Abstract
Roots are considered to be a vital organ system of plants due to their involvement in water and nutrient uptake, anchorage, propagation, storage functions, secondary metabolite (including hormones) biosynthesis, and accumulation. Crops are strongly dependent on the availability of nitrogen in soil and on the efficiency of nitrogen utilization for biomass production and yield. However, knowledge about molecular responses to nitrogen fluctuations mainly derives from the study of model species. Nitric oxide (NO) has been proposed to be implicated in plant adaptation to environment, but its exact role in the response of plants to nutritional stress is still under evaluation. Recently a novel role for NO production and scavenging, thanks to the coordinate spatio-temporal expression of nitrate reductase and non-symbiotic hemoglobins, in the maize root response to nitrate, has been postulated. This control of NO homeostasis is preferentially accomplished by the cells of the root transition zone (TZ) which seems to represent the most nitrate responsive portion of maize root. The TZ is already known to function as a sensory center able to gather information from the external environment and to re-elaborate them in an adequate response. These results indicate that it could play a central role also for nitrate sensing by roots. A lot of work is still needed to identify and characterize other upstream and downstream signals involved in the “nitrate-NO” pathway, leading to root architecture adjustments and finally to stress adaptation.
Keywords: Zea mays L., nitrate, root, transition zone, nitric oxide
To search for nutrients and water, roots need to efficiently explore large soil volumes. To this aim they generate complex root systems, allowing them to maximize their resource allocation efficiency.1
Despite the vital importance of roots, the difficulty in accessing intact root systems for analysis, particularly under field conditions, have slowed down the breeding programs for plant’s adaptation to environmental restrictions.2,3 The capacity of plants to take up nutrients and water is mainly determined by changes in the architecture of the root system.1
Three major processes affect the overall architecture of the root system: the rate of cell division, the rate of cell differentiation, and the extent of expansion and elongation of cells.4-6 Disturbs in any of these 3 processes can affect the whole root-system architecture and the capacity of plants to survive and develop in adverse environments (Giehl et al.7 and references therein).
The root system results from the coordinated control of both genetic endogenous programs (regulating growth and organogenesis) and the action of abiotic and biotic environmental stimuli.8,9 The dynamic control of the overall root system architecture (RSA) throughout time finally determines root plasticity and allows plants to efficiently adapt to environmental constraints.10
The soil-environment from which plants extract nutrients and water is extremely heterogeneous, both spatially and temporally.11 Among the nutrients present in soil, nitrate (NO3−) may vary by an order of magnitude within centimeters or over the course of a day.12 The effects of NO3− on the root system are complex and depend on several factors, such as the concentration available to the plant, the endogenous nitrogen status and the sensitivity of the species.10,13,14
A considerable part of the studies aimed to unravel the mechanisms controlling RSA growth and development in response to nitrate have been focused on lateral roots (LR),8,13,15-20 while the nitrate-regulation of the primary root growth is still unclear. Beside NO3−, auxin has been demonstrated to strongly affect and control the LR development,21-24 and an increasing number of studies suggests an overlap between auxin and NO3− signaling pathways in controlling LR development.25-33
NO3− has a Doubtful Role in Regulating the Growth of Primary Roots
Despite the high amount of reports published on nitrate effects on root elongation, the lack of univocal results makes it difficult to clearly decipher this response (Table 1). In Arabidopsis thaliana, inhibition of primary root growth has been observed when nitrate is applied homogeneously at high concentrations (50 mM) for 7 d, but not in a range between 0.1 and 10 mM.35 On the contrary, in this same species Linkohr et al.36 showed an inhibition of primary root elongation with the increase of nitrate concentration already beyond 0.01 mM, but in this case seedlings were grown in the nutrient medium either for 17 or 18 d. This is in contrast with results previously obtained by Zhang and Forde,34 which did not observe changes in primary root length in a range of nitrate concentrations from 0.01 mM to 100 mM.
