Abstract
Background
Patients with subclinical hypothyroidism (SHT) are common in clinical practice. However, the clinical significance of SHT, including prognosis, has not been established. Further clarifying SHT will be critical in devising a management plan and treatment guidelines for SHT patients. Thus, the aim of this study was to investigate the prognostic factors of SHT.
Methods
We reviewed the medical records of Korean patients who visited the endocrinology outpatient clinic of Severance Hospital from January 2008 to September 2012. Newly-diagnosed patients with SHT were selected and reviewed retrospectively. We compared two groups: the SHT maintenance group and the spontaneous improvement group.
Results
The SHT maintenance group and the spontaneous improvement group had initial thyroid-stimulating hormone (TSH) levels that were significantly different (P=0.035). In subanalysis for subjects with TSH levels between 5 to 10 µIU/mL, the spontaneous improvement group showed significantly lower antithyroid peroxidase antibody (anti-TPO-Ab) titer than the SHT maintenance group (P=0.039). Regarding lipid profiles, only triglyceride level, unlike total cholesterol and low density lipoprotein cholesterol, was related to TSH level, which is correlated with the severity of SHT. Diffuse thyroiditis on ultrasonography only contributed to the severity of SHT, not to the prognosis. High sensitivity C-reactive protein and urine iodine excretion, generally regarded as possible prognostic factors, did not show any significant relation with the prognosis and severity of SHT.
Conclusion
Only initial TSH level was a definite prognostic factor of SHT. TPO-Ab titer was also a helpful prognostic factor for SHT in cases with mildly elevated TSH. Other than TSH and TPO-Ab, we were unable to validate biochemical prognostic factors in this retrospective study for Korean SHT patients.
Keywords: Subclinical hypothyroidism, Thyrotropin, Thyroid peroxidase antibody, Lipids, C-reactive protein, Iodine
INTRODUCTION
In clinical practice, it is not uncommon for patients to present with high serum thyroid-stimulating hormone (TSH) levels but normal free thyroxine (fT4) levels [1]. Such subclinical hypothyroidism (SHT) is known to occur in 3% to 8% of the total population, although each study reported various results according to their definition of normal TSH level and geographic/ethnic differences of their subjects [2,3,4,5,6]. Unlike overt hypothyroidism (OHT) with elevated TSH levels and decreased fT4 levels, SHT is generally asymptomatic, with the diagnosis made solely from laboratory results. Diagnosis of SHT has been recently growing due to a popularized screening test for thyroid function. As a result, concerns about the natural course and prognosis of SHT and long-term consequences of a persistent subclinical hypothyroid state are on the rise. However, the clinical significance of SHT has not been fully characterized. A comprehensive understanding of the clinical significance of SHT will aid in the establishment of a management plan and treatment guidelines for SHT patients.
In this study, we report our experience with 327 SHT patients and aim to define the prognostic factors of SHT.
METHODS
Subjects
We reviewed the medical records of Korean patients who visited the endocrinology outpatient clinic of Severance Hospital from January 2008 to September 2012. Among them, patients who showed subclinical hypothyroid state, defined in this study as TSH >5 µIU/mL and fT4 between 0.73 and 1.95 ng/dL, were selected. A retrospective study was conducted and data were gathered on age, sex, fT4, TSH, thyroid peroxidase antibody (TPO-Ab), lipid profile, high sensitivity C-reactive protein (hsCRP), urine iodine, as well as ultrasonography (US) findings at diagnosis. According to the independent and subjective decision making of five endocrinologists, patients were either administered levothyroxine or no medication based on the following criteria: subject's clinical presentation, chance of pregnancy in woman of childbearing age, and initial laboratory findings. In the retrospective analysis, we found that female subjects with lower fT4, higher TSH level, higher anti-TPO Ab titer, lower urinary iodine were more frequently prescribed levothyroxine (except for TSH, all others were statistically different between two groups).
During follow-up, thyroid function of patients who were kept under observation had shifted to euthyroid, subclinical hypothyroid, or overt hypothyroid state. The remaining subjects were given levothyroxine supplements immediately after the diagnosis and they all had developed a euthyroid state by the follow-up. Regular check-up to determine fT4, TSH, TPO-Ab, lipid profile, and hsCRP status were conducted (the check-up did not include urine iodine and US evaluation). To determine the prognostic factors of SHT, we compared two groups; one group maintained a subclinical hypothyroid state (the SHT maintenance group) and another group improved to a euthyroid state spontaneously (the spontaneous improvement group). To evaluate the benefit of treatment in the SHT, we analyzed the data for the levothyroxine supplement group. Patients with a history of taking antithyroid drugs or thyroid hormone replacement, pregnant women, and those with a previous thyroid disease history or those having other comorbidities that could affect lipid profile and hsCRP, such as diagnosed diabetes mellitus or hyperlipidemia, were excluded.
Thyroid function test and thyroid autoantibodies
Serum fT4 levels were measured using the AMERLEX-MAB* FT4 kits (Trinity Biotech PLC, Wicklow, Ireland), and serum TSH levels were measured by IRMA (TSH-CTK-3, SORIN Biomedica, Saluggia, Italy). The reference ranges were 0.73 to 1.95 ng/dL for fT4 and 0.4 to 5.0 µIU/mL for TSH. Serum TPO-Ab was detected by B.R.A.H.M.S TPO-Ab RIA (B.R.A.H.M.S. AG, Hennigsdorf, Germany), with 60 U/mL set as the upper normal limit.
