Abstract
Exposing roots of plants to hypoxic conditions is known to greatly improve their anoxic stress tolerance. We previously showed that pre-treatment of wheat seedlings with an ethylene precursor, 1-aminocyclopropanecarboxylic acid (ACC), enhanced their tolerance of oxygen-deficient conditions. Although ACC-pretreated seminal roots of wheat seedlings grown under oxygen-deficient conditions avoided root tip death, they elongated very little. In the present study, we assessed the effects of ethylene on the responses of adventitious roots of wheat seedlings to oxygen-deficient conditions. Lysigenous aerenchyma formation in the adventitious roots of wheat seedlings pretreated with ACC appeared to reduce tip death under oxygen-deficient conditions, but the adventitious roots, like the seminal roots, hardly elongated. We also found that adventitious roots that emerge in oxygen-deficient conditions continued to elongate even under such conditions. The adventitious roots emerged in oxygen-deficient conditions were found to have thicker root diameters than those emerged in aerated conditions. These results suggest that the adventitious roots with thicker root diameters can better cope with oxygen-deficient conditions. Measurements of the area of the lysigenous aerenchyma confirmed that the increased root diameters have a greater amount of air space generated by lysigenous aerenchyma formation.
Keywords: Adventitious root, ethylene, lysigenous aerenchyma, root thickness, wheat (Triticum aestivum L.)
The principal cause of damage to plants grown in waterlogged soil is inadequate supply of oxygen to submerged tissues.1 Internal transport of oxygen from shoots to roots is essential for the survival and functioning of roots under waterlogged soil.2,3 Plants have no active oxygen dispersal mechanisms for long-distance transport of oxygen.4 Cytoplasmic streaming is relatively ineffective for long-distance transport, so internal oxygen transport to submerged tissues is dominated by oxygen diffusion.4 To adapt to waterlogging in soil, some gramineous plants develop lysigenous aerenchyma, which is formed by the creation of longitudinal gas spaces as a result of death and the subsequent lysis of some cells, in the root cortex.5-7 In some wetland plant species, root lysigenous aerenchyma is constitutively formed under well-drained soil conditions, and its formation can be further enhanced during soil waterlogging.5-7 On the other hand, in non-wetland plants, such as wheat (Triticum aestivum L.), lysigenous aerenchyma is not generally formed under well-drained soil conditions, but is induced by poor aeration.5-7 Generally, the induction of aerenchyma formation takes several hours after exposure to waterlogged conditions.8 Therefore, exposure to waterlogged conditions can severely damage the roots of non-wetland plants before the induction of sufficient amounts of aerenchyma. Gramineous plants, such as wheat, produce two distinct root systems during their development.9 Seminal roots, which emerge from the embryo upon seed germination, serve to take up water and nutrients during seedling development, while adventitious (nodal) roots, which originate from nodes, function as the permanent root system of gramineous plants.9 In non-wetland plants, such as wheat, prolonged waterlogging kills the initial root system (both seminal and adventitious roots),10 which is then replaced by newly emerged adventitious roots. Therefore, the adaptive responses of adventitious roots to waterlogging might be more important than those of seminal roots for survival in waterlogged soil.
Exposing roots of wheat seedlings to hypoxic conditions greatly improves their anoxic stress tolerance.11 Similarly, pre-treatment of an ethylene precursor, 1-aminocyclopropanecarboxylic acid (ACC), enhanced the tolerance of wheat seedlings to oxygen-deficient conditions.12 Ethylene is involved in inducible aerenchyma formation in roots of some gramineous plants.5-7 Recently, we demonstrated that ethylene-induced lysigenous aerenchyma formation in seminal roots helps wheat seedlings to tolerate stagnant deoxygenated conditions (which mimic oxygen-deficient conditions in waterlogged soils).12 The seminal root tips of wheat seedlings grown under stagnant conditions died within 3 d. Although ACC-pretreated seminal roots of wheat seedlings grown under stagnant conditions avoided root tip death, they elongated very little.12 Non-ACC-pretreated wheat seedlings appeared to suffer severe damage under stagnant conditions before the emergence of adventitious roots, but adventitious roots that emerged in stagnant conditions were highly adapted to stagnant conditions as shown by their continued growth under stagnant conditions.12 Together, these observations suggested that the adventitious roots emerged in stagnant conditions have some special features to adapt to oxygen-deficient conditions. In the present study, we assessed the effects of ethylene on the responses of adventitious roots to oxygen-deficient conditions. To this end, we subjected wheat seedlings to stagnant conditions with or without ACC pre-treatment and analyzed elongation rates, viability and lysigenous aerenchyma formation in adventitious roots. In addition, we compared the anatomical and morphological features of adventitious roots emerging in aerated and stagnant conditions. To reduce the number of variables, we examined only the first emerging adventitious roots, i.e., ones that emerged at the same developmental stage.
Adventitious roots of wheat seedlings first emerged at 10 d after growth under aerated conditions.12 To assess the effects of ethylene on the responses of adventitious roots to oxygen-deficient conditions, 11-d-old wheat (cv Bobwhite line SH 98 26) seedlings were transferred to aerated conditions with or without ACC (20 μM) treatment and grown for 2 d. Subsequently, 13-d-old seedlings were transferred to stagnant conditions and grown for 3 d (Fig. 1A). We previously found that the adventitious roots that emerge in stagnant conditions have some anatomical and morphological features that are distinct from those of adventitious roots that emerge in aerated conditions.12 To compare these features in the adventitious roots at the same stage of development, 9-d-old wheat seedlings were transferred to stagnant conditions and grown for 7 d (Fig. 1A). We defined the seedlings with and without ACC pre-treatments as the -ACC seedlings and the +ACC seedlings, as well as the seedlings grown under stagnant conditions for 7 d as the STAG seedlings (Fig. 1A). It should be noted that the adventitious roots of the -ACC seedlings and the +ACC seedlings emerged in aerated conditions, and those of the STAG seedlings emerged in stagnant conditions. In both conditions, the adventitious roots first emerged at 10 d after sowing (Fig. 1A). The average lengths of 13-d-old adventitious roots of the -ACC, +ACC, and STAG seedlings were 68 mm, 58 mm and 55 mm, respectively. During an additional 3 d growth under stagnant conditions, the adventitious roots of the STAG seedlings elongated approximately 20 mm, whereas those of the -ACC seedlings and the +ACC seedlings hardly elongated under stagnant conditions (Fig. 1B). The 2,3,5-triphenyltetrazolium chloride (TTC) reduction assay has been used to evaluate the viability of plant tissues quantitatively by detecting absorbance of reduced TTC, a red formazan derivative.13 The values of TTC reduction (OD520) at 0 mm to 10 mm from the adventitious root tips of the -ACC seedlings were significantly reduced under stagnant conditions, whereas the value of TTC reduction in the +ACC seedlings was kept higher as was the case with the STAG seedlings (Fig. 1C). These results indicate that the adventitious roots of the +ACC seedlings were more adaptive to stagnant conditions than those of the -ACC seedlings, whereas the adventitious roots emerged in stagnant conditions had higher adaptive capacity to stagnant conditions than those emerged in aerated conditions with or without ACC treatment.