Table 1. Overview of the papers reporting results on primary root (PR) response to nitrate treatments.
| Authors | Species | Treatments | Effect on PR length |
|---|---|---|---|
| Zhang and Forde 34 | Arabidopsis thaliana | Seedlings were grown on agar plates containing a range of NO3- concentration (0.01–100mM) and the lengths of the primary roots were measured after 14d. | No effects |
| Signora et al. 35 | Arabidopsis thaliana | Seedlings were grown on agar plates containing a range of NO3- concentrations (0.1–50mM). The lengths of the primary roots were recorded after 7d. | No effects (0.1–10mM) Inhibition (> 50mM) |
| Linkohr et al. 36 | Arabidopsis thaliana | Seedlings were grown either for 17 or 18d on agar plates containing a range of NO3- concentrations (0.01–1.0mM). The lengths of the primary roots were collected after the treatments. | Inhibition |
| Walch-Liu and Forde 13 | Arabidopsis thaliana | Primary root growth was measured 9d after transfer of 5-d-old seedlings to segmented plates where NO3- (0.05–5mM) was present only in the bottom segment (localized treatments). | Stimulation |
| Gifford et al. 37 | Arabidopsis thaliana | Seedlings were grown on agar plates containing a range of NO3- concentration (0–20mM). The primary root lengths were measured after 12d. | Stimulation |
| Celis-Arámburo et al. 14 | Capsicum chinense Jacq. | Seedlings were grown on agar plates with 0.01mM NO3- and transferred to segmented. NO3- concentrations in the middle segment were adjusted to 0.01–10mM (localized treatments). For the homogeneous treatment the concentration was 1mM NO3-. The primary root lengths were recorded after 10d. | Inhibition |
| Yendrek et al. 38 | Medicago truncatula | Plants were grown on a N-free medium for 1 wk, transferred to plates with increasing concentrations of NO3- (1–20–50mM) and grown for 3 wk. The lengths of the primary roots were recorded after the treatments. | Inhibition |
| Tian et al. 39 | Zea mays L. | Plants were grown in nutrient solution containing several NO3- concentration (0.05–20mM). The lengths of the primary roots were recorded after 12d. | Inhibition (> 5mM) |
| Tian et al. 40 | Zea mays L. | Seedlings were incubated in the solutions containing different concentrations of NO3- (0.05–20mM) and the root length was measured after 12d of incubation. | No effects (0- 0.5mM) Inhibition (> 5mM) |
| Zhao et al. 41 | Zea mays L. | Seedlings were grown in varying concentrations of NO3- (0.1–10mM) for 7d and then exposed to 0.1 and 1mM NO3- for 48h. The root length was measured after the incubation. | Inhibition |
| Manoli et al. 68 | Zea mays L. | Primary root growth of 8-d-old seedlings grown in 6 different solutions (1mM NO3-, - NO3- and NO-donors/scavengers) were monitored for 24–48h. | Stimulation |
However, if nitrate supply was localized only to the apex, primary root growth of a number of Arabidopsis accessions was significantly stimulated, even if to a different extent according to the line responsiveness.13 More recently Gifford et al.37 demonstrated a stimulatory effect on primary root elongation in Arabidopsis seedlings grown for 12 d on a nitrate concentration ranging from 0 to 20 mM. Conversely, a reduction of primary root growth has been observed in both Capsicum chinense Jacq.,14 and Medicago truncatula38 in response to a prolonged exposure to nitrate.
In maize (Zea mays L.) a consistent inhibitory effect on primary root length was observed by Tian and co-authors after 12 d of growth at a nitrate concentration of 20 mM.39 A few years later a more detailed study was published by the same authors who demonstrated that nitrate concentrations lower than 0.5 mM had no effect on elongation of primary, seminal, and crown roots, while concentrations above 5 mM affected more significantly the root elongation after 12 d of treatment.40 Moreover, by investigating the effect of different nitrate concentrations on root cell sizes, they found that high concentrations of nitrate had no effect on the length of the meristem, but did result in reduced cell elongation in the root elongation zone. Interestingly, the different types of roots considered in this study displayed different sensitivities to high nitrate, suggesting a specific regulation for each of them.40
Unlike what is known on the nitrate regulation of lateral root development, the mechanisms underlying the nitrate effects on primary root elongation are still controversial and poorly known. Future studies are thus needed to try to shed light on this aspect that could highly affects plant adaptation to an external environment characterized by a spatio-temporal non constant nutrient accessibility.