Biochemistry
Serum total cholesterol, triglyceride, and high density lipoprotein cholesterol (HDL-C) were measured with a Hitachi modular 7600 (Hitachi, Tokyo, Japan) automated clinical chemistry analyzer. The reference values used were 100 to 220 mg/dL for total cholesterol, 44 to 150 mg/dL for triglyceride, and 40 to 400 mg/dL for HDL-C. Serum low density lipoprotein cholesterol (LDL-C) was calculated using the formula: LDL-C=total cholesterol-HDL-C-(triglyceride/5.0). Serum hsCRP was analyzed using the same automated chemistry analyzer, and the reference values applied were 0 to 3.0 mg/L.
Determination of urinary iodine excretion
Fasting spot urine samples were collected from subjects and analyzed by potentiometric methods using the Metrohm pH/ion meter model 692. Urinary iodine excretion was expressed as µmoL iodine/g creatinine. The normal urinary iodine excretion range given was 8.6 to 41.3 µmol/g of creatinine.
US evaluation of the thyroid gland
US evaluation of the thyroid gland was performed with an HDI 3000 or HDI 5000 system (Philips Medical Systems, Bothell, WA, USA) or an Acuson Sequoia 512 system (Siemens Medical Solutions, Mountain View, CA, USA). One of three radiologists with 4, 6, and 10 years of experience in thyroid imaging performed a real-time US exam and interpreted the results. US features of diffuse thyroiditis (DT) were defined using the generally accepted standards of diffuse parenchymal hypoechogenicity or a heterogeneous echogenic pattern of the thyroid gland. If focal lesions were accompanied with DT, for example those suggestive of focal thyroiditis or a benign nodule, we conducted fine needle aspiration and included only the lesions confirmed as focal thyroiditis with lymphocytic infiltration.
Statistical analysis
Results are expressed as the mean value with standard deviation, the number of subjects with the percentage (%) or median (minimum-maximum). All data were analyzed using IBM SPSS version 21.0 (IBM Co., Armonk, NY, USA). Between two groups (either the SHT maintenance and the spontaneous improvement groups, the observation and levothyroxine supplement groups, or the DT+ and - groups), mean values were compared using t test and sex ratio using Pearson chi-square test. Between initial and follow-up parameters in each of the three groups, mean values were compared using paired t test.
RESULTS
Baseline characteristics of the SCH maintenance, spontaneous improvement, and levothyroxine supplement group
Table 1 shows the baseline clinical and biochemical characteristics of three groups. The data from 68 patients of the SHT maintenance group (maintained subclinical hypothyroid state without medication), 70 of the spontaneous improvement group (improved to euthyroid state without medication), and 189 of the levothyroxine supplement group (which attained a euthyroid state with medication) were analyzed. The mean follow-up period was 10.1 months for the SHT maintenance group, 9.6 months for the spontaneous improvement group, and 13.1 months for the levothyroxine supplement group.
Table 1.
Baseline Characteristics of the Subclinical Hypothyroidism Maintenance, Spontaneous Improvement, and Levothyroxine Supplement Group
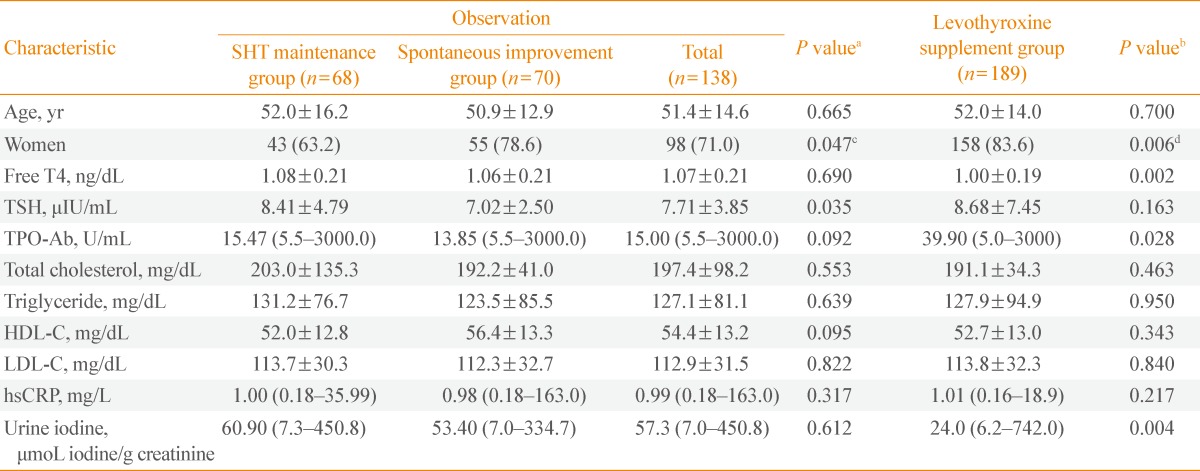
Data are mean±standard deviation, number (%), or median (minimum-maximum).
SHT, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO-Ab, anti-thyroid peroxidase antibody; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
aP values for the comparison of the mean values between SHT maintenance and Spontaneous improvement group by t test; bP values for the comparison of the sex ratio by Pearson's chi-square test; cP values for the comparison of the mean values between Observation and Levothyroxine supplement group by using t test; dP values for the comparison of the sex ratio by Pearson's chi-square test.
Women showed a better prognosis in the course of SHT (P=0.047). In addition, initial TSH level was significantly different (8.41±4.79 µIU/mL in the SHT maintenance group and 7.02±2.50 µIU/mL in the spontaneous improvement group; P=0.035). Except TSH level and sex ratio, there were no statistically significant differences in age, fT4, TPO-Ab, lipid profile, hsCRP, and urine iodine. We performed further analysis on the subpopulation, according to TSH levels (Table 2). For subjects with TSH between 5 to 10 µIU/mL, those in the spontaneous improvement group showed significantly lower TPO-Ab titer than the SHT maintenance group (P=0.039).
Table 2.
Baseline Characteristics of SHT Maintenance and Spontaneous Improvement Group in the Subjects with TSH between 5 to 10 µIU/mL
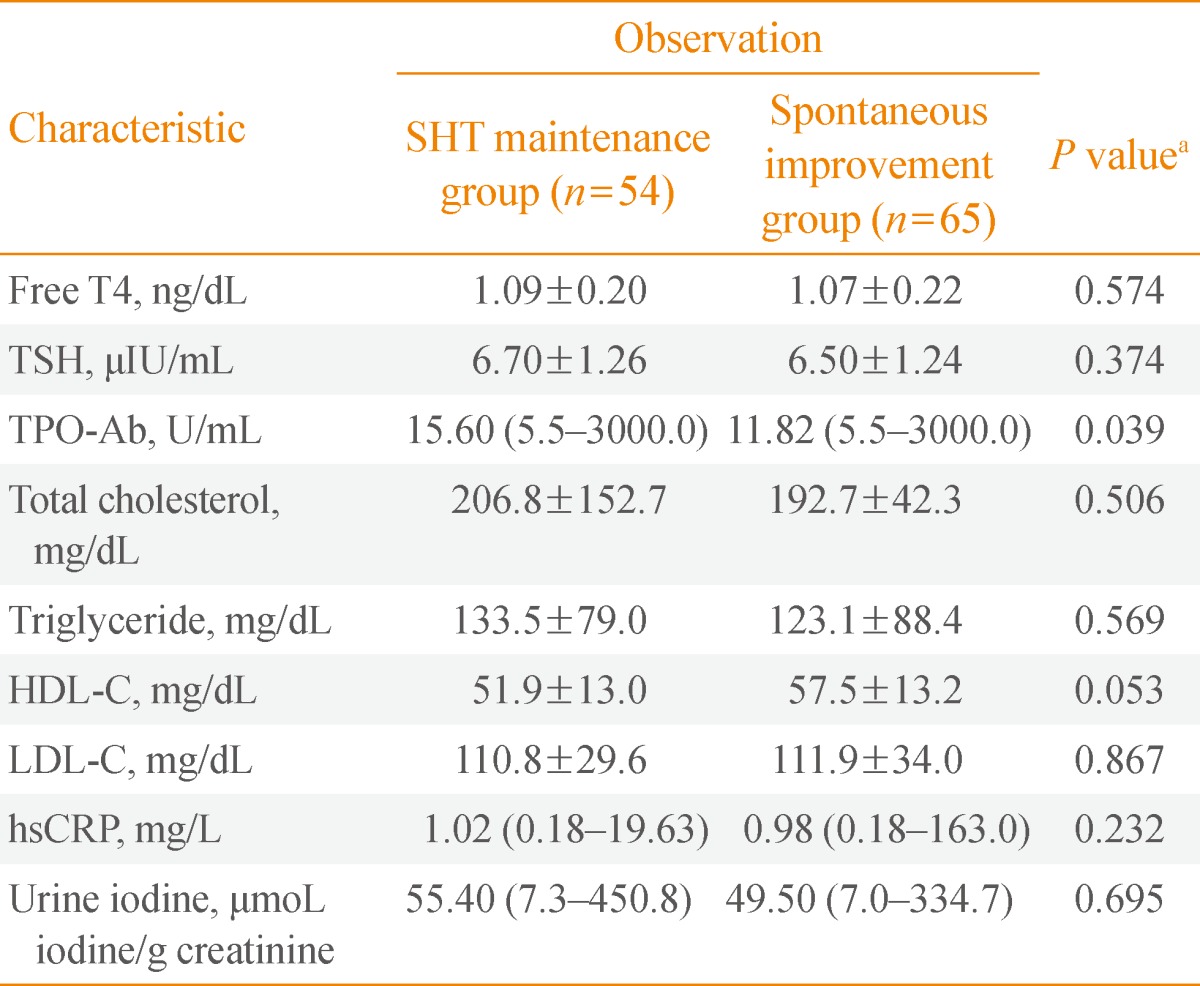
Data are mean±standard deviation or median (minimum-maximum).
SHT, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO-Ab, anti-thyroid peroxidase antibody; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
aP values for the comparison of the mean values by using t test.
Baseline characteristics of the levothyroxine supplement group were compared with those of the observed population (the SHT maintenance and spontaneous improvement groups) (Table 1). SHT patients with lower fT4, higher TPO-Ab titer, and lower urine iodine were more likely to be considered for supplement therapy by the doctor. Additionally, the levothyroxine supplement group had a higher proportion of women than the observation group.
Comparison between initial and follow-up parameters in the SHT maintenance and spontaneous improvement group
Table 3 shows the changes in parameters during follow-up (about 10 months) in the SHT maintenance and spontaneous improvement group. As expected, none of the measured parameters showed any changes during follow-up in the SHT maintenance group. In the spontaneous improvement group with TSH levels that had returned to normal, there were no significant improvements in TPO-Ab and hsCRP. TPO-Ab titer showed only a decreasing trend. Analysis of lipid profiles showed triglyceride levels decreasing significantly during follow-up (127.6±88.5 mg/dL vs. 100.0±56.9 mg/dL; P=0.004).