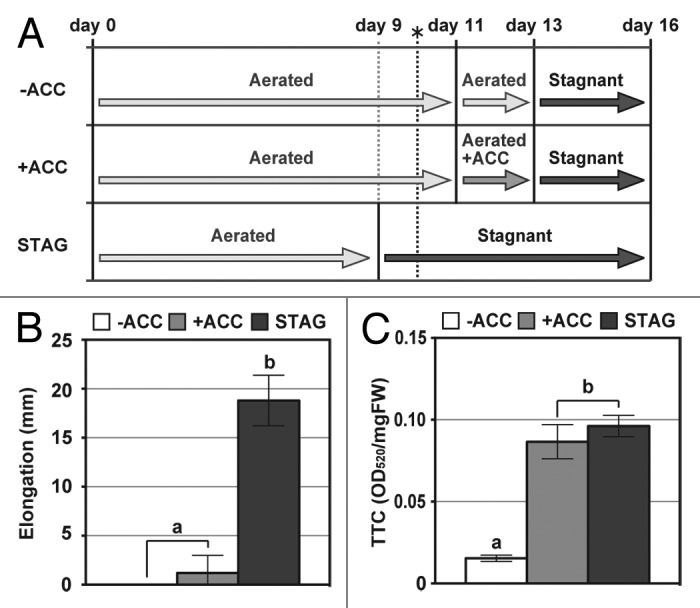
Figure 1. Elongation of adventitious roots and viability of adventitious root tips in wheat seedlings. (A) Growth conditions. Eleven-day-old aerobically-grown wheat seedlings were transferred to aerated nutrient solution with or without 20 μM ACC. After 2 d, these seedlings were transferred to stagnant solution and grown for 3 d. While 9-d-old wheat seedlings were transferred to stagnant solution and grown for 7 d. Stagnant solution contained 0.1% (w/v) dissolved agar and was deoxygenated (dissolved oxygen, < 0.5 mgL−1) prior to use by flushing with N2 gas. The broken line with the asterisk indicates when the emergence of the first adventitious roots was observed (day 10). (B) Elongation of adventitious roots during 3 d growth under stagnant conditions (from day 13 to day 16). (C) Cell viability (TTC reduction) in adventitious roots at 0 to 10 mm from the root tips of the wheat seedlings immediately after 3 d growth under stagnant conditions (day 16). The methods are described in more detail by Yamauchi and colleagues.12 Values are means (n = 5) ± SD. Different lower-case letters denote significant differences among the conditions (P < 0.05, one-way ANOVA and then Tukey’s test for multiple comparisons). -ACC; the -ACC seedlings, +ACC ; the +ACC seedlings, STAG; the STAG seedlings, TTC; 2,3,5-Triphenyltetrazolium chloride.
Root cross-sections for each position of the adventitious roots were photographed using a light microscope (Fig. 2A), and then the percentages of each cross-section occupied by aerenchyma were determined. Aerenchyma formation hardly occurred at 10 mm from the adventitious root tips of the -ACC seedlings, whereas 6.0% and 14.2% of aerenchyma was observed at 10 mm from the adventitious root tips of the +ACC seedlings and the STAG seedlings, respectively (Fig. 2B). Although the percentages of aerenchyma at 30 mm and 50 mm from the adventitious root tips were comparable between the -ACC seedlings and the +ACC seedlings (30 mm: -ACC; 8.1%, +ACC; 10.0%: 50 mm: -ACC; 12.8%, +ACC; 13.1%), the percentages of aerenchyma were significantly higher in the adventitious roots of the STAG seedlings (30 mm: 17.5%, 50 mm: 20.1%)(Fig. 2B). These results suggest that ethylene-induced lysigenous aerenchyma formation at the tip part of the adventitious roots helped to prevent the tip death under stagnant conditions. This was consistent with our previous study for seminal roots of wheat seedlings pre-treated with ACC.12
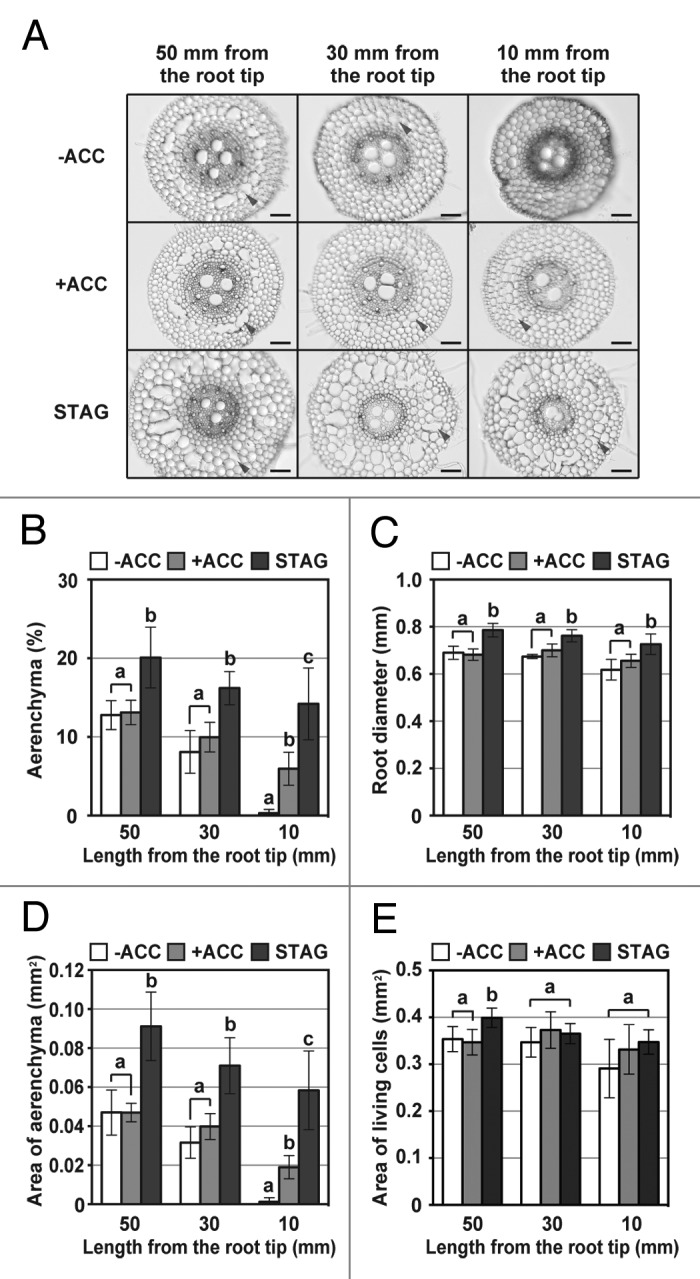
Figure 2. Aerenchyma formation and thickness of adventitious roots in wheat seedlings. (A) Cross-sections. Distances from the root tips are displayed on the top of figures. Lysigenous aerenchyma is indicated by a gray arrowhead. Bar = 100 μm. Cross-sections were prepared by hand-sectioning with a razor blade. Each section was photographed using a light microscope with a CCD camera. The percentages of each cross-section occupied by aerenchyma (B), the diameters of roots (C), the area of aerenchyma (D), and the area of living cells (E) along the adventitious roots of wheat seedlings immediately after 3 d growth under stagnant conditions (day 16). The percentages and area of each cross-section occupied by aerenchyma, and the diameters of the roots in each cross section were determined using ImageJ software (Ver. 1.43u, US National Institutes of Health, Bethesda, MD, USA). The methods are described in more detail by Yamauchi and colleagues.12 Values are means (n = 5) ± SD. Different lower-case letters denote significant differences among the conditions (P < 0.05, one-way ANOVA and then Tukey’s test for multiple comparisons). -ACC; the -ACC seedlings, +ACC ; the +ACC seedlings, STAG; the STAG seedlings.
Even though ACC enhanced lysigenous aerenchyma formation in the adventitious roots, root elongation of the +ACC seedlings was almost completely inhibited under stagnant conditions. To understand why the adventitious roots of the STAG seedlings can continue to elongate under stagnant conditions, we closely observed their morphological features, and noticed that the adventitious roots of the STAG seedlings were somewhat thicker than those of the +ACC seedlings and the -ACC seedlings. Then we measured diameters of the transverse sections obtained from each position of the adventitious roots. At 10 mm and 30 mm from the root tips, the adventitious root diameters were 0.62 mm and 0.67 mm for the -ACC seedlings, and 0.65 mm and 0.70 mm for the +ACC seedlings, respectively (Fig. 2C). Although no significant differences were observed, the diameters of the adventitious roots of the +ACC seedlings at 10 mm and 30 mm from the root tips (these parts were formed during the ACC pre-treatment) were slightly thicker than those of the -ACC seedlings (Fig. 2C). Interestingly, the adventitious root diameters of the STAG seedlings were significantly longer than the -ACC seedlings and the +ACC seedlings at all positions examined (Fig. 2C). The adventitious root diameters of the STAG seedlings were 0.73 mm, 0.76 mm, and 0.79 mm for the positions at 10 mm, 30 mm and 50 mm from the root tips, respectively (Fig. 2C). Together, these results indicate that the adventitious roots that emerged in stagnant conditions were thicker than those emerged in aerated conditions.
The capacity for longitudinal oxygen diffusion in the submerged tissues is determined by anatomical, morphological and physiological characteristics.3 Lysigenous aerenchyma and root thickness affect the porosities (i.e., air volumes) in roots.3,14,15 To reveal a possible relationship between the lysigenous aerenchyma and root thickness, we measured the area of aerenchyma in transverse sections (Fig. 2D). Although the percentage of aerenchyma at 10 mm from the adventitious root tips of the STAG seedlings was 2.4-fold higher than that of the +ACC seedlings (Fig. 2B), the area of aerenchyma of the STAG seedlings was more than 3-fold higher than that of the +ACC seedlings (Fig. 2D). Similar trends were obtained for the positions at 30 mm and 50 mm from the adventitious root tips (Fig. 2D). To further study the possible advantages of the thicker root diameters for the adaptation of adventitious roots to oxygen-deficient conditions, we measured the area of living cells in the transverse sections obtained from each position of the adventitious roots (Fig. 2E). Despite the thicker root diameters, the area of living cells at 10 mm and 30 mm from the adventitious roots of the STAG seedlings were comparable with those of the -ACC seedlings and the +ACC seedlings (Fig. 2E). Together, these results indicate that the thicker root diameters of the STAG seedlings increased the volume of lysigenous aerenchyma.
In the present study, we showed that adventitious roots of wheat seedlings that emerge in stagnant conditions have thicker root diameters and larger air spaces (i.e., aerenchyma) than adventitious roots that emerge in aerated conditions. This would clearly accelerate the transport of oxygen to the root tips. Moreover, it is known that aerenchyma formation in roots results in a reduction of oxygen demand of roots.2,16 The area of living cells was comparable between the STAG seedlings and the +ACC seedlings, further supporting the notion that the thick root diameters are essential to the adaptation of adventitious roots to oxygen-deficient conditions. Stagnant treatment increased root diameters in some wetland plants, and the thicker roots were often associated with larger air volumes in their roots.14 On the other hand, stagnant treatment appeared to severely damage wheat seedlings because there was not enough time for the emergence of the adaptive adventitious roots.12 Therefore, the initial root system of wheat, a non-wetland plant, appears to differ from that of wetland plants in its response to oxygen-deficient conditions in waterlogged soil, mainly by increasing root diameters and enlarging the aerenchyma.
In rice, adventitious root emergence under submergence is stimulated by ethylene, which accumulates through both increased ethylene biosynthesis and physical entrapment.17,18 Ethylene accumulation at the root-shoot junction (nodes) where adventitious roots emerged, might also stimulate the formation of the adventitious roots in wheat seedlings.12 The diameters of the adventitious roots of the +ACC seedlings were slightly thicker than those of the -ACC seedlings, implying that ethylene is also involved in controlling the thickness of adventitious roots. It should be noted that the adventitious roots of the +ACC seedlings examined in the present study first emerged in aerated conditions. Therefore, the thickness of those roots might not be the same as the thickness of adventitious roots that emerge in stagnant conditions. In other words, the thickness of roots may be predominantly determined during the formation of root primordia or during early root growth. Further studies are needed to reveal the mechanisms that control root thickness in response to oxygen-deficient conditions in waterlogged soil.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Hirokazu Takahashi, Shunsaku Nishiuchi and Kohtaro Watanabe for stimulating discussions. This work was partly supported by a grant from the Bio-oriented Technology Research Advancement Institution (Promotion of Basic Research Activities for Innovative Biosciences) and a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan [Genomics-based Technology for Agricultural Improvement (GMO1005b)] to MN. TY is supported by postdoctoral fellowship from the Japan Society for the Promotion of Science.
References
- 1.Colmer TD, Voesenek LACJ. . Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 2009; 36:665 - 81; http://dx.doi.org/ 10.1071/FP09144 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong W. . Aeration in higher plants. Adv Bot Res 1980; 7:225 - 332; http://dx.doi.org/ 10.1016/S0065-2296(08)60089-0 [DOI] [Google Scholar]
- 3.Colmer TD. . Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 2003; 26:17 - 36; http://dx.doi.org/ 10.1046/j.1365-3040.2003.00846.x [DOI] [Google Scholar]
- 4.Armstrong W, Armstrong J. . Plant internal oxygen transport (diffusion and convection) and measuring and modeling oxygen gradients. Plant Cell Monographs 2014; 21:267 - 97; http://dx.doi.org/ 10.1007/978-3-7091-1254-0_14 [DOI] [Google Scholar]
- 5.Jackson MB, Armstrong W. . Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1999; 1:274 - 87; http://dx.doi.org/ 10.1111/j.1438-8677.1999.tb00253.x [DOI] [Google Scholar]
- 6.Evans DE. . Aerenchyma formation. New Phytol 2003; 161:35 - 49; http://dx.doi.org/ 10.1046/j.1469-8137.2003.00907.x [DOI] [Google Scholar]
- 7.Takahashi H, Yamauchi T, Colmer TD, Nakazono M. . Aerenchyma formation in plants. Plant Cell Monographs 2014; 21:247 - 65; http://dx.doi.org/ 10.1007/978-3-7091-1254-0_13 [DOI] [Google Scholar]
- 8.Nishiuchi S, Yamauchi T, Takahashi H, Kotula L, Nakazono M. . Mechanisms for coping with submergence and waterlogging in rice. Rice 2012; 5:2; http://dx.doi.org/ 10.1186/1939-8433-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briske DD. Grazing management: an ecological perspective. Portland, Oregon: Timber Press; 1991; Developmental morphology and physiology of grasses; 11-26. [Google Scholar]
- 10.Wiengweera A, Greenway H. . Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. J Exp Bot 2004; 55:2121 - 9; http://dx.doi.org/ 10.1093/jxb/erh232; PMID: 15310817 [DOI] [PubMed] [Google Scholar]
- 11.Waters I, Morrel S, Greenway H, Colmer TD. . Effects of anoxia on wheat seedlings II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J Exp Bot 1991; 42:1437 - 47; http://dx.doi.org/ 10.1093/jxb/42.11.1437 [DOI] [Google Scholar]
- 12.Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. . Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 2014; 65:261 - 73; http://dx.doi.org/ 10.1093/jxb/ert371; PMID: 24253196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steponkus PL, Lanphear FO. . Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol 1967; 42:1423 - 6; http://dx.doi.org/ 10.1104/pp.42.10.1423; PMID: 16656672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. . Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 2000; 23:1237 - 45; http://dx.doi.org/ 10.1046/j.1365-3040.2000.00628.x [DOI] [Google Scholar]
- 15.Visser EJW, Bögemann GM, Van de Steeg HM, Pierik R, Blom CWPM. . Flooding tolerance of Carex species in relation to field distribution and aerenchyma formation. New Phytol 2000; 148:93 - 103; http://dx.doi.org/ 10.1046/j.1469-8137.2000.00742.x [DOI] [PubMed] [Google Scholar]
- 16.Justin SHFW, Armstrong W. . The anatomical characteristics of roots and plant response to soil flooding. New Phytol 1987; 106:465 - 95; http://dx.doi.org/ 10.1111/j.1469-8137.1987.tb00153.x [DOI] [Google Scholar]
- 17.Sauter M. . Root responses to flooding. Curr Opin Plant Biol 2013; 16:282 - 6; http://dx.doi.org/ 10.1016/j.pbi.2013.03.013; PMID: 23608517 [DOI] [PubMed] [Google Scholar]
- 18.Steffens B, Sauter M. . Role of ethylene and other plant hormones in orchestrating the responses to low oxygen conditions. Plant Cell Monographs 2014; 21:117 - 32; http://dx.doi.org/ 10.1007/978-3-7091-1254-0_7 [DOI] [Google Scholar]