The Root Transition Zone
The root apex represents the first part of the plant getting in touch with unknown regions of the soil, and it functions as a dynamic sensory organ, able to both perceive the external environment and to adequately reorganize the root growth in response to the stimuli received.42
In 1990 Baluška et al.43 invented the term transition zone to describe a unique part of the maize root apex, in which cells after leaving the meristem and before entering the elongation zone undergo slow isotropic-like growth, but do not still elongate, in fact resembling meristematic cells in many aspects. In particular, the apical part (distal) of this region seems to be characterized mainly by cells that optionally can reenter the cell cycle, whereas cells of the basal (proximal) part of this zone are able to readily enter into the fast cell elongation region.42,44,45 This developmental feature could be differentially regulated at the opposite root flanks, providing the root apices with an effective mechanism to re-orientate growth in response to environmental stimuli.44
The transition zone is a unique zone being competent for integration of diverse endogenous and exogenous signals, and translating them into adaptive differential growth responses. It plays crucial functions for the perception and response to a range of external factors, as for example mechanical stimuli42 and aluminum toxicity.46-49
This capability seems to be, at least in part, linked to the complex system of a polar auxin transport circuit.42 Actually, since 1993 it has been evidenced that cells belonging to this zone are strongly auxin-responsive and accomplish dramatic rearrangements of the cytoskeleton, being subjected to a series of fundamental changes in their cytoarchitecture.50
A recent study conducted on maize demonstrated that the transition zone plays central roles in both sensing and adapting to root hypoxia.51 The authors also observed that the oxygen deprivation of roots induces local NO emission in the TZ, that is essential for the successful acclimation of the entire maize root to oxygen deprivation.51
A number of experimental data globally indicates that the transition zone of the root may be considered as a sort of sensory center, enabling the root apex to continuously monitor environment parameters and to trigger appropriate responses.51-63 Future studies will be needed to deepen the role of this unique root zone in translating the external stimuli in motoric responses.
Nitrate Affects Root Elongation through NO-Elicited Actions
Recently nitric oxide (NO) was proposed to be involved in the regulation of the nitrate-dependent primary root growth.41 The authors showed that high nitrate supply may reduce IAA levels and subsequently inhibits NO synthase activity, leading to a decrease in the endogenous NO level, which serves as a trigger to elicit nitrate-dependent root growth. A regulatory role for NO in the inhibition of primary root growth has also been suggested in tomato64 and Arabidopsis.65,66
Furthermore, a recent study performed in maize provided evidences that NO is produced by nitrate reductase (NR) as an early response to nitrate supply and that the coordinated induction of non-symbiotic hemoglobins (nsHbs) could finely regulate the NO steady-state.67,68 Both nitric oxide biosynthesis and gene regulation were preferentially accomplished by cells of the transition zone of roots, which would seem the most nitrate responsive portion of maize root.68 NsHbs play important roles in plant physiology by regulating a number of downstream physiological events involved in plant developmental processes and stress responses, also interacting with many hormonal signaling (for a review see refs 69,70). They catalyze the conversion of nitric oxide to nitrate, contributing to the control of nitric oxide homeostasis in plant cells. They should be considered to be as important as NO generation in regulating in planta NO signaling.70
Moreover, in this same study,68 a stimulatory effect of a low concentration of nitrate (1 mM) on root elongation after 1–2 days of treatment was measured in very young seedlings. Nevertheless, when an inhibitor of nitrate reductase activity (tungstate) or a nitric oxide scavenger (cPTIO) were supplied together with nitrate, no effects on root elongation were observed. On the contrary the treatment of nitrate-depleted roots with a low concentration (10 µM) of a nitric oxide donor (SNP) stimulated root elongation to an extent similar to that measured after nitrate supply. These results strongly suggest that the mechanism through which nitrate affects root elongation is dependent on nitric oxide, as also observed by Zhao et al.41 even if these authors found some different and in some way opposite results. This apparently contradictory finding could derive from the very different experimental plans and growth conditions utilized in these 2 works, making difficult to compare results obtained. Furthermore, our unpublished results suggest that nitrate is able to affect root elongation in a contrasting mode according to their concentration, acting as a stimulatory signal for concentrations equal or below 1 mM, and as a negative regulator at higher concentrations, suggesting the existence of a multifaceted concentration/time-dependent mechanism of regulation of root growth by nitrate availability.
