Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) project is a joint initiative of the societies of toxicological pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP). Its aim is to develop an internationally-accepted nomenclature for proliferative and non-proliferative lesions in laboratory rodents. A widely accepted international harmonization of nomenclature in laboratory animals will decrease confusion among regulatory and scientific research organizations in different countries and will provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists. The purpose of this publication is to provide a standardized nomenclature for classifying microscopical lesions observed in the integument of laboratory rats and mice. Example colour images are provided for most lesions. The standardized nomenclature presented in this document and additional colour images are also available electronically at http://www.goreni.org. The nomenclature presented herein is based on histopathology databases from government, academia, and industrial laboratories throughout the world, and covers lesions that develop spontaneously as well as those induced by exposure to various test materials. (DOI: 10.1293/tox.26.27S; J Toxicol Pathol 2013; 26: 27S–57S)
Keywords: diagnostic pathology, histopathology, nomenclature, rodent pathology, integumentary system, skin; rodent
Abbreviations: BSTP, British Society of Toxicological Pathologists; EMA/CHMP, European Medicines Agency/Committee for Medicinal Products for Human Use ; ESTP, European Society of Toxicologic Pathology; FDA/CDER, Food and Drug Administration, Center for Drug Evaluation and Research; H&E, hematoxylin and eosin; INHAND, International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice; JSTP, Japanese Society of Toxicologic Pathology; OECD, Organisation for Economic Co-operation and Development; STP, Society of Toxicologic Pathology; Tg.AC, zetaglobin promoted v-Ha-ras.
Introduction
This manuscript provides a standardized nomenclature for classifying microscopic lesions observed in the skin and its appendages, namely hair follicles, sebaceous glands, apocrine glands and eccrine glands. It does not cover claws. Lesions in the Zymbal's gland, mammary gland, preputial and clitoral gland are covered in a separate manuscript (106Rudmann et al., 2012). Lesions in the dermis are covered only if not discussed in the manuscript about soft tissue (48Greaves et al., 2013), in the manuscript about the nervous system (66Kaufmann et al., 2012) or in the manuscript about the cardiovascular system (12Berridge et al., in preparation).
Ontology, anatomy and function of the rodent skin
The integument derives from the embryonal ectoderm and the underlying primitive mesenchyme. Its ontogeny depends on a multitude of complex ectodermal-mesenchymal interactions and is further influenced by the migration of neuroectoderm-derived melanocytes. The integument is considered the largest organ of the body and functions as a barrier against the environment. As such it protects from external chemical, physical and microbiological agents. In addition, the skin and its appendages function as a reservoir of electrolytes, water, vitamins, lipids, carbohydrates, and proteins. The skin possesses immunological and metabolic competence and is essential for the production of vitamin D.
The basic anatomy of the rodent skin is largely comparable to that of other mammals, but species-specific differences need to be taken into account when evaluating the morphology of the skin and its appendages in biomedical research. The reader is referred to standard texts on mouse and rat anatomy (54Krinke, 2004; 59Hofstetter et al., 2006).
The number of transgenic, conditional, and spontaneous mutant mice that display skin and hair abnormalities is increasing rapidly (86Nakamura et al., 2001; 87Nakamura et al., 2002). The researcher analyzing such mice is referred to detailed descriptions of mouse skin and hair follicle anatomy (98Paus et al., 1999; 135Yamanishi, 1998; 84Mueller-Roever et al., 2001; 118Sundberg et al., 2005) and to general reviews about the phenotyping procedure itself (142Zeiss et al., 2012).
Common diseases of the rodent skin
Based on its direct interaction with the environment, the skin is subject to many spontaneous and housing-related diseases. This emphasizes the importance of husbandry, particularly if dealing with delicate animal models. The pathologist evaluating the integument from experimental studies in rodents should be aware of such conditions. Morphological lesions should be described using the nomenclature listed herein. However, spontaneous diseases of the rodent skin should not be diagnosed on the histomorphology of the lesions alone but in combination with clinical data. In a pathology report, the disease terms listed below could be used as 'syndromes' to summarize and interpret morphological lesions.
Alopecia: There are several strains of mice and fewer in rats that display a congenital form of hair loss, due to abnormal hair follicle formation. The hallmark morphological lesion is 'abnormal development' of hair follicles. A detailed description of these forms of alopecia is beyond the scope of this manuscript, and the reader is referred to textbooks and reviews covering this issue (120Sundberg, 1994;86 Nakamura et al., 2001). Alopecic mice frequently used in biomedical research are hairless mice (Hr) and nude (Foxn1/nu) mice. Several mutations in the hairless (Hr) gene, encoding a transcriptional co-repressor, have been identified in mice, all resulting in hairlessness in homozygous animals. Outbred SKH1 mice are the most widely used hairless mice. Alopecia develops after a single cycle of relatively normal hair growth and is caused by dysplasia of hair follicles, which lose their ability to form hair shafts and transform into large intradermal cysts (9Benavides et al., 2009). Mutations in the nude (Foxn1) gene, encoding a member of the winged helix/forkhead family of transcription factors, lead to macroscopic nudity and thymic dysgenesis in mice and rats. Relatively normal hair follicles develop that still produce hair shafts; however, presumably because of a lack of some hair keratins, the hair shafts that are generated twist and coil in the hair follicle infundibulum, which becomes dilated. Because hair shafts fail to penetrate the epidermis, the animals appear alopecic (79Mecklenburg et al., 2001).
Hair loss in haired rodents can also occur due to external trauma that breaks existing hair shafts, or due to inflammatory or degenerative processes that impair formation of new hair shafts. It is not always easy to distinguish between these two processes and detailed clinical or histopathological investigations may be needed (80Mecklenburg, 2009). Alopecia due to barbering occurs frequently in group-housed mice. It occurs mostly in males, and less dominant animals are primarily affected. Genetic components may also be involved (63Kalueff et al., 2006). Overcrowding needs to be considered as a contributing factor in dominance bevavior (69Kurien et al., 2005; 63Kalueff et al., 2006). Mechanical denuding of facial hair is a consequence of improperly constructed feeder openings or watering devices and is a differential diagnosis for barbering (101Percy and Barthold, 2007). Hair loss due to external trauma is morphologically characterized by a lack of hair shafts from hair follicle infundibula outward and is frequently associated with erosion/ulcer of the epidermis and inflammation in the dermis.
C3H mice are known to develop a spontaneous follicular inflammation leading to follicular necrosis which resembles alopecia areata in humans (77McElwee et al., 2003).