Table 3.
Comparison between the Parameters at Initial Presentation and Follow-up of the SHT Maintenance and Spontaneous Improvement Group
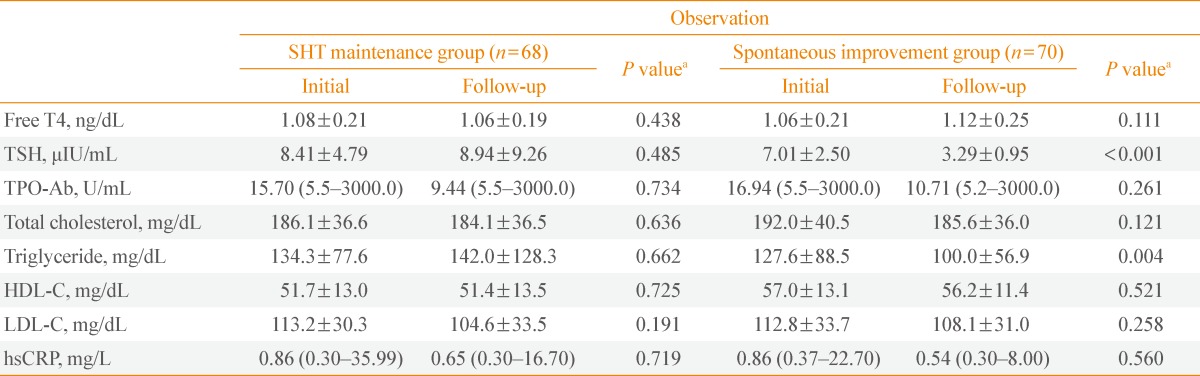
Data are mean±standard deviation or median (minimum-maximum).
SHT, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO-Ab, anti-thyroid peroxidase antibody; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
aP values for the comparison of the mean values between initial and follow-up parameters by using paired t test.
Comparison between initial and follow-up parameters in the levothyroxine supplement group
Table 4 shows the changes in parameters during follow-up in the levothyroxine supplement group. We compared the parameters at the time their thyroid function reached euthyroid state to those at diagnosis. TPO-Ab titer and triglyceride level significantly decreased during follow-up (for TPO-Ab, median value, 40.06 U/mL vs. 33.97 U/mL, P=0.009; for triglyceride, mean value, 128.2±97.4 µIU/dL vs. 105.3±52.5 µIU/dL, P=0.002, respectively). hsCRP titer did not show significant changes.
Table 4.
Comparison between the Parameters at Initial Presentation and Follow-up of Levothyroxine Supplement Group
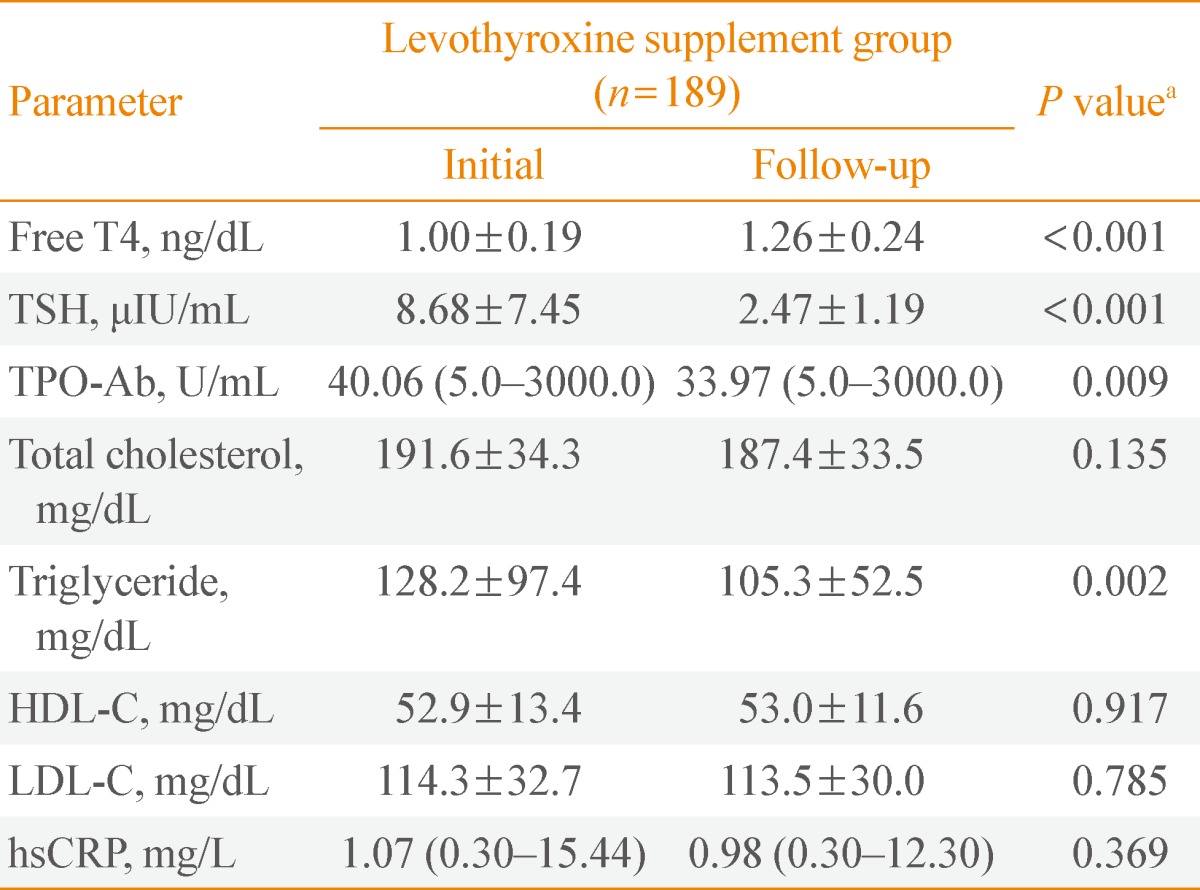
Data are mean±standard deviation or median(minimum-maximum).
SHT, subclinical hypothyroidism; TSH, thyroid-stimulating hormone; TPO-Ab, anti-thyroid peroxidase antibody; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
aP values for the comparison of the mean values between initial and follow-up parameters by using paired t test.
US finding in SHT
The existence of DT on US at diagnosis was not significantly different between the SHT maintenance group and spontaneous improvement group (28.3% of the SHT maintenance group and 21.7% of the spontaneous improvement group; P=0.144). However, when we looked into the clinical and laboratory characteristics at diagnosis according to US finding (Table 5), the existence of DT was associated with higher TSH, TPO-Ab, and triglyceride (P=0.035, P<0.01, and P=0.017, respectively) at that time.
Table 5.
The Presentation of SHT according to US Finding
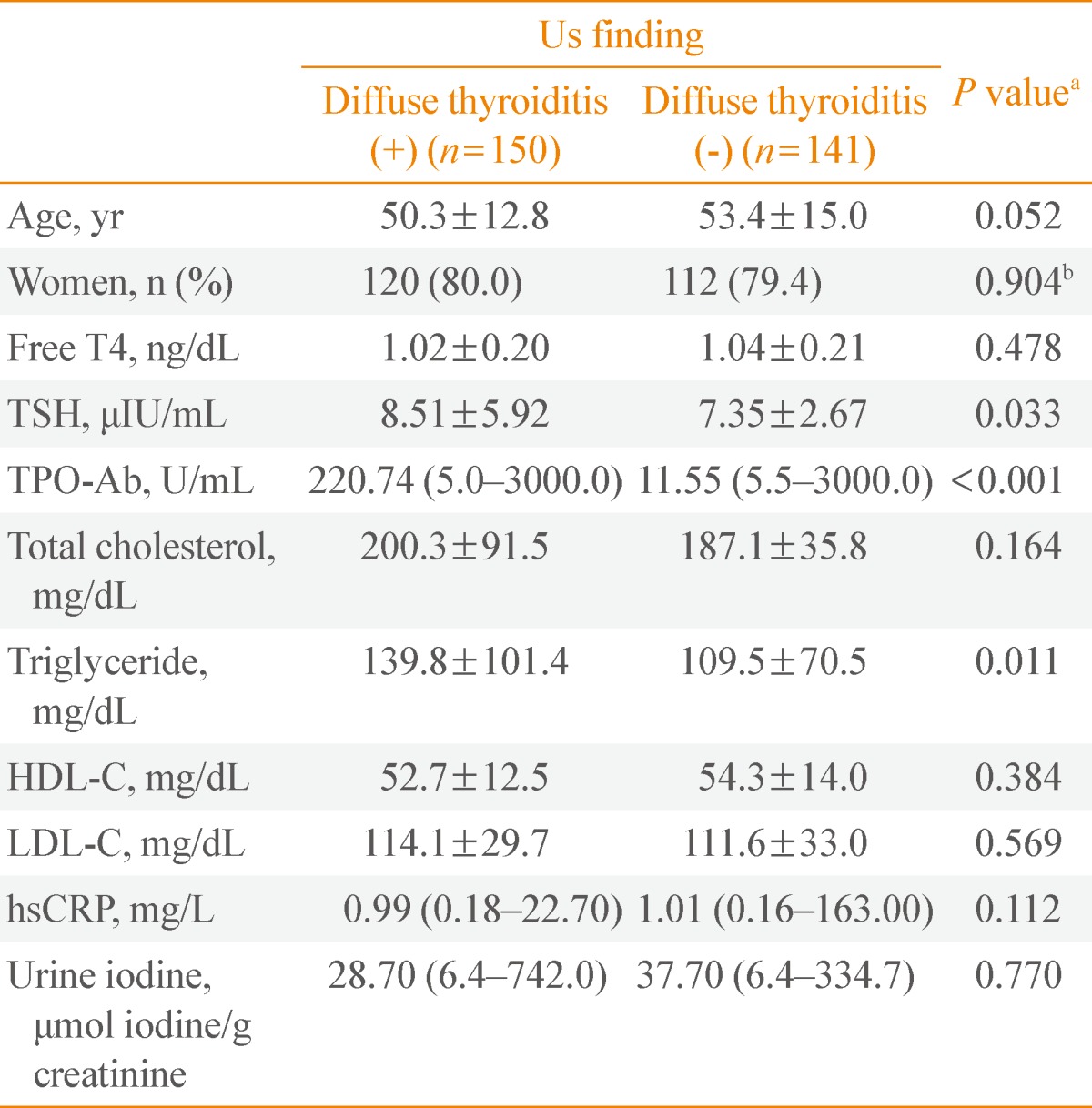
Data are mean±standard deviation, number (%) or median (minimum-maximum).
SHT, subclinical hypothyroidism; US, ultrasohographic; TSH, thyroid-stimulating hormone; TPO-Ab, anti-thyroid peroxidase antibody; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
aP values for the comparison of the mean values of two groups by using t test; bP values for the comparison of the Sex ratio of two groups by using Pearson's chi-square test.
DISCUSSION
Some SHT patients spontaneously recover thyroid function without specific treatment, others maintain a subclinical hypothyroid state without improvement, while others progress to an overt hypothyroid state requiring thyroid hormone supplement. Therefore, a general understanding of the natural course of SHT, prognostic factors, clinical consequences of a persistent subclinical hypothyroid state, and the effect of levothyroxine supplement, is necessary for clinicians in planning SHT management strategies, including determination of the necessity and appropriate time of treatment.
Until now, various studies have regarded TSH level and TPO-Ab titer as the most reliable clinical parameters in predicting the progression of SHT or euthyroidism to OHT. In a 1995 cohort study conducted over the course of 20 years in the United Kingdom [7], the odds ratios (ORs) (with 95% confidence intervals [CIs]) of developing hypothyroidism in euthyroid subjects with raised serum TSH alone (TSH >2 µIU/mL) were OR, 8; 95% CI, 3 to 20 for women; and OR, 44; 95% CI, 19 to 104 for men. In cases with positive antithyroid antibodies alone, the odds ratios were OR 8 (95% CI, 5 to 15) for women and OR 25 (95% CI, 10 to 63) for men [7]. In subjects with both elevated serum TSH and positive antithyroid antibodies, the ORs were 38 (95% CI, 22 to 65) for women and OR 173 (95% CI, 81 to 370) for men. In another 4-year cohort study conducted in the United States [8], one-third of subjects with SHT developed OHT over the course of the study. The initial TSH level of all subjects who became overtly hypothyroid was above 20 µIU/mL, and 80% of all of subjects, regardless of initial TSH level, also showed high TPO-Ab titer. Another prospective long-term study of 82 female patients with SHT also showed that higher initial TSH concentrations and positive TPO-Ab allowed initial risk stratification for the development of overt thyroid failure within 10 years [9]. Other prospective studies conducted since 2000 also have proved that both elevated TSH level and the presence of TPO-Ab are poor prognostic factors of SHT.
In this study, to determine the prognostic factors of SHT, we compared two groups, the SHT maintenance group and the spontaneous improvement group. Comparison of numerous parameters including age, sex, fT4, TSH, TPO-Ab, lipid profile, hsCRP, urine iodine, and the presence of DT on US at initial presentation found that women had a more favorable prognosis than men and, among biochemical parameters considered for prognostic factors in many studies, only initial TSH level was significantly different. It is well-known that women have higher prevalence of both SHT and OHT than men. However, sexual difference of disease prognosis has not been established. Subjects with higher TSH level seemed to maintain a subclinical hypothyroid state, while subjects with lower TSH level showed a tendency to spontaneously improve. Our result is consistent with a study of 107 elderly SHT patients in Spain, which reported that TSH concentration was the most powerful predictor for the outcome of SHT [10]. TPO-Ab, known as the other apparent major predictor of OHT and SHT in several other studies, was not shown to be a significant predictor in our pool of SHT patients. However, when we performed further subanalysis for subjects with TSH between 5 to 10 µIU/mL, those in the spontaneous improvement group showed significantly lower TPO-Ab titer than the SHT maintenance group. Therefore, we can conclude that TPO-Ab titer is also a useful predictor for SHT. Although TSH level is the most powerful prognostic factor in the overall SHT population, TPO-Ab titer can be used as a predictor in SHT with mildly elevated TSH (between 5 to 10 µIU/mL). This can be explained, at least partially, by the fact that TPO-Ab titer contributes to prognosis earlier than TSH in the pathogenesis of SHT. TPO-Ab titer can be regarded as an earlier prognostic factor that is especially helpful in mild SHT. However, TSH level is the strongest prognostic factor, regardless of TPO-Ab titer, in advanced SHT.
We also investigated the changes in parameters during follow-up in each group. As for TPO-Ab changes, in the levothyroxine treatment group, TPO-Ab titer was decreased significantly. In contrast, in the spontaneous improvement group, there was no significant improvement of TPO-Ab titer, which exhibited a decreasing trend. Given that most of the subjects in the spontaneous improvement group were those who had relatively low TPO-Ab titer (only seven subjects of the spontaneous improvement group had TPO-Ab titer >60 U/mL) and that the subjects in the levothyroxine treatment group had higher TPO-Ab titer, we cannot generalize our results across all SHT subjects. However, our results at least demonstrate a benefit of levothyroxine supplement in SHT for the purpose of decreasing TPO-Ab, thus decreasing autoimmunity and the parenchymal pathologic process.
Regarding lipid profiles, currently there is no conclusive evidence regarding the effect of SHT on lipid profile. In an observational cohort study in the United States, lipid levels including total cholesterol, LDL-C and triglyceride increased in a graded fashion as thyroid function declined [11]. On the other hand, in another randomized placebo-controlled study in SHT where patients were randomly assigned to levothyroxine therapy or placebo and re-evaluated after 6 months of euthyroidism, triglyceride levels remained similar regardless of TSH levels, although total cholesterol and LDL-C levels were lowered by levothyroxine therapy and did correlate with baseline TSH levels [12]. The study concluded that only LDL-C levels are increased specifically and reversibly in association with SHT. Another study in Switzerland reported found that SHT subjects showed borderline elevated LDL-C and similar total cholesterol and triglyceride concentrations compared to controls matched for age, sex, and body mass index [13]. In our study, in both the spontaneous improvement group and the levothyroxine treatment group, triglyceride level decreased significantly during follow-up. However, most of these patients did not have hypertriglyceridemia (triglyceride >150 mg/dL) at diagnosis, so the change should be interpreted as the decrease of mean triglyceride level, not the improvement of hypertriglyceridemia. Unlike most previous studies stating total cholesterol and LDL-C as lipid profiles correlating with changes in thyroid function, an apparent correlation between triglyceride level and subclinical hypothyroid state was shown in this study. Therefore, we suggest that triglyceride level should not be ignored in evaluating SHT at least in Korean patients.
There have been several studies examining the correlation between hsCRP, a low grade inflammatory marker, and OHT or SHT. In several small-sized overseas studies, some investigators reported that patients with SHT had significantly higher levels of serum hsCRP [14,15] and others observed no significant differences [16]. Recently, there was a report in more than 900 elderly Korean patients finding that CRP did not show any differences between the subclinical hypothyroid and the euthyroid group [17]. Because hsCRP has recently been established as a potential marker for coronary heart disease, hsCRP elevation in SHT may imply cardiologic problems associated with SHT, although this has not yet been confirmed. However, in this study, hsCRP did not show any significant differences at diagnosis between the SHT maintenance group and the spontaneous improvement group or any significant change during follow-up in all three groups.
Recently, dietary iodine excess has been found to be associated with decreased thyroid function. Iodine is the most notable environmental factor in thyroid dysfunction and also is an indicator of vulnerability to other possible harmful environmental factors [18]. However, in this study, higher iodine concentration in the body, as quantified by urinary iodine excretion, was not associated with poor prognosis of SHT. A 1-year observational study of SHT in Denmark reported a significant positive correlation between TSH and urinary iodine excretion in monthly measurements [19]. Furthermore, high urinary iodine excretion predicted high TSH and TPO-Ab titer during the following month. In another study of adults in an iodine-rich area in Japan [20], hypothyroidism was more prevalent in subjects who were negative for antithyroid antibodies who had high urinary iodine excretion compared with those with normal urinary iodine excretion. They reported significantly higher TSH and lower fT4 levels in SHT patients with high urinary iodine excretion and concluded that the prevalence of hypothyroidism in iodine sufficient areas may be associated with the amount of iodine ingested. Based on our study, however, restriction of excessive iodine intake, which is a possible prognostic factor, if so, maybe only modifiable prognostic factor, has only weak evidence supporting its use in SHT management at present, at least in Korean subjects. As serial measurements of urinary iodine excretion after initial laboratory tests were not conducted; however, we cannot rule out the possibility that the altered iodine status during follow-up may influence the clinical course of our subclinical hypothyroid subjects.
Decreased or irregular heterogeneous echogenicity on US is a characteristic finding in diffuse thyroid disease, including both OHT and SHT [21,22,23,24]. However, the value of DT on US as a predictor of SHT has not been established until recently. In a 3-year follow-up study of 117 cases of mild SHT in Brazil, the likelihood of a progression toward OHT and improvement to euthyroidism, respectively, were similar between patients with positive TPO-Ab and/or US alteration and patients with negative TPO-Ab but with positive US alteration, thus suggesting that US findings may be useful in determining the prognosis of mild SHT [25]. In a retrospective study of Korean SHT patients, which aimed to see the difference of response to levothyroxine replacement according to autoantibody status and US finding, patients who initially showed DT on US, regardless of thyroid autoantibody level, showed poor response after levothyroxine replacement [26]. This report suggested the possibility that the DT pattern on thyroid US can serve as a prognostic factor when combined with other known parameters. In our study, the existence of DT on US at diagnosis was not significantly different between the SHT maintenance group and the spontaneous improvement group. Therefore, this observation indicates that DT on US only reflects the severity of the hypothyroid state at the time of US evaluation, which is indicated by higher TSH and TPO-Ab. It could not predict the prognosis of SHT.
This study has several limitations. First, because this is a retrospective study, the impacts and powers of the results are weakened. Second, the study population was not representative of the entire Korean population because the pool of patients was derived from a single tertiary health care center. For the most part, the care of SHT patients can be handled by local physicians at primary health care clinics. Therefore, the number of eligible participants in our study was small compared with the relatively many OHT patients. The third limitation is that the follow-up period was not long enough (less than 13.0 months). Without apparent progression to OHT, patients typically return to the local clinic for follow-up. Fourth, because we lacked additive data, such as body mass index and premenopause/postmenopause state, we were unable to interpret our data using these epidemiologic factors, which may have affected the prognosis of SHT. Lastly, as mentioned above, as urinary iodine excretion was not measured serially, altered iodine status during follow-up may have influenced the clinical course of our SHT subjects.
In conclusion, only initial TSH level was a definite prognostic factor of SHT. TPO-Ab titer was also a helpful prognostic factor for SHT cases with mildly elevated TSH (between 5 to 10 µIU/mL). Regarding lipid profiles, only triglyceride level, unlike total cholesterol and LDL-C, was correlated with TSH level, a measure of the severity of SHT. Findings of DT on US only contributed to the severity of SHT, not to its prognosis. hsCRP and urine iodine excretion, generally regarded as possible prognostic factors, were not related in any significant way to the prognosis and severity of SHT. Except for TSH and TPO-Ab, we were unable to validate any other biochemical prognostic factors in this retrospective analysis. In the future, a large-scaled, prospective trial will be needed to determine the definite prognostic factors of SHT.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Fowler PB, Swale J, Andrews H, Ikram H, Banim SO. Grades of hypothyroidism. Br Med J. 1973;2:178. doi: 10.1136/bmj.2.5859.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Riniker M, Tieche M, Lupi GA, Grob P, Studer H, Burgi H. Prevalence of various degrees of hypothyroidism among patients of a general medical department. Clin Endocrinol (Oxf) 1981;14:69–74. doi: 10.1111/j.1365-2265.1981.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann J. Prevalence of hypothyroidism in the elderly in Germany. A pilot study. J Endocrinol Invest. 1981;4:327–330. doi: 10.1007/BF03349452. [DOI] [PubMed] [Google Scholar]
- 5.Bilous RW, Tunbridge WM. The epidemiology of hypothyroidism: an update. Baillieres Clin Endocrinol Metab. 1988;2:531–540. doi: 10.1016/s0950-351x(88)80052-1. [DOI] [PubMed] [Google Scholar]
- 6.Kostoglou-Athanassiou I, Ntalles K. Hypothyroidism: new aspects of an old disease. Hippokratia. 2010;14:82–87. [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal MJ, Hunt WC, Garry PJ, Goodwin JS. Thyroid failure in the elderly. Microsomal antibodies as discriminant for therapy. JAMA. 1987;258:209–213. [PubMed] [Google Scholar]
- 9.Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221–3226. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 10.Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89:4890–4897. doi: 10.1210/jc.2003-032061. [DOI] [PubMed] [Google Scholar]
- 11.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 12.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:1533–1538. doi: 10.1210/jcem.87.4.8378. [DOI] [PubMed] [Google Scholar]
- 13.Althaus BU, Staub JJ, Ryff-De Leche A, Oberhansli A, Stahelin HB. LDL/HDL-changes in subclinical hypothyroidism: possible risk factors for coronary heart disease. Clin Endocrinol (Oxf) 1988;28:157–163. doi: 10.1111/j.1365-2265.1988.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Sharma TK, Kaushik GG, Sharma S, Vardey SK, Sinha M. Subclinical hypothyroidism and its association with cardiovascular risk factors. Clin Lab. 2011;57:719–724. [PubMed] [Google Scholar]
- 15.Tuzcu A, Bahceci M, Gokalp D, Tuzun Y, Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive c-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J. 2005;52:89–94. doi: 10.1507/endocrj.52.89. [DOI] [PubMed] [Google Scholar]
- 16.Toruner F, Altinova AE, Karakoc A, Yetkin I, Ayvaz G, Cakir N, Arslan M. Risk factors for cardiovascular disease in patients with subclinical hypothyroidism. Adv Ther. 2008;25:430–437. doi: 10.1007/s12325-008-0053-7. [DOI] [PubMed] [Google Scholar]
- 17.Park YJ, Lee EJ, Lee YJ, Choi SH, Park JH, Lee SB, Lim S, Lee WW, Jang HC, Cho BY, Woo JI, Kim KW. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Arch Gerontol Geriatr. 2010;50:e68–e73. doi: 10.1016/j.archger.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Chung HK. Environmental factors and thyroid dysfunction. Endocrinol Metab. 2012;27:191–193. [Google Scholar]
- 19.Karmisholt J, Laurberg P. Serum TSH and serum thyroid peroxidase antibody fluctuate in parallel and high urinary iodine excretion predicts subsequent thyroid failure in a 1-year study of patients with untreated subclinical hypothyroidism. Eur J Endocrinol. 2008;158:209–215. doi: 10.1530/EJE-07-0407. [DOI] [PubMed] [Google Scholar]
- 20.Konno N, Makita H, Yuri K, Iizuka N, Kawasaki K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab. 1994;78:393–397. doi: 10.1210/jcem.78.2.8106628. [DOI] [PubMed] [Google Scholar]
- 21.Loy M, Cianchetti ME, Cardia F, Melis A, Boi F, Mariotti S. Correlation of computerized gray-scale sonographic findings with thyroid function and thyroid autoimmune activity in patients with Hashimoto's thyroiditis. J Clin Ultrasound. 2004;32:136–140. doi: 10.1002/jcu.20008. [DOI] [PubMed] [Google Scholar]
- 22.Rago T, Chiovato L, Grasso L, Pinchera A, Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dsfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763–769. doi: 10.1007/BF03343925. [DOI] [PubMed] [Google Scholar]
- 23.Schiemann U, Avenhaus W, Konturek JW, Gellner R, Hengst K, Gross M. Relationship of clinical features and laboratory parameters to thyroid echogenicity measured by standardized grey scale ultrasonography in patients with Hashimoto's thyroiditis. Med Sci Monit. 2003;9:MT13–MT17. [PubMed] [Google Scholar]
- 24.Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jorgensen T. The association between hypoechogenicity or irregular echo pattern at thyroid ultrasonography and thyroid function in the general population. Eur J Endocrinol. 2006;155:547–552. doi: 10.1530/eje.1.02255. [DOI] [PubMed] [Google Scholar]
- 25.Rosario PW, Bessa B, Valadao MM, Purisch S. Natural history of mild subclinical hypothyroidism: prognostic value of ultrasound. Thyroid. 2009;19:9–12. doi: 10.1089/thy.2008.0221. [DOI] [PubMed] [Google Scholar]
- 26.Shin DY, Kim EK, Lee EJ. Role of ultrasonography in outcome prediction in subclinical hypothyroid patients treated with levothyroxine. Endocr J. 2010;57:15–22. doi: 10.1507/endocrj.k09e-154. [DOI] [PubMed] [Google Scholar]