NO is considered a key regulator of plant developmental processes and defense (for a reviews see refs 70-74), although the mechanism and direct targets of NO action remain largely unknown (for a review see ref. 75 and references therein).
In the case of NO-dependent nitrate regulation of root elongation, the downstream events triggering the root to elongate have still to be identified. Cytoskeletal proteins seem to represent a highly probable molecular target for NO signal76-78 and accumulating evidences place NO among the key elements in the control of a number of cytoskeleton-mediated processes in plants, such as root growth and development,79 guard cell dynamic,80 vesicle trafficking,76 pollen,81 and root hair tip growth,82 or gravitropic bending.83 In particular, Kasprowicz et al.76 demonstrated that the actin-dependent endocytosis and organization of the actin cytoskeleton are modulated by NO levels in maize root apices, according to cell-type and developmental stage with the most remarkable effects noticed at level of the transition zone. Thus, the involvement of cytoskeletal rearrangements in the NO-mediated nitrate regulation of primary root elongation is highly conceivable.
Moreover, since NO and auxin act synergically to control diverse aspects of root biology (for a review see Freschi et al.84) and lateral root development in response to nitrate is strongly auxin dependent,85 a role of NO as a coordinator of nitrate and auxin signaling to control the overall root response to the anion cannot be excluded. The involvement of nitric oxide homeostasis control in the root elongation response to nitrate68 adds a novel component to the complicated puzzle of the root adaptation to nitrate fluctuations in soil (Fig. 1). Furthermore, the prominent role of the maize transition zone in the accomplishment of this sensing pathway widens the range of signal/molecules which are sensed and decoded by this particular region of root, which seems to transversally operate in translating a large number of endogenous and exogenous clues in motoric behavior.
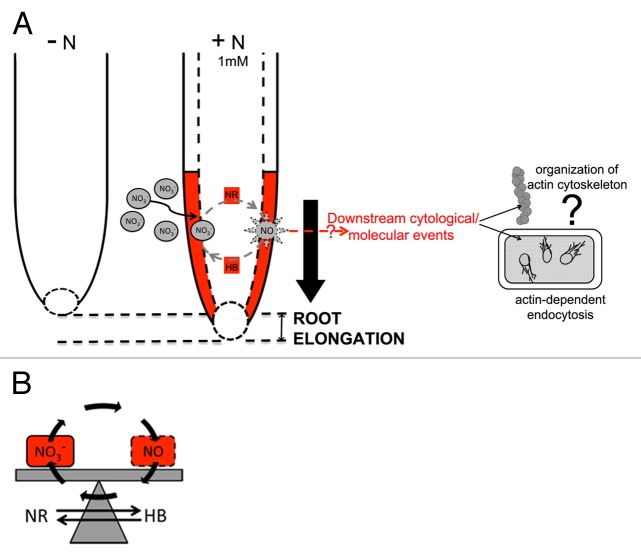
Figure 1. Model of the NO-mediated nitrate regulation of primary root elongation. (A) The transfer of seedlings from a NO3--deprived media to a NO3--supplied solution results in a elongation of the primary root. The stimulatory effect of NO3- (1mM) was demonstrated to be dependent on the control of nitric oxide (NO) homeostasis thank to the coordinate regulation of cytosolic nitrate reductase (NR) and non-symbiotic hemoglobins (nsHbs) (B).67,68 The preferential localization and the strong transcriptional responsiveness of both NR and nsHbs in the transition zone of the apex straightened the hypothesis of a role of this root portion in translating the environmental stimuli in developmental response.67,68 Because of the role of NO in several cytoskeleton-mediated processes in plants,76-83 the actin-dependent endocytosis and the organization of the actin cytoskeleton are proposed as candidates in transducing the NO-dependent nitrate regulation of root elongation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by ex-60% 2011–2012–2013 Università di Padova.
Glossary
Abbreviations:
- cPTIO
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- IAA
indole-3-acetic acid
- SNP
sodium nitroprusside
References
- 1.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–7. doi: 10.1016/S1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 2.de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007;12:474–81. doi: 10.1016/j.tplants.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JP. Roots of the second green revolution. Aust J Bot. 2007;55:493–512. doi: 10.1071/BT06118. [DOI] [Google Scholar]
- 4.Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- 5.Scheres B, Benfey P, Dolan L. Root development. In: The Arabidopsis book. Somerville CR, Meyerowitz EM, eds. Rockville: America Society of Plant Biologists, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang K, Feldman LJ. Regulation of root apical meristem development. Annu Rev Cell Dev Biol. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- 7.Giehl RF, Gruber BD, von Wirén N. It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot. 2014;65:769–78. doi: 10.1093/jxb/ert421. [DOI] [PubMed] [Google Scholar]
- 8.Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- 9.Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant Soil. 2009;321:153–87. doi: 10.1007/s11104-009-9929-9. [DOI] [Google Scholar]
- 10.Gruber BD, Giehl RFH, Friedel S, von Wirén N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013;163:161–79. doi: 10.1104/pp.113.218453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom AJ, Frensch J, Taylor AR. Influence of inorganic nitrogen and pH on the elongation of maize seminal roots. Ann Bot. 2006;97:867–73. doi: 10.1093/aob/mcj605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson LE, Bloom AJ. Root distribution in relation to soil nitrogen availability in field-grown tomatoes. Plant Soil. 1990;128:115–26. doi: 10.1007/BF00011100. [DOI] [Google Scholar]
- 13.Walch-Liu P, Forde BG. Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J. 2008;54:820–8. doi: 10.1111/j.1365-313X.2008.03443.x. [DOI] [PubMed] [Google Scholar]
- 14.Celis-Arámburo TdeJ, Carrillo-Pech M, Castro-Concha LA, Miranda-Ham MdeL, Martínez-Estévez M, Echevarría-Machado I, Celis-ArámburoTde J Exogenous nitrate induces root branching and inhibits primary root growth in Capsicum chinense Jacq. Plant Physiol Biochem. 2011;49:1456–64. doi: 10.1016/j.plaphy.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol. 2002;53:203–24. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 16.Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci U S A. 2006;103:19206–11. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benková E, Bielach A. Lateral root organogenesis - from cell to organ. Curr Opin Plant Biol. 2010;13:677–83. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Bennett T, Scheres B. Root development-two meristems for the price of one? Curr Top Dev Biol. 2010;91:67–102. doi: 10.1016/S0070-2153(10)91003-X. [DOI] [PubMed] [Google Scholar]
- 19.De Smet I. Lateral root initiation: one step at a time. New Phytol. 2012;193:867–73. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- 20.Jung JK, McCouch S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front Plant Sci. 2013;4:186. doi: 10.3389/fpls.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7:847–59. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 22.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–8. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.De Smet I. Lateral root initiation: one step at a time. New Phytol. 2012;193:867–73. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- 24.Van Norman JM, Xuan W, Beeckman T, Benfey PN. To branch or not to branch: the role of pre-patterning in lateral root formation. Development. 2013;140:4301–10. doi: 10.1242/dev.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci U S A. 1999;96:6529–34. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol. 2002;53:203–24. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Chen F, Zhang F, Mi G. Auxin transport from shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Sci. 2005;169:894–900. doi: 10.1016/j.plantsci.2005.06.007. [DOI] [Google Scholar]
- 28.Beeckman T, Friml J. Nitrate contra auxin: nutrient sensing by roots. Dev Cell. 2010;18:877–8. doi: 10.1016/j.devcel.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell. 2010;18:927–37. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, An X, Cheng L, Chen F, Bao J, Yuan L, Zhang F, Mi G. Auxin transport in maize roots in response to localized nitrate supply. Ann Bot. 2010;106:1019–26. doi: 10.1093/aob/mcq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutiérrez RA. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2010;107:4477–82. doi: 10.1073/pnas.0909571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiba T, Kudo T, Kojima M, Sakakibara H. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot. 2011;62:1399–409. doi: 10.1093/jxb/erq410. [DOI] [PubMed] [Google Scholar]
