Abstract
Tyrosine kinase inhibitors have dramatically improved the treatment of chronic myeloid leukemia. Recent evidence revealed that some patients with chronic myeloid leukemia can stop imatinib without relapse after achieving a complete molecular response. This review discusses the possible predictive markers to identify these patients who can stop imatinib without relapse.
Keywords: chronic myeloid leukemia, imatinib, NK cells, cytotoxic T lymphocytes, predictive marker, immunosurvelliance
Imatinib, a tyrosine kinase inhibitor, has dramatically improved the treatment of chronic myeloid leukemia (CML). Recent evidence has revealed that some patients with CML can safely discontinue imatinib therapy without relapse, particularly after achieving a complete molecular response. This review discusses the possible immunosurveillance predictive markers useful to discriminate patients who may stop imatinib therapy without eliciting disease recurrence.
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder of hematopoietic stem cells caused by formation of the BCR-ABL1 chimeric gene encoding an aberrant tyrosine kinase with oncogenic activity.1 Tyrosine kinase inhibitors (TKIs) are the current standard-of-care treatment for patients with CML. Imatinib (Glivec®) is the first TKI used to treat chronic-phase CML, replacing conventional interferon α (IFNα) administration.2 However, discontinuation of TKI therapy usually causes rapid disease relapse, presumably due to the reactivation of dormant CML stem cells that are resistant to TKI-induced leukemic cell ablation. TKI therapy is therefore considered to be necessary throughout the lifetime of the patient although an indefinite intake of TKI causes concerns about long-term safety, tolerability, drug resistance, and costs. If CML can be cured permitting safe cessation of an expensive drug treatment, such as imatinib, then both personal and governmental medical expenses could be expected to dramatically decrease without sacrificing patient care. Of note, recent accumulating evidence indicates that some CML patients can stop imatinib treatment without suffering disease relapse after achieving a complete molecular response (CMR).3 Therefore, there is currently a strong need for specific predictive markers that could precisely determine which patients can discontinue therapy without experiencing relapse. To date, several markers have been reported. Physiological variables associated with resistance to relapse include: male sex, low Sokal risk score, shorter time to BCR-ABL1 negativity, longer duration of CMR before discontinuation, and longer duration of imatinib therapy.3,4 However, further investigation of this issue in larger clinical studies encompassing more patients is necessary to prove reliability.
It has been previously reported that 41% of imatinib-treated CML patients with CMR lasting more than 2 y can safely discontinue treatment without relapse.3 In another study, a unique subset of CML patients also demonstrated maintenance of CMR after imatinib discontinuation and yet, intriguingly, high sensitivity quantitative polymerase chain reaction assay revealed that these patients harbored persistent BCR-ABL1 translocated DNA.5 Thus, it may not be necessary to continue imatinib therapy indefinitely, and some CML patients can stop imatinib without apparent disease relapse, despite the presence of persistent residual CML cells. This evidence strongly suggests that although TKI therapy plays a central role in minimizing BCR-ABL1–positive CML cells, other endogenous factors could also be vital for restraining CML cells even in the absence of TKIs. Among such native anticancer effectors are immune cells mediating immunosurveillance. Increasing evidence suggests that natural killer (NK) cells play an important role in controlling growth of CML cells and sustaining CMR.6-9 Recently, CML patients who sustained a CMR after imatinib discontinuation were shown to exhibit higher levels of functional NK cells than either normal (non-diseased) subjects or CML patients who did not sustain a CMR but did maintain a major molecular response for more than 2 y with continuing imitinib therapy (Fig. 1A).7 In accordance with this report, increased counts of NK cells have also been reported for IFNα-treated CML patients who were able to discontinue treatment without relapse.8 The essential role of NK cells in constraining CML relapse has also been demonstrated by implantation of NK cells into the bone marrow of irradiated recipient mice, revealing that NK cells are able to control the growth of CML cells in vivo through missing self-recognition.9 The effect of NK cells was considered to be mediated, at least in part, by targeting leukemia-initiating stem cells.9 Although off-target effects secondarily induced by imatinib therapy may be involved in triggering activation of NK cells as has been previously reported in gastrointestinal stromal tumor patients, the molecular mechanisms by which NK cells are activated in CML patients undergoing imatinib treatment remain to be clarified.
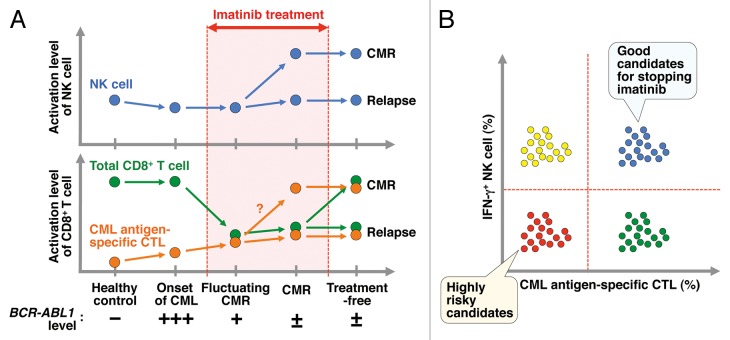
Figure 1. Predictive immune cell markers for identifying patients who can stop imatinib without relapse. (A) Hypothetical kinetics regarding the activation level of natural killer (NK) cells, total CD8+ T cells, and chronic myeloid leukemia (CML) antigen-specific cytotoxic T lymphocyte (CTLs). Total CD8+ T cells appear to be more susceptible to imatinib than NK cells. It is predicted that patients who have sustained and higher levels of activated NK cells and/or CML antigen–specific CTLs can safely stop imatinib without relapse. (B) Combined prediction using multiple markers, such as the presence of IFNγ+ NK cells and CML antigen–specific CTLs, could be a more reliable strategy.
Cytotoxic T lymphocyte (CTL) responses are also attractive candidates for predictive markers of relapse risk following TKI discontinuation, but there have been few reports of this occurrence so far. This is presumably due to the attenuation of CTL responses that may be more sensitive than NK cells to TKI-mediated inhibition of off-target kinases. For instance, one such off-target kinase, lymphocyte-specific protein tyrosine kinase (LCK), binds to CD4+ and CD8+ T cells and plays an indispensable signaling role in the selection and maturation of stimulated T cells. CML patients have lower numbers of total CD8+ T cells while undergoing imatinib treatment. However, after imatinib discontinuation this lymphocyte number returns to the homeostatic level of normal healthy controls (Fig. 1A).7 However, alloreactive CTLs have been demonstrated to mediate curative anti-leukemic effects in allogeneic hematopoietic stem cell transplantation. Moreover, one of several mechanisms by which IFNα controls CML is considered to be via the enhancement of CTL responses against CML antigens, such as the leukemia-associated antigen serine protease, proteinase-3.10 Therefore, taken together, these results indicate that further detailed analyses of CML antigen-specific CTL responses, for example using several sensitive human leukocyte antigen tetramer–peptide complexes, could help to predict those patients who can safely stop imatinib therapy.
In conclusion, in addition to the depth of CMR achieved and the patients genetic background, the presence of relatively abundant and functional NK cells is a good prognostic marker for safe discontinuation of imatinib.6,7 Moreover, CTLs specific for CML antigens such as BCR-ABL1 or proteinase-3 would also be good candidates for predictive markers. However, to further confirm reliability, the development and availability of highly sensitive human leukocyte antigen tetramer–peptide complexes that recognize unique CML antigen-specific CTLs are necessary tools for such analyses. Moreover, longitudinal studies before and after discontinuation of imatinib involving a larger patient cohort are warranted, kinetic analyses that may reveal a combination of these multiple markers offering a more reliable and attractive strategy to stratify CML patients according to predicted outcome (Fig. 1B).
Disclosure of Potential Conflicts of Interest
K.O. received research support from Bristol–Myers Squibb KK and Novartis KK, served as consultant and advisor for Novartis KK, Bristol-Myers Squibb KK, and Ariad, and received honoraria for lecture fees from Novartis KK and Bristol–Myers Squibb KK. This study was supported in part by the Private University Strategic Research-Based Support Project: Epigenetics Research Project Aimed at General Cancer Cure using Epigenetic Targets and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Glossary
Abbreviations:
- CML
chronic myeloid leukemia
- CMR
complete molecular response
- CTL
cytotoxic lymphocyte
- IFN
interferon
- NK
natural killer
- TKI
tyrosine kinase inhibitor
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–56. [PubMed] [Google Scholar]
- 2.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 3.Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, et al. Intergroupe Français des Leucémies Myéloïdes Chroniques Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Kyo T, Maeda Y, Sugihara T, Usuki K, Kawaguchi T, Usui N, Okamoto S, Ohe Y, Ohtake S, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97:903–6. doi: 10.3324/haematol.2011.056853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, Slader C, Field C, Dang P, Filshie RJ, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24:1719–24. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 6.Ohyashiki K, Katagiri S, Tauchi T, Ohyashiki JH, Maeda Y, Matsumura I, Kyo T. Increased natural killer cells and decreased CD3(+)CD8(+)CD62L(+) T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br J Haematol. 2012;157:254–6. doi: 10.1111/j.1365-2141.2011.08939.x. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi I, Yoshimoto T, Katagiri S, Mizuguchi J, Tauchi T, Kimura Y, Inokuchi K, Ohyashiki JH, Ohyashiki K. Sustained upregulation of effector natural killer cells in chronic myeloid leukemia after discontinuation of imatinib. Cancer Sci. 2013;104:1146–53. doi: 10.1111/cas.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreutzman A, Rohon P, Faber E, Indrak K, Juvonen V, Kairisto V, Voglová J, Sinisalo M, Flochová E, Vakkila J, et al. Chronic myeloid leukemia patients in prolonged remission following interferon-α monotherapy have distinct cytokine and oligoclonal lymphocyte profile. PLoS One. 2011;6:e23022. doi: 10.1371/journal.pone.0023022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kijima M, Gardiol N, Held W. Natural killer cell mediated missing-self recognition can protect mice from primary chronic myeloid leukemia in vivo. PLoS One. 2011;6:e27639. doi: 10.1371/journal.pone.0027639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burchert A, Müller MC, Kostrewa P, Erben P, Bostel T, Liebler S, Hehlmann R, Neubauer A, Hochhaus A. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010;28:1429–35. doi: 10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]