. The protein was shown to localize to the early endosomes and, to a much lower extent, to the PM. Chemical treatments revealed that this picture is a result of active processes of endocytosis and export.4 Internalization of PM-localized IRT1 is a ubiquitination-dependent process. Ubiquitination-resistant IRT1 has enhanced stability and its expression in plants causes metal overaccumulation.4,5 Recently, the E3 ubiquitin ligase IRT1 DEGRADATION FACTOR1 (IDF1) was shown to regulate the internalization of PM-localized IRT1.6
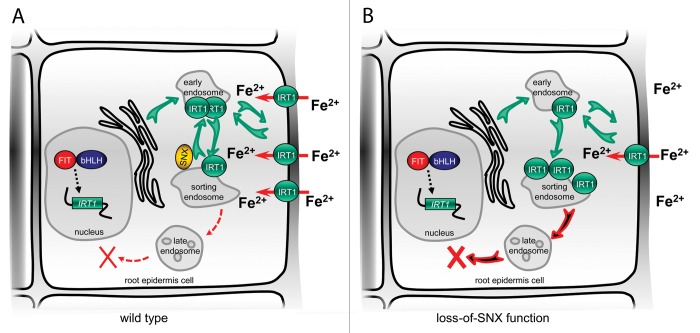
Following the internalization from the PM, the fate of endocytosed IRT1 is decided in the endosomes. The protein can be either targeted for degradation, or reused and sent for another round of activity to the PM (Fig. 1A). This sorting step involves more than one type of endosomal compartments and is dependent on the SORTING NEXIN (SNX) protein family.7 IRT1 and SNX1 colocalize in a subpopulation of the trans-Golgi Network that represents the plant sorting endosome. The subsequent retrograde transport of IRT1 back to the secretory pathway is dependent on the SNX action (Fig. 1A). In the absence of SNX, IRT1 is targeted for degradation, probably due to excessive accumulation in the sorting endosome (Fig. 1B). This represents a case different to other transmembrane proteins, like the Brassica oleracea SRK3 receptor kinase, which in its non-active state tends to accumulate in VPS29-labeled sorting endosomes without undergoing degradation.8 Newly synthesized IRT1 is able to reach the PM through the secretory pathway independently of the SNX1-labeled sorting endosome. That is why snx mutants retain capability to partially induce their iron uptake machinery under iron deficiency (Fig. 1B). However, since the amounts of PM-localized IRT1 depend on the streams of both newly synthesized and recycled IRT1, the perturbed IRT1 retrograde trafficking in the absence of SNX results in reduced amounts of transporter at the PM and reduced iron uptake efficiency (Fig. 1B).
Figure 1. Role of SNX proteins in IRT1 trafficking and stability. (A) In wild type, under iron deficiency the newly synthesized IRT1 protein follows the secretory pathway and, passing through the early endosomes, enters the PM where it imports bivalent iron from the root apoplast. From the PM IRT1 is internalized and sent through the early endosome to the sorting endosome, where a decision needs to be made. IRT1 is either targeted for degradation (red punctate arrows) through the late endosome, or travels back to the early endosome in a SNX-dependent manner, to be sent again to the PM. (B) In the absence of SNX proteins, IRT1 molecules cannot exit the sorting endosome and are degraded (red-and-black arrows). Only the newly-synthesized IRT1 molecules can reach the PM. As a consequence of the lower number of transporters at the PM, reduced amounts of iron are imported into the cell.
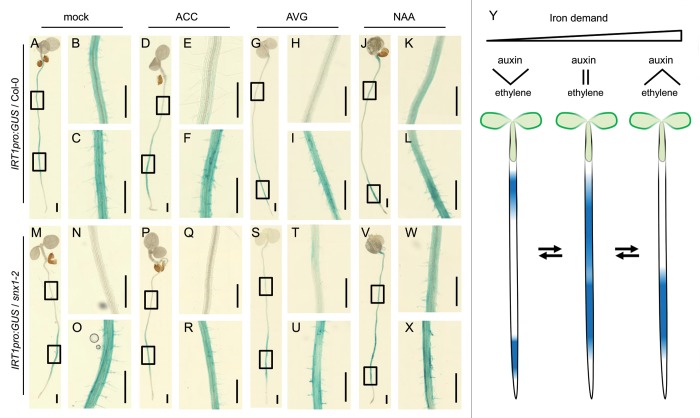
In response to the disturbed iron homeostasis in snx mutants, expression of iron uptake-related genes is changed, leading to higher IRT1 expression levels compared with the wild type. At the whole-root level, this increased expression is concentrated in just a part of the differentiation zone containing the newly developed root hairs,7 which we designate the early differentiation zone. Since the hormones ethylene and auxin were shown to positively influence iron deficiency responses,9,10,11,12 we wanted to test their role in influencing the zone where IRT1 is expressed, and thus the region where iron uptake occurs. We used 8 d-old iron-deficient wild-type (Col-0) and snx1–2 plants expressing the IRT1pro:GUS cassette.3,7 Plants were grown upright on agar plates containing iron-deficient Hoagland medium7 and were transferred for two hours to liquid growth medium, which remained untreated (mock control) or contained either 10µM 1-Aminocyclopropane-1-carboxylic acid (ACC, an ethylene biosynthesis donor), 20 µM Aminoethoxyvinylglycine (AVG, an ethylene synthesis inhibitor) or 10µM 2-(1-Naphthyl)acetic acid (NAA, a synthetic auxin). We chose these relatively short-term treatments in order to better observe the progress, rather than the end effect, of hormone action. Visualization of β-glucuronidase (GUS) activity was described previously.7 Three independent experiments were performed with a minimum of five analyzed plants per condition per experiment. In Col-0 background, ethylene treatment caused the concentration of IRT1 promoter activity in the early differentiation zone (Fig. 2D-F). The situation resembled the mock-treated snx1–2 condition (Fig. 2M-O), suggesting that the change of IRT1 expression in the absence of SNX might be due to enhanced ethylene influence. This is supported also by the fact that in snx1–2 background ethylene did not cause any significant changes in comparison to the mock-treated control (Fig. 2P-R). After AVG treatment, a prolonged staining duration was required, due to the documented AVG inhibitory effects on IRT1 expression.11,12 The IRT1 promoter activity zone was strongly shifted toward the root tip (Fig. 2G-I). In snx1–2 background, a slight reappearance of IRT1 expression could be observed in shootward direction from the early differentiation zone (Fig. 2S-U), showing a partial phenotype reversal when ethylene amounts are reduced. A full recovery is not possible, since the two hours of treatment represent an intermediate state before the complete switch off of the IRT1 promoter. Auxin treatment in Col-0 background caused a clear separation of IRT1 expression into two zones, which tended to move away from each other toward the hypocotyl and toward the root tip, respectively (Fig. 2J-L). The latter fully resembles the situation after ethylene depletion (Fig. 2G and I). Our auxin results are similar to the ones reported by Seguela et al.,13 where a longer (24h) auxin exposure resulted in the concentration of IRT1 promoter activity in two foci, one at the base of the root and one at the root tip. In the snx1–2 mutant background, auxin application extended the IRT1 expression domain in shootward direction, without however affecting the expression in the early differentiation zone (Fig. 2V-X). As a result, the situation resembled the IRT1 promoter activity in roots of mock-treated iron-deficient Col-0 background plants (Fig. 2A-C). Taken together, these results show that the extent and localization of IRT1 expression in the root is regulated by an interplay between the hormones ethylene and auxin. We propose a model where the role of ethylene is to ensure the expression and synthesis of the transporter in the early differentiation zone, while auxin tends to push IRT1 expression outwards of this zone (Fig. 2Y). This might reflect the demands of the plant under the changing iron availability. When iron is generally available and under weak iron deficiency, auxin dominates over ethylene and IRT1 is only expressed in small limited zones ensuring that the plant requirement is met without wasting resources. This is consistent with the observed IRT1 promoter expression pattern under sufficient iron.7 In this case, the majority of the root is free for other processes, such as the ongoing developmental program or uptake of other nutrients. When iron is present in very low amounts, the enhanced ethylene biosynthesis and signaling12,14 adds to the auxin effects and ensures the spatial expansion of IRT1 expression, and therefore provides a larger iron uptake surface. If plant iron levels become critical, as in prolonged absence of environmental iron or reduced plant uptake capability, stronger ethylene influence overrides the role of auxin and concentrates IRT1 expression in the early differentiation zone. There, a combination of factors, including developmental prerequisites and external nutrient accessibility, might enable iron uptake with the highest possible efficiency (Fig. 2Y). Also, the concentration of iron uptake in a limited portion of the root allows the plant to save resources when coping with the increased levels of other IRT1-transported metals, such as Mn, Zn, and Co.15 In the specific case of snx mutants, external auxin treatment restores the auxin-ethylene balance and the broadly spread IRT1 expression (Fig. 2V-X). It also has to be noted that the documented involvement of SNX1 in the trafficking of the PIN2 auxin transporter16,17,18 might additionally influence the auxin-ethylene spatial regulation of IRT1 expression in snx mutants.
Figure 2. Role of ethylene and auxin in the spatial regulation of IRT1 expression. (A-X) GUS staining of representative plants expressing the IRT1pro:GUS cassette in Col-0 and snx1–2 genetic background. Treatments were made as indicated. The mock treatment was iron-deficient Hoagland medium with 0.1% ethanol. For each genotype and treatment, three images are shown: a seedling overview and two magnifications of regions indicated with rectangles on the overview image. (Y) Ethylene and auxin play opposing roles when defining the expression of IRT1 along the root depending on the plant iron demands. Under sufficient iron supply or mild iron deficiency, auxin dominates over ethylene causing IRT1 expression in the extremities of the root (left). The balance between the two hormones leads to an almost continuous IRT1 presence when iron is insufficient (center). Under severe iron limitation, dominant ethylene presence causes the concentration of IRT1 expression in the early differentiation zone (right).
In the future, these findings need to be put in the context of a developing root, where hormones, including auxin, are involved in the lateral root formation,19 which is influenced by, but also benefits the iron status of the plant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Catherine Curie (INRA Montpellier) for the IRT1pro:GUS line,3 Dr. Thierry Gaude (ENS Lyon) for the snx1–2 mutant16 and Angelika Anna for technical assistance. This research was supported by the Saarland University and the Heinrich-Heine University.
Glossary
Abbreviations:
- ACC
1-Aminocyclopropane-1-carboxylic acid (an ethylene biosynthesis donor)
- AVG
Aminoethoxyvinylglycine (an ethylene synthesis inhibitor)
- GUS
β-glucuronidase
- IDF1
IRT1 DEGRADATION FACTOR1
- IRT1
IRON-REGULATED TRANSPORTER1
- NAA
2-(1-Naphthyl)acetic acid (a synthetic auxin)
- SNX1
SORTING NEXIN1
- PM
plasma membrane
References
- 1.Ivanov R, Brumbarova T, Bauer P. Fitting into the harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Mol Plant. 2012;5:27–42. doi: 10.1093/mp/ssr065. [DOI] [PubMed] [Google Scholar]
- 2.Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A. 1996;93:5624–8. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–33. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, Vert G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci U S A. 2011;108:E450–8. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkeb L, Mukherjee I, Chatterjee I, Lahner B, Salt DE, Connolly EL. Iron-induced turnover of the Arabidopsis IRON-REGULATED TRANSPORTER1 metal transporter requires lysine residues. Plant Physiol. 2008;146:1964–73. doi: 10.1104/pp.107.113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin LJ, Lo JC, Chen GH, Callis J, Fu H, Yeh KC. IRT1 degradation factor1, a ring E3 ubiquitin ligase, regulates the degradation of iron-regulated transporter1 in Arabidopsis. Plant Cell. 2013;25:3039–51. doi: 10.1105/tpc.113.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanov R, Brumbarova T, Blum A, Jantke AM, Fink-Straube C, Bauer P. SORTING NEXIN1 is required for modulating the trafficking and stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell. 2014 doi: 10.1105/tpc.113.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov R, Gaude T. Endocytosis and endosomal regulation of the S-receptor kinase during the self-incompatibility response in Brassica oleracea. Plant Cell. 2009;21:2107–17. doi: 10.1105/tpc.108.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010;154:810–9. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García MJ, Suárez V, Romera FJ, Alcántara E, Pérez-Vicente R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant Physiol Biochem. 2011;49:537–44. doi: 10.1016/j.plaphy.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 11.García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot. 2010;61:3885–99. doi: 10.1093/jxb/erq203. [DOI] [PubMed] [Google Scholar]
- 12.Lingam S, Mohrbacher J, Brumbarova T, Potuschak T, Fink-Straube C, Blondet E, Genschik P, Bauer P. Interaction between the bHLH transcription factor FIT and ETHYLENE INSENSITIVE3/ETHYLENE INSENSITIVE3-LIKE1 reveals molecular linkage between the regulation of iron acquisition and ethylene signaling in Arabidopsis. Plant Cell. 2011;23:1815–29. doi: 10.1105/tpc.111.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Séguéla M, Briat JF, Vert G, Curie C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J. 2008;55:289–300. doi: 10.1111/j.1365-313X.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 14.Romera FJ, Alcantara E, De La Guardia MD. Ethylene Production by Fe-deficient Roots and its Involvement in the Regulation of Fe-deficiency Stress Responses by Strategy I Plants. Ann Bot (Lond) 1999;83:51–5. doi: 10.1006/anbo.1998.0793. [DOI] [Google Scholar]
- 15.Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/A:1026438615520. [DOI] [PubMed] [Google Scholar]
- 16.Jaillais Y, Fobis-Loisy I, Miège C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–9. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 17.Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci U S A. 2008;105:17812–7. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanzawa T, Shibasaki K, Numata T, Kawamura Y, Gaude T, Rahman A. Cellular auxin homeostasis under high temperature is regulated through a sorting NEXIN1-dependent endosomal trafficking pathway. Plant Cell. 2013;25:3424–33. doi: 10.1105/tpc.113.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giehl RF, Lima JE, von Wirén N. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell. 2012;24:33–49. doi: 10.1105/tpc.111.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]