- 33.Mounier E, Pervent M, Ljung K, Gojon A, Nacry P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014;37:162–74. doi: 10.1111/pce.12143. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–9. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- 35.Signora L, De Smet I, Foyer CH, Zhang H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J. 2001;28:655–62. doi: 10.1046/j.1365-313x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- 36.Linkohr BI, Williamson LC, Fitter AH, Leyser HM. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002;29:751–60. doi: 10.1046/j.1365-313X.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 37.Gifford ML, Banta JA, Katari MS, Hulsmans J, Chen L, Ristova D, Tranchina D, Purugganan MD, Coruzzi GM, Birnbaum KD. Plasticity regulators modulate specific root traits in discrete nitrogen environments. PLoS Genet. 2013;9:e1003760. doi: 10.1371/journal.pgen.1003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yendrek CR, Lee YC, Morris V, Liang Y, Pislariu CI, Burkart G, Meckfessel MH, Salehin M, Kessler H, Wessler H, et al. A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J. 2010;62:100–12. doi: 10.1111/j.1365-313X.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 39.Tian QY, Chen FJ, Zhang FS, Mi GH. Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil. 2005;100:1–12. [Google Scholar]
- 40.Tian Q, Chen F, Liu J, Zhang F, Mi G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J Plant Physiol. 2008;165:942–51. doi: 10.1016/j.jplph.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhao DY, Tian QY, Li LH, Zhang WH. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot. 2007;100:497–503. doi: 10.1093/aob/mcm142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baluška F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci. 2010;15:402–8. doi: 10.1016/j.tplants.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Baluška F, Kubica S, Hauskrecht M. Postmitotic ‘isodiametric’ cell growth in the maize root apex. Planta. 1990;181:269–74. doi: 10.1007/BF00195876. [DOI] [PubMed] [Google Scholar]
- 44.Verbelen JP, De Cnodder T, Le J, Vissenberg K, Baluška F. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav. 2006;1:296–304. doi: 10.4161/psb.1.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baluška F, Mancuso S. Root apex transition zone as oscillatory zone. Front Plant Sci. 2013;4:354. doi: 10.3389/fpls.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–63. doi: 10.1104/pp.116.1.155. [DOI] [Google Scholar]
- 47.Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex. short-term effects on the distal part of the transition zone. Plant Physiol. 1999;119:1073–82. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol. 2000;122:945–56. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Illés P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovečka M. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot. 2006;57:4201–13. doi: 10.1093/jxb/erl197. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–10. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mugnai S, Azzarello E, Baluška F, Mancuso S. Local root apex hypoxia induces NO-mediated hypoxic acclimation of the entire root. Plant Cell Physiol. 2012;53:912–20. doi: 10.1093/pcp/pcs034. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa H, Evans ML. Induction of curvature in maize roots by calcium or by thigmostimulation: role of the postmitotic isodiametric growth zone. Plant Physiol. 1992;100:762–8. doi: 10.1104/pp.100.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winch S, Pritchard J. Acid-induced cell wall loosening is confined to the accelerating region of the root growing zone. J Exp Bot. 1999;50:1481–7. doi: 10.1093/jxb/50.338.1481. [DOI] [Google Scholar]
- 54.Baluška F, Volkmann D, Barlow PW. Specialized zones of development in roots: view from the cellular level. Plant Physiol. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu Y, Cosgrove DJ. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot. 2000;51:1543–53. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- 56.Mancuso S, Boselli M. Characterisation of the oxygen fluxes in the division, elongation and mature zones of Vitis roots: influence of oxygen availability. Planta. 2002;214:767–74. doi: 10.1007/s004250100670. [DOI] [PubMed] [Google Scholar]
- 57.Ober ES, Sharp RE. Electrophysiological responses of maize roots to low water potentials: relationship to growth and ABA accumulation. J Exp Bot. 2003;54:813–24. doi: 10.1093/jxb/erg060. [DOI] [PubMed] [Google Scholar]
- 58.Mancuso S, Barlow PW, Volkmann D, Baluška F. Actin turnover-mediated gravity response in maize root apices: gravitropism of decapped roots implicates gravisensing outside of the root cap. Plant Signal Behav. 2006;1:52–8. doi: 10.4161/psb.1.2.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancuso S, Marras AM. Adaptative response of Vitis root to anoxia. Plant Cell Physiol. 2006;47:401–9. doi: 10.1093/pcp/pcj007. [DOI] [PubMed] [Google Scholar]
- 60.Amenós M, Corrales I, Poschenrieder C, Illés P, Baluška F, Barceló J. Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant Cell Physiol. 2009;50:528–40. doi: 10.1093/pcp/pcp013. [DOI] [PubMed] [Google Scholar]
- 61.Sobol M, Kordyum E. Distribution of calcium ions in cells of the root distal elongation zone under clinorotation. Microgravity Sci Technol. 2009;21:179–85. doi: 10.1007/s12217-008-9045-0. [DOI] [Google Scholar]
- 62.Marciano DPRO, Toledo-Ramos F, Neiva-Alvim M, Magalhaes JR, Costa França MG. Nitric oxide reduces the stress effects of aluminum on the process of germination and early root growth of rice. J Plant Nutr Soil Sci. 2010;173:885–91. doi: 10.1002/jpln.200900312. [DOI] [Google Scholar]
- 63.Mugnai S, Marras AM, Mancuso S. Effect of hypoxic acclimation on anoxia tolerance in Vitis roots: response of metabolic activity and K+ fluxes. Plant Cell Physiol. 2011;52:1107–16. doi: 10.1093/pcp/pcr061. [DOI] [PubMed] [Google Scholar]
- 64.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–5. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 65.Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J. Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol. 2010;51:1612–26. doi: 10.1093/pcp/pcq117. [DOI] [PubMed] [Google Scholar]
- 66.Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci U S A. 2011;108:18506–11. doi: 10.1073/pnas.1108644108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trevisan S, Manoli A, Begheldo M, Nonis A, Enna M, Vaccaro S, Caporale G, Ruperti B, Quaggiotti S. Transcriptome analysis reveals coordinated spatiotemporal regulation of hemoglobin and nitrate reductase in response to nitrate in maize roots. New Phytol. 2011;192:338–52. doi: 10.1111/j.1469-8137.2011.03822.x. [DOI] [PubMed] [Google Scholar]
- 68.Manoli A, Begheldo M, Genre A, Lanfranco L, Trevisan S, Quaggiotti S. NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J Exp Bot. 2014;65:185–200. doi: 10.1093/jxb/ert358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill RD. Non-symbiotic haemoglobins-What’s happening beyond nitric oxide scavenging? AoB Plants. 2012;2012:pls004. doi: 10.1093/aobpla/pls004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJ, Hebelstrup KH, Gupta KJ. Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants. 2013;5:pls052. doi: 10.1093/aobpla/pls052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–74. doi: 10.1016/S1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 72.Wojtaszek P. Nitric oxide in plants. To NO or not to NO. Phytochemistry. 2000;54:1–4. doi: 10.1016/S0031-9422(00)00056-X. [DOI] [PubMed] [Google Scholar]
- 73.Beligni MV, Lamattina L. Nitric oxide: a non-traditional regulator of plant growth. Trends Plant Sci. 2001;6:508–9. doi: 10.1016/S1360-1385(01)02156-2. [DOI] [PubMed] [Google Scholar]
- 74.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–36. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 75.Simontacchi M, García-Mata C, Bartoli CG, Santa-María GE, Lamattina L. Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep. 2013;32:853–66. doi: 10.1007/s00299-013-1434-1. [DOI] [PubMed] [Google Scholar]
- 76.Kasprowicz A, Szuba A, Volkmann D, Baluška F, Wojtaszek P. Nitric oxide modulates dynamic actin cytoskeleton and vesicle trafficking in a cell type-specific manner in root apices. J Exp Bot. 2009;60:1605–17. doi: 10.1093/jxb/erp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Chen T, Zhang C, Hao H, Liu P, Zheng M, Baluška F, Šamaj J, Lin J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytol. 2009;182:851–62. doi: 10.1111/j.1469-8137.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 78.Yao LL, Pei BL, Zhou Q, Li YZ. NO serves as a signaling intermediate downstream of H₂O₂ to modulate dynamic microtubule cytoskeleton during responses to VD-toxins in Arabidopsis. Plant Signal Behav. 2012;7:174–7. doi: 10.4161/psb.18768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolbert Z, Bartha B, Erdei L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J Plant Physiol. 2008;165:967–75. doi: 10.1016/j.jplph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot. 2008;59:165–76. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 81.Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–14. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 82.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–70. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freschi L. Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci. 2013;4:398. doi: 10.3389/fpls.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laskowski M. Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. J Exp Bot. 2013;64:2609–17. doi: 10.1093/jxb/ert155. [DOI] [PubMed] [Google Scholar]