Ulcerative dermatitis: Chronic ulcerative dermatitis in mice can result from barbering in group-housed mice, but it can also be caused by self-trauma/overgrooming. In the C57BL/6 substrain, chronic ulcerative dermatitis is a common skin problem. Female animals are predisposed, and there is marked seasonal variation in the disease frequency with a peak in spring and fall. Furthermore, the severity and prevalence of the disease within a colony are dependent on nutritional and husbandry factors. Lesions occur along the dorsum and in the cervicothoracic area. Microscopically, there is epidermal erosion/ulcer with crusting and dermal inflammation. A hypersensitivity reaction has been proposed as pathogenesis (65Kastenmayer et al., 2006), although recent investigations suggest follicular developmental abnormalities as the primary pathogenesis with secondary rupture of severely affected follicles. The dermis may contain granulomatous inflammation (119Sundberg et al., 2011). A secondary infection with Staphylococcus spp. or Streptococcus spp. may be associated with neutrophilic inflammation. Ulcerative dermatitis is less common in rats than in mice, and has mainly been described in the Sprague Dawley strain (5Ash, 1971; 37Fox et al., 1977; 127Wagner et al., 1977).
Ulcerative pododermatitis in rats: Epidermal erosion/ulcer at the footpads occurs frequently in rats housed in wire cages, especially in strains of rats with higher body weights, such as some diabetic phenotypes. Initial traumatic abrasions and secondary infections may lead to dermal granulomatous inflammation of the foot pads, known as "bumblefoot" (82Morrow et al., 1977).
Necrotizing dermatitis of the tail: A ring-like constriction of the tail skin with dermal hemorrhage, thrombosis, edema and full-thickness necrosis is known as 'ringtail' in mice and rats. Epidermal hyperkeratosis and secondary infection occur frequently. This condition has historically been associated with low environmental humidity (<40%) and high temperatures (>80°F/27°C). Poor diet, hydration status, genetic susceptibility, and other predisposing factors may also be involved (71Lawson and Churchman, 1993; 25Crippa et al., 2000; 101Percy and Barthold, 2007).
Auricular chondritis: A granulomatous inflammation in the dermis in the ear pinnae associated with chondrolysis has been described in rats (78McEwen and Barsoum, 1990). The ear pinnae present with intradermal nodules and crusting or erosion/ulcer of the overlying epidermis. The etiology is unknown.
Dermatophytosis (syn. Favus, ringworm): Dermatophytes such as Trichophyton spp. and Microsporum spp. can infect mice and rats (8Balsari et al., 1981) and might be transmitted to humans. Although most infections are subclinical, alopecia, crusting, and dermal inflammation may occur. Inflammatory cell infiltration of the hair follicle is present and dermatophytes are recognized as arthrospores and hyphae along the hair shafts. The organisms can be stained with periodic acid-Shiff (PAS) or GMS.
Demodicosis: Mice and rats are susceptible to Demodex musculi, although infestations with clinical symptoms are very rare in immunocompetent animals (128Walberg et al., 1981; 56Hill et al., 1999). However, clinical disease may occur in transgenic mice lacking mature T cells and NK cells (101Percy and Barthold, 2007). The hallmark lesion is inflammation of hair follicles and dermis in association with elongate mites that are easily detected within the follicular infundibulum.
Acariasis: Fur mites include Myobia musculi (most clinically significant), Radfordia affinis, and Myocoptes musculinis in mice and Radfordia ensifera in rats (26Csiza and McMartin, 1976; 62Jungmann et al., 1996; 100Peper, 1994). Myocoptes musculinis is the most common fur mite and frequently occurs as a mixed infestation with Myobia musculi (101Percy and Barthold, 2007). Mite infestation leads to hypersensitivity resulting in gross clinical manifestations that vary from focal alopecia to widespread ulcerative dermatitis. Lesions are mostly located along the dorsum, including the head and are characterized by dermal inflammation, as well as epidermal hyperplasia and hyperkeratosis. Mites are easily found within the stratum corneum.
Hyperkeratosis in nude mice: Nude mice infected with Corynebacterium bovis may develop a diffuse orthokeratotic epidermal hyperkeratosis associated with a sparse superficial dermal inflammation. Small gram-positive rods can be demonstrated in the hyperkeratotic stratum corneum (22Clifford et al., 1995).
Ectromelia in mice: The Ectromelia virus is the cause of mousepox, an epizootic disease with high mortality. The virus is transmitted via direct animal-to-animal contact and cutaneous trauma. While some strains such as C3H, DBA and BALB/c are highly susceptible to the virus, others such as C57BL/6 are not. Infected animals present with an acute swelling of the extremities caused by follicular necrosis and dermal inflammation. The necrotic keratinocytes contain pale, slightly eosinophilic intracytoplasmic inclusion bodies (129Wallace and Buller, 1985; 29Dick et al., 1996).
Dermatotoxicology in rodents
Because of its function as an outer barrier, the skin is exposed to a wide variety of toxic agents. These can harm the skin directly, causing irritation or corrosion, or can cause immune-mediated toxic effects. A special mechanism of toxicity is phototoxicity, which is due to the interaction of a toxic agent with ultraviolet light (52Haschek et al., 2010).
Direct toxicity is caused by direct cell damage of epidermal keratinocytes or by a direct activation of the effector pathway of the immune system. It is often referred to as 'non-immunological activation', since it occurs independently of antibodies. Activation of mast cells or complement or prostaglandin synthesis results in reversible damage to the skin referred to as 'irritation'. It usually occurs within 4 hours following topical application of the irritating substance. Histologically, skin irritation is characterized by dermal inflammation and epidermal hyperkeratosis and hyperplasia, associated with variable other epidermal changes such as erosion/ulcer, necrosis or vesicle. If the damage to the skin is irreversible, the lesion is clinically referred to as 'corrosion'. Corrosion is characterized by full thickness necrosis of the epidermis penetrating into the underlying dermis.
Immune-mediated cutaneous toxicity can be subdivided into anaphylactic reactions mediated by IgE antibodies (type I hypersensitivity), immune-complex reactions mediated by IgG or IgM antibodies (type III hypersensitivity), and delayed-type (type IV) hypersensitivity reactions mediated by effector T cells. While type I and type III hypersensitivity reactions are mostly caused by toxic agents that are taken up systemically, type IV hypersensitivity reactions usually occur with toxic agents in direct contact with the skin. Type IV hypersensitivity reactions in the skin are frequently referred to as 'allergic contact dermatitis' and present clinically with pruritus, erythema, edema, and development of papules, vesicles or bullae. The ability of a compound to initiate type IV hypersensitivity ('skin sensitization') is usually investigated in the guinea pig (92OECD, 1992); however, the local lymph node assay in mice is gradually replacing traditional hypersensitivity testing in guinea pigs (94OECD, 2010a, 952010b, 962010c). Skin sensitization lesions are characterized by dermal inflammation and various epidermal changes ranging from hyperplasia to erosion/ulcer. These lesions are not reliably different from those seen with skin irritation by histomorphology alone.
Additional forms of immune-mediated dermal toxicity include 'erythema multiforme' and 'toxic epidermal necrolysis'. These represent a continuum of reactions, presumably caused by cytotoxic T cells that induce apoptosis or necrosis of epidermal keratinocytes (52Haschek et al., 2010). The predominant histological finding is epidermal necrosis, either single cell type (erythema multiforme) or full thickness type (toxic epidermal necrolysis).
Phototoxicity can occur due to direct reaction of a toxic agent with ultraviolet light or indirectly if the toxic agent alters endogenous proteins thus making them reactive with ultraviolet light. Phototoxicity can be immune-mediated ('photoallergy') or non-immunological ('photoirritation'). For pharmaceutical chemicals, the assessment for any need to conduct experimental photosafety testing is mandatory in both the European Union (24CPMP/SWP, 2002) and the United States of America (35FDA/CDER, 2003). Although there are in vitro test systems to determine the general reactivity of a chemical with UV light, in vivo testing in hairless mice may still be necessary. This is particularly true when testing the potential for carcinogenesis due to a chemical in association with ultraviolet light ('photocarcinogenesis') (36Forbes, 1996). Histologically, ultraviolet light-induced skin lesions are not distinguishable from other toxic skin changes.
Testing of topically applied chemicals for acute dermal irritation/corrosion is typically performed in rabbits (93OECD, 2002). However, as outlined in the supplement to the OECD test guideline 404 on dermal irritation/corrosion testing (93OECD, 2002), consideration of existing data, structure activity relationships, physiochemical properties of the test item and testing in validated in vitro and ex vivo systems are recommended, before an in vivo study is conducted. Several in vitro testing methods for skin corrosion have been validated such as the transcutaneous electrical resistance test, the human skin model test, and the reconstructed human epidermis test. Acute and chronic cutaneous and systemic toxicity of a topically applied chemical is usually investigated in rats, rabbits or guinea pigs (89OECD, 1987, 901981a, 911981b), although minipigs are now becoming more frequently used, due to the high degree of morphological and physiological similarity between porcine skin and human skin (74Mahl et al., 2006).
The carcinogenic risk of a chemical after its topical administration is traditionally investigated in rats. In recent years, however, the Tg.AC (zetaglobin promoted v-Ha-ras) transgenic mouse has become a popular alternative to the rat. The skin of the Tg.AC mouse is genetically initiated, thus the induction of epidermal papillomas in response to dermal or oral exposure to a chemical agent acts as a reporter phenotype for the carcinogenicity of the test chemical. In Tg.AC mouse bioassays, the test agent is typically applied topically for up to 26 weeks, and the number of tumors in the treated area is counted weekly (31Dunson et al., 2000). If chemicals are applied topically to the skin, their effect is influenced not only by their primary mode of toxicity but also by the processes of absorption and metabolism. The outer surface of the skin has an oily coating of sebum from the sebaceous glands which forms a barrier to polar water soluble compounds. Lipophilic nonpolar compounds more readily pass through the surface epithelium and the intercellular spaces, and can also enter through the hair follicles and sebaceous glands. Absorption is further affected by the integrity and thickness of the epidermis and dermal vascularization. Microsomal enzymes in keratinocytes are able to metabolize topically applied chemicals, thus rendering them inactive or active. For instance, dimethylbenz(a)anthracene (DMBA) becomes a potent skin carcinogen after metabolic activation by keratinocytes (99Peckham and Heider, 1999). Some systemically administered chemicals, particularly some antiproliferative anticancer drugs and cytokines, can induce skin inflammation, atrophy and necrosis (45Greaves, 2000).
For neoplasms in the epidermis and cutaneous adnexa, it might be difficult to determine whether they are spontaneous or related to treatment. In that case, combining the incidence of different tumors with similar histogenesis might provide additional value, particularly if progression from benign to malignant neoplasms is known such as for squamous cell papilloma/carcinoma, basal cell tumor/carcinoma or sebaceous gland adenoma/carcinoma (17Bruner et al., 2001; 15Brix et al., 2010).
Alopecia, i.e. the loss of hair, is a frequent finding in rodent toxicity studies. Although it can be caused by excessive barbering or other external trauma (see Common diseases of the rodent skin), alopecia can also be a sequel of systemic toxicity induced by insufficient supply of nutrients or due to hair cycle disturbances caused by hormonal dysregulation. While the underlying pathogenesis cannot always be determined, histological examination of the alopecic skin will usually differentiate among an abnormality in hair follicle cycling, atrophy of cutaneous adnexa and inflammation or necrosis of the hair follicle. Hypertrichosis, i.e. increased occurrence of hair (also known as 'hirsutism'), is not covered in this manuscript, because it rarely occurs in rodent toxicity studies. However, in nude mice, visible hair growth can be stimulated by treatment with cyclosporine (109Sawada et al., 1987) or recombinant keratinocyte growth factor (27Danilenko et al., 1995).
Nomenclature
It is common practice in diagnostic dermatopathology to approach cutaneous lesions histopathologically by a method known as `pattern analysis` (1Ackerman, 1978). The main aspect of this approach is the use of a scanning magnification (i.e. 1.0 – 2.5x objective) followed by a deliberate, orderly, logical description of the lesion. The latter is facilitated by an analysis and description of the individual anatomic compartments of the skin. Despite the difference in objectives, this approach is also extremely helpful in experimental dermatopathology. Therefore,the nomenclature that is suggested to be used for the description of lesions in the rat and mouse integument is separated into the compartments 'EPIDERMIS', 'CUTANEOUS ADNEXA', and 'DERMIS AND SUBCUTIS'.
Normal morphology
SKIN – EPIDERMIS
Epidermal thickness varies greatly among species. Even within one species, the epidermal thickness will vary between body regions, and within one region will vary depending on physiological parameters such as the hair cycle stage and gender (50Hansen et al., 1984; 6Azzi et al., 2005). Mouse epidermis is approximately 10 µm thick, and rat epidermis is 10 to 20 µm thick. Human epidermis is 50 to 120µm thick. The greater epidermal thickness in man is due to the presence of more cell layers. Rete pegs, a prominent feature of human epidermis, are not observed in normal rodent skin. Rodent and human skin also differ with respect to skin adnexae. Human haired skin contains both eccrine and apocrine glands, while these structures are absent from the haired skin of rats and mice.
The epidermis is derived from ectoderm. The epidermis is a stratified epithelium characterized by a unique differentiation process known as keratinization (syn: cornification). Keratinization is due to formation of intracellular tonofilaments, composed of cytokeratins. Cytokeratins are usually found in pairs of a type I cytokeratin and a type II cytokeratin. The composition of cytokeratins changes during the differentiation process. As an example, epidermal basal cell keratinocytes express the cytokeratin pairs 5 and 14, while suprabasal epidermal keratinocytes lose these cytokeratins, but express cytokeratins 1 and 10 instead (40Galvin et al., 1989). Cells are continuously lost from the epidermal surface by desquamation, and new cells are continuously produced in the basal epidermal layer by proliferation of transit amplifying cells which arise from stem cells and divide a finite number of times until they become differentiated. The basal layer of the epidermis is connected to the underlying connective tissue via a basement membrane and adhesion structures called 'hemidesmosomes'. Differentiating cells from the stratum basale move upwards into the stratum spinosum, which represents the thickest layer of the normal human epidermis. Keratinocytes within the stratum spinosum change shape from polyhedral to flattened as they move upward. With further differentiation, keratinocytes develop intracellular keratohyalin granules. These granules contain the protein filaggrin, which is important for cross-linking cytokeratin filaments. Keratohyalin granules are readily visible in H&E-stained histological sections and are characteristic of the stratum granulosum. As cross-linking of cytokeratin filaments occurs, the cell membrane is transformed into a 'cornified envelope', the nucleus vanishes, and lipid-containing lamellar bodies are formed. Lipids from these lamellar bodies fill the intercellular spaces of the stratum corneum and surround terminally differentiated keratinocytes (corneocytes). Corneocytes eventually desquamate into the environment. Keratinocytes within the stratum basale, stratum spinosum and stratum granulosum are connected to each other via desmosomes and adherens junctions. Because the normal epidermis of rodents is only 2-4 cells thick, the stratum spinosum and stratum granulosum are not usually visible; however, these layers become evident in hyperplastic epidermis.
Interspersed among keratinocytes are antigen-presenting cells ('Langerhans cells'), Merkel cells (neuroendocrine cells interacting with nerve endings), T cells, and melanocytes (99Peckham and Heider, 1999). The epidermis has an important function in the skin immune system (72Loser and Beissert, 2007). There are known species differences with regard to the distribution of immunocompetent cells within the epidermis. Rodents, for example, exhibit many gamma/delta T-cells in epithelia such as the epidermis, while in humans these cells predominate in lymph nodes, not the epithelium (107Salerno and Dieli, 1998).
Local epidermal thickenings in mouse skin that are associated with a tylotrich hair follicle and that are composed of many Merkel cell-neurite complexes are known as 'Haarscheibe' (114Smith, 2004) (Figure 1).
Figure 1.
Mouse skin. Normal 'Haarscheibe' (Courtesy of R. Herbert).
Melanocytes in the skin of mice are located in the epidermis in some glabrous areas of the body and within hair follicles of the trunk. They derive from neural crest cells that migrate to the epidermis in early development and become interdigitated between keratinocytes. These cells reside only briefly in the epidermis of the trunk (88Noonan et al., 2000). Melanoblasts may also reside in the dermis where they can proliferate and cause pigmented neoplasms (64Kanno, 1989).
SKIN – CUTANEOUS ADNEXA
The skin would not be able to fulfill its numerous functions without cutaneous adnexa. Cutaneous adnexa all derive from the ectodermal component of the skin and consist of hair follicles, sebaceous glands, apocrine glands, and eccrine glands. Claws represent further cutaneous adnexa in rodents and are the analogues of nails in humans. For reporting purposes, it is recommended to use 'cutaneous adnexa' as a primary locator and 'hair follicles', 'sebaceous glands', 'apocrine glands', 'eccrine glands' and 'claws' as secondary locators.
Mice and rats have up to 8000 hairs per cm2 of skin. Hair follicles can be divided into primary hair follicles (also referred to as 'tylotrich' or 'guard' hair follicles) and secondary hair follicles (also referred to as 'non-tylotrich' or 'wool' hair follicles). Primary hair follicles have large sebaceous glands, prominent innervations, a prominent blood supply, and they are associated with a focal epidermal thickening, known as the 'Haarscheibe'. In contrast, secondary hair follicles are smaller and have small sebaceous glands. Eccrine glands in rodents are restricted to the foot pads.
In the rodent, secondary hair follicles are the predominant type (about 70%) in animals that are kept indoors (83Meyer, 2009). If evaluating morphological defects of hair follicles, it is important to understand the morphology of hair follicle morphogenesis and cycling, which has been described in detail (98Paus et al., 1999; 135Yamanishi, 1998; 84Mueller-Roever et al., 2001; 118Sundberg et al., 2005). The hair cycle may also functionally influence the skin response towards topically applied chemicals (3Argyris, 1963). Hair shafts that are produced by hair follicles in mice are divided based on their morphology into zigzags, auchenes, awls and monotrichs (30Dry, 1926). In addition to the primary and secondary hairs of the trunk, rodents have a number of specialized hair follicle types with unique histomorphologic features. Included in these specialized hair follicle types are vibrissae (large tactile hairs of the face/ muzzle featuring blood sinuses and trigeminal nerve innervation), cilia (eyelashes, hairs with specialized sebaceous glands called 'Meibomian glands'), perinanal/ genital hairs (also larger with unique sebaceous secretions functioning in marking/ territorial scent behaviors), tail hairs, and possibly others (120Sundberg, 1994).
Hair follicles can be divided into three morphologically and functionally different segments: The upper segment is represented by the follicular infundibulum, which is composed of a central lumen that is filled with hair shafts and keratin and that is lined by a stratified squamous epithelium which is continuous with the interfollicular epidermis. The middle segment is known as the 'isthmus'. It is a very complex and still not completely understood structure that plays an important role in hair follicle cycling. The isthmus is lined by an epithelial sheath with distinct tricholemmal keratinization and contains the opening of the sebaceous gland duct. The deepest segment is known as the 'inferior portion'. It is again a very complex structure that contains the hair bulb at its lower end. The hair bulb is composed of epithelial matrix cells. These cells produce the hair shaft and encircle mesenchymal cells of the dermal papilla. Matrix cell differentiation and upward migration build the inner root sheath and the hair shaft, while the outer root sheath is supposedly formed from basal cells deriving from the isthmus region. While the upper segment remains largely unchanged throughout the hair cycle, the middle segment undergoes striking morphological changes throughout the hair cycle, and the lower segment disintegrates and almost completely vanishes during the catagen and telogen phase, with complete separation of the dermal papilla from the epithelial matrix cells. During the course of the synchronized hair cycle in rodents, there is a marked change in skin thickness.
Sebaceous glands derive from the follicular isthmus and are composed of a glandular and a ductal component. The duct is lined by keratinizing squamous epithelium. Peripheral glandular cells are basaloid. They differentiate towards the glandular centre by accumulating lipid droplets within their cytoplasm. Cell rupture and disintegration result in 'holocrine' secretion of sebum.
Rodents do not have thermoregulatory apocrine sweat glands. Eccrine glands in mice and rats are limited to the footpads (99Peckham and Heider, 1999; 122Taylor et al. 2012).
Lesions of other specialized cutaneous adnexa such as Zymbal's gland, auditory sebaceous gland, and circumanal gland are covered in other manuscripts of the INHAND initiative.
SKIN – DERMIS AND SUBCUTIS
Dermis and subcutis (syn. hypodermis, subdermis) are of mesodermal origin. They provide the tensile strength to the skin, yet they are also responsible for its elasticity and flexibility. The extracellular matrix of the dermis mainly consists of type I, III, V, and VI collagen fibers, accompanied by reticular and elastic fibers, and embedded in a dermal ground substance (composed of glycosaminoglycans, proteoglycans, hyaluronic acid, and dermatan sulphate). The extracellular matrix is formed by fibroblasts and contains blood vessels and nerves. The hypodermis is characterized by densely-packed adipocytes.
Morphological lesions in the dermis and subcutis are similar to lesions in other soft tissues. Therefore, the reader is referred to the manuscript on proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle and mesothelium (48Greaves et al., 2013). Vascular lesions in the dermis are covered by the manuscript on cardiovascular abnormalities in rats and mice (12Berridge et al., in preparation).
Non-proliferative lesions
SKIN – EPIDERMIS
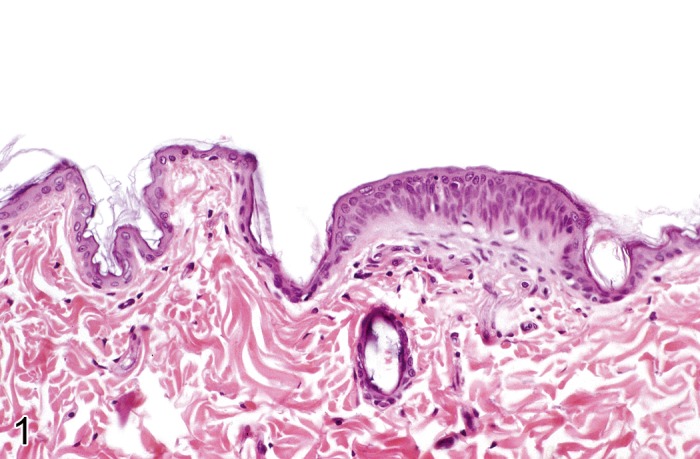
Atrophy, epidermal (Figure 2)
Figure 2.
Mouse skin: Atrophy, epidermal. Reduced thickness of all noncornified epidermal layers. Note concurrent atrophy of cutaneous adnexal structures and dermis (Courtesy of R. Herbert).
Synonyms: Epidermal thinning.
Pathogenesis/cell of origin
• Result of reduced basal cell turnover.
Diagnostic features
• Decreased thickness of all noncornified epidermal layers.
• Reduced number and size of nucleated epidermal keratinocytes.
Differential diagnoses
• Erosion/ ulcer: Loss of superficial epidermal cell layers (erosion) or complete loss of epidermis (ulceration).
• Necrosis, epidermal: Loss of cellular detail.
Comment: Atrophy in laboratory rodents is difficult to detect, because the normal epidermis in mice and rats is only 2 to 4 cell layers. Atrophy of the epidermis can occur in association with underlying expansile masses or with chemicals such as corticosteroids that reduce the metabolic activity of epidermal keratinocytes. Partial ischemia and severe malnutrition have also been cited as possible causes of epidermal atrophy. Epidermal atrophy has been described as a spontaneous finding in B6C3F1 mice. (51Hargis and Ginn, 2007; 113Slaga et al., 1975; 99Peckham and Heider, 1999)
Erosion/ ulcer (Figure 3)
Figure 3.
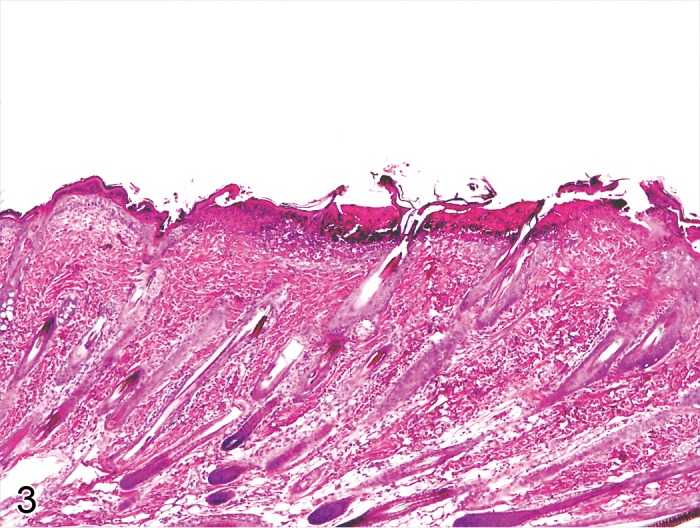
Rat skin: Erosion/ulcer. Complete loss of epidermis with disruption of the basement membrane (Courtesy of E.T. Adams, from NTP image database).
Pathogenesis/cell of origin
• Loss of epidermal cell layers.
Diagnostic features
• Loss of superficial epidermal cell layers (erosion) or complete loss of epidermis (ulceration).
• In case of ulceration, the basement membrane is disrupted.
Differential diagnoses
• Necrosis, epidermal: Loss of cellular detail.
• Atrophy, epidermal: Decreased thickness of all noncornified epidermal cell layers.
Comment: Erosion and ulcer are a continuum. Erosions are nearly always caused by external trauma, mostly from scratching. Ulcerations are also often caused by external trauma, but may also derive from internal sources such as necrotizing dermatitis (see 'Necrosis, epidermal') or vesicular dermatitis (see 'Vesicle') with detachment of the entire epidermis. Toxic epidermal ulceration needs to be differentiated from spontaneous ulcerative dermatitis, which is known to occur in certain strains of mice and rats (see 'Common diseases of the rodent skin'). Ulceration is nearly always associated with dermal inflammation. As external pathogens get direct access to the dermis, neutrophils are usually heavily involved in the inflammatory reaction and accumulate in the upper dermis. The exposed dermis is often covered with cellular debris and exudate (crust). (65Kastenmayer et al., 2006; 134Wuepper et al., 1975; 99Peckham and Heider, 1999)
Necrosis, epidermal (Figure 4)
Figure 4.
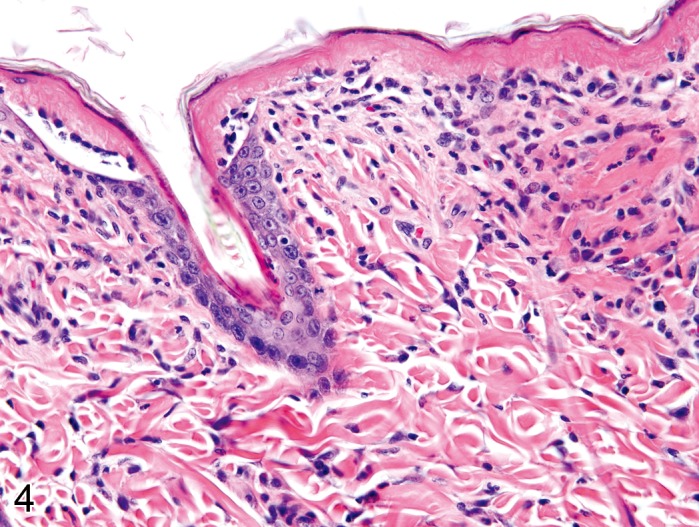
Rat skin: Necrosis, epidermal, full thickness type. Complete loss of cellular detail from large parts of the epidermis, focally with cleft formation (Courtesy of R. Herbert).
Modifiers: Full thickness type, Single cell type
Synonyms: Necrolysis (full thickness type), Sunburn cells (see Comment)
Pathogenesis/cell of origin
• Non-specific cell death of epidermal keratinocytes.
Diagnostic features
Full thickness type
• Complete loss of cellular detail from the epidermis.
• Often associated with separation of epidermis from dermis (necrolysis).
Single cell type
• Individual keratinocytes show hyaline hypereosinophilic cytoplasm and nuclear pyknosis.
• Necrotic keratinocytes may be surrounded by lymphocytes (satellitosis).
Differential diagnoses
• Erosion/ ulcer: Loss of superficial epidermal cell layers (erosion) or complete loss of epidermis (ulcer).
Comment: Apoptosis should no longer be used as a diagnostic term unless molecular techniques have confirmed the apoptotic pathway. Single necrotic epiermal cells are therefore refered to as necrosis, single cell type rather than apoptosis. Necrosis of epidermal keratinocytes occurs in a continuum of spontaneous and experimentally-induced lesions. Single cell type necrosis of epidermal keratinocytes is typical for 'erythema multiforme', whereas full thickness necrosis is typical for 'toxic epidermal necrolysis'. Single cell type necrotic keratinocytes in ultraviolet light-exposed epidermis are known as 'sunburn cells'. The term 'dyskeratosis' is frequently misused to describe apoptotic keratinocytes, because keratinocytes undergo a process of programmed cell death during terminal keratinisation, which cannot reliably be differentiated from apoptosis. (139Young, 1987; 49Gross et al., 2005)
Edema, intracellular, epidermal (Figure 5)
Figure 5.
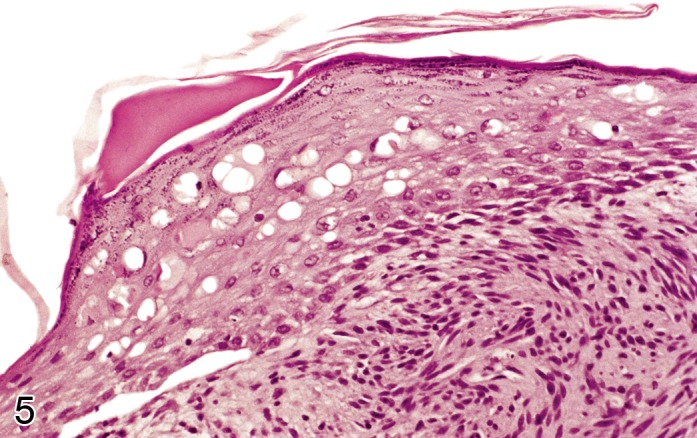
Rat skin: Edema, intracellular, epidermal. Increased size of epidermal keratinocytes with cytoplasmic pallor and displacement of the nucleus to the periphery of the cell (Courtesy of E.T. Adams, from NTP image database).
Synonyms: Hydropic degeneration, Vesicular degeneration, Vacuolar degeneration, Ballooning degeneration, Reticular degeneration
Pathogenesis/cell of origin
• Intracellular fluid accumulation.
Diagnostic features
• Increased cell size.
• Cytoplasmic pallor and presence of intracellular vacuoles.
• Displacement of the nucleus to the periphery of the cell.
Differential diagnoses
• Edema, intercellular, epidermal: Widening of intercellular spaces without disruption of epidermal architecture.
• Vesicle: Fluid-filled cavity within or beneath the epidermis.
Comment: Intracellular edema is defined by the occurrence of intracellular vacuoles. It is mostly associated with reversible cell injury indicating alterations of the membranes, mitochondria and endoplasmic reticulum with subsequent changes in fluid balance. However, there is a continuum to irreversible cell damage. If intracellular edema occurs in the basal layer of the epidermis it is usually named 'hydropic degeneration' or 'vacuolar degeneration'. If occurring in suprabasal epidermal cell layers, it is usually named 'ballooning degeneration'. Severe cytoplasmic swelling may result in the rupture of keratinocytes and in the formation of intraepidermal vesicles (see 'Vesicle'). (51Hargis and Ginn, 2007)
Edema, intercellular, epidermal (Figure 6)
Figure 6.
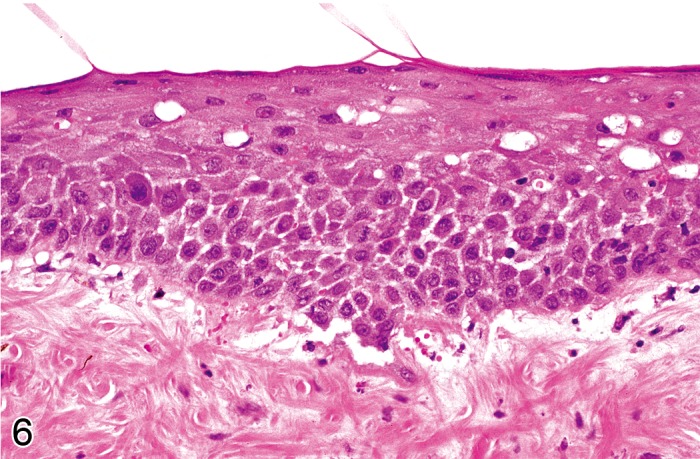
Mouse skin: Edema, intercellular, epidermal. Widening of intercellular spaces with accentuation of intercellular desmosomes. Note concurrent intracellular edema in keratinocytes of the upper stratum spinosum (Courtesy of E.T. Adams, from NTP image database).
Synonyms: Spongiosis
Pathogenesis/cell of origin
• Intercellular edema within the epidermis.
Diagnostic features
• Widening of intercellular spaces.
• Accentuation of intercellular desmosomes.
Differential diagnoses
• Edema, intracellular, epidermal: Intracellular fluid accumulation with increased cell size, cytoplasmic pallor and displacement of the nucleus to the periphery.
• Vesicle: Fluid-filled cavity within or beneath the epidermis.
Comment: Spongiosis results from widening of the intercellular spaces by edema; however the keratinocytes remain connected to each other via desmosomal attachments. Severe intercellular edema of the epidermis may be associated with rupture of desmosomes and formation of intraepidermal vesicles. Intercellular edema is a common feature of skin inflammation. (49Gross et al., 2005; 51Hargis and Ginn, 2007)
Vesicle (Figure 7)
Figure 7.
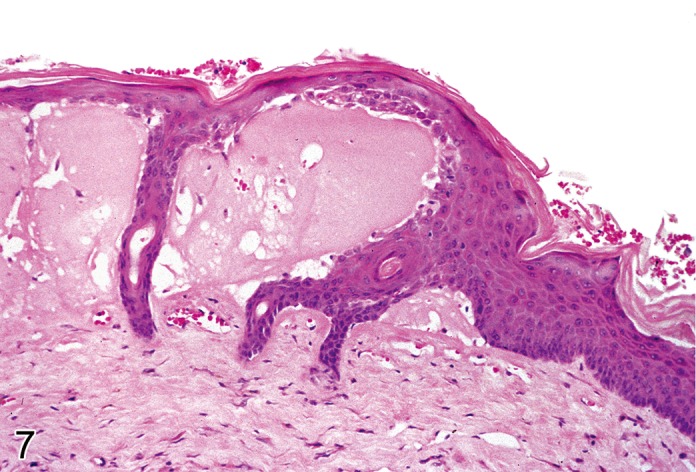
Mouse skin: Vesicle. Disruption of epidermal architecture with open spaces resulting from vesicular degeneration (Courtesy of E.T. Adams, from NTP image database).
Synonyms: Bulla, Cleft, Reticular degeneration
Pathogenesis/cell of origin
• Loss of cohesion between epidermal keratinocytes or between epidermis and dermis, resulting in the accumulation of fluid within a cavity.
Diagnostic features
• Disruption of epidermal architecture with open intercellular spaces.
• Fluid-filled cavities within or beneath the epidermis.
• Cavities do not contain inflammatory cells.
Differential diagnoses
• Edema, intracellular, epidermal: Intracellular edema with increased cell size, cytoplasmic pallor and displacement of the nucleus to the periphery.
• Edema, intercellular, epidermal: Widening of intercellular spaces without disruption of epidermal architecture.
• Pustule: Intraepidermal or subepidermal cavity filled with inflammatory cells.
Comment: Vesicles can result from immune mediated injury (e.g. loss of desmosomal attachments known as 'acantholysis') or as a result of epidermal or dermal edema as a consequence of poxvirus infection, frictional trauma, or burns. Intraepidermal vesicles may develop from severe intercellular edema and/or severe intracellular edema with rupture of keratinocytes. These are also known as 'reticular degeneration'. Vesicles have been described in association with hydrogen peroxide treatment. (60Jeong et al., 2010)
Infiltrate, inflammatory cell, epidermal
Modifiers: Lymphocytic, Mononuclear, Neutrophilic, Eosinophilic
Synonyms: Exocytosis
Pathogenesis/cell of origin
• Infiltration of leukocytes into the epidermis.
Diagnostic features
• Leukocytes are present in between epidermal keratinocytes.
• Usually associated with dermal inflammation.
• Frequently associated with hyperkeratosis and intercellular edema.
Lymphocytic
• Lymphocytes predominate.
Mononuclear
• Lymphocytes and macrophages predominate.
Neutrophilic
• Neutrophils predominate.
Eosinophilic
• Eosinophils predominate.
Differential diagnoses
• Pustule: leukocytes accumulate in a cavity.
Comment: The epidermis is non-vascularized, hence inflammatory cells in the epidermis all derive from the dermal compartment. Most infiltrations are neutrophilic and /or eosinophilic in nature. In these cases, bacteria, fungi or parasites should be looked for in the stratum corneum. Monomorphic lymphocytic infiltrates within the epidermis could point towards an epitheliotrophic lymphoma (syn. 'mycosis fungoides'). (126Veldman and Feliciani, 2008)
Pustule (Figures 8A and 8B)
Figure 1.
A) Rat skin: Pustule. Intraepidermal cavity filled with neutrophils and cellular debris (Courtesy of E.T. Adams). B) Rat skin: Pustule. Intracorneal and subcorneal accumulation of degenerate neutrophils and cellular debris forming a crust (Courtesy of R. Herbert).
Modifiers: Lymphocytic, Mononuclear, Neutrophilic, Eosinophilic
Synonyms: Microabscess
Pathogenesis/cell of origin
• Focal accumulation of leukocytes within the epidermis.
Diagnostic features
• Intraepidermal or subepidermal cavity filled with inflammatory cells.
• Mostly degenerate neutrophils and/or eosinophils.
• Frequently associated with cellular debris and intercellular edema.
Lymphocytic
• Lymphocytes predominate.
Mononuclear
• Lymphocytes and macrophages predominate.
Neutrophilic
• Neutrophils predominate.
Eosinophilic
• Eosinophils predominate.
Differential diagnoses
• Infiltrate, inflammatory cell, epidermal: Leukocytes are diffusely distributed throughout the epidermis without formation of a cavity.
• Vesicle: Fluid filled space within the epidermis that is not filled with leukocytes.
Comment: Intraepidermal pustules are a frequent sequel of superficial skin inflammation. Pustules can be further named according to the predominant leukocyte population, i.e. neutrophilic, eosinophilic or lymphocytic. Lymphocytic pustules occur in epitheliotrophic lymphoma (syn. 'mycosis fungoides'). Pustules that contain isolated rounded keratinocytes with a normal nucleus are referred to as 'acantholytic pustules'. This is a common feature in pemphigus diseases. (126Veldman and Feliciani, 2008)
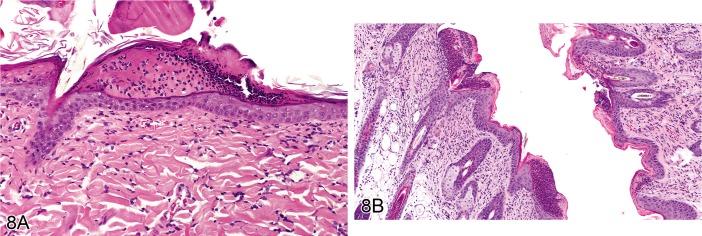
Hyperkeratosis, epidermal (Figures 9A and 9B)
Figure 9.
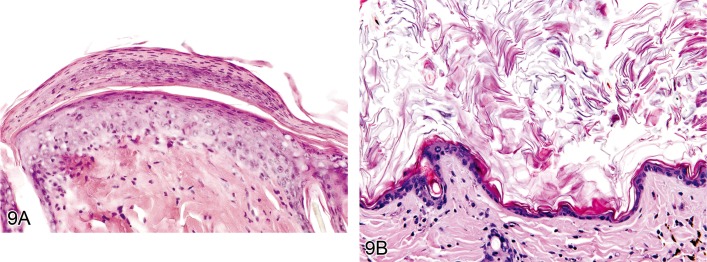
A) Rat skin: Hyperkeratosis, epidermal, parakeratotic. Increased thickness of the stratum corneum with nucleated corneocytes (Courtesy of E.T. Adams, from NTP image database). B) Mouse skin: Hyperkeratosis, epidermal, orthokeratotic. Increased thickness of the stratum corneum with non-nucleated corneocytes (Courtesy of E.T. Adams, from NTP image database).
Modifiers: Orthokeratotic, Parakeratotic, Crust
Pathogenesis/cell of origin
• Alteration in epidermal cell turnover and differentiation of superficial keratinocytes.
Diagnostic features
• Increased thickness of the stratum corneum.
Orthokeratotic
• Normal non-nucleated corneocytes.
Parakeratotic
• Corneocytes are nucleated.
Crust
• Desiccated accumulation of inflammatory cells, erythrocytes, epithelial squames and clotted plasma proteins.
Differential diagnoses
• Hyperplasia, epidermal: Increased thickness of the non-keratinized layers of the epidermis.
Comment: Hyperkeratosis is a common sequel of chronic epidermal disease and is caused by increased turnover of epidermal cells or decreased desquamation of corneocytes. Hyperkeratosis can be a sign of skin irritancy. It also occurs in association with ulcerative dermatitis of the tail, acariasis and due to Corynebacterium bovis infections in nude mice (section 1.2) (45Greaves 2000)
SKIN – CUTANEOUS ADNEXA
Atrophy, adnexal (Figures 10A and 10B)
Figure 10.
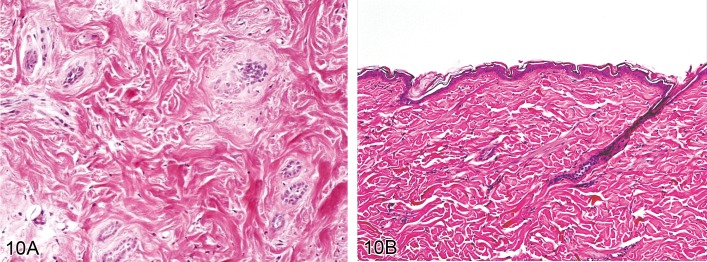
A) Rat skin: Atrophy, adnexal. Small remnants of keratinocyte strands are surrounded by a thickened connective tissue sheath. Induced by bleomycin treatment. (Courtesy of J. Yamate). B) Rat skin: Atrophy, adnexal. Hair follicles and sebaceous glands are markedly reduced in size beyond a normal telogen stage. Induced by corticosteroid treatment (Courtesy of J. Yamate).
Synonyms: Alopecia, Fading follicles
Pathogenesis/cell of origin
• Loss of cells from the pilosebaceous unit or skin glands.
Diagnostic features
• Hair follicles and sebaceous glands are markedly reduced in size beyond a normal telogen stage.
• Small remnants of keratinocyte strands are surrounded by a thickened connective tissue sheath.
• Most hair follicles will have lost their hair shaft, but occasional presence of hair shafts is possible.
• There may be accompanying dermal atrophy or scarring.
Differential diagnoses
• Dysplasia, adnexal: Abnormalities in the shape of the hair follicle and/or the hair shaft with no evident reduction in size.
• Necrosis, adnexal: Degeneration of hair follicle keratinocytes that may be associated with distortion of the hair follicle.
Comment: Hair follicles lose cells when they undergo regression in the catagen stage of the hair cycle. Therefore, hair follicle atrophy must be distinguished from catagen and telogen stages of the physiological hair cycle. Hair follicle atrophy is a loss of cells beyond the physiological telogen stage. Hair follicle atrophy can be caused by a number of different compound classes such as anti-proliferatives and steroid hormones. It can also be linked to dermal ischemia caused by vasculopathy and some autoimmune conditions. Genetically engineered mice may display a loss of eccrine glands from the foot pads, resembling anhidrotic ectodermal dysplasia in humans. (20Cerundolo and Mecklenburg, 2009; 122Taylor et al. 2012)
Dysplasia, adnexal (Figures 11A and 11B)
Figure 11.
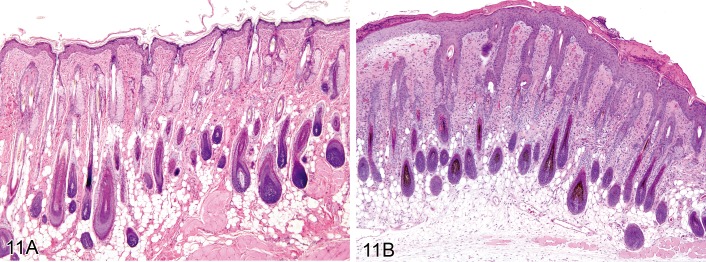
A) Mouse skin: Dysplasia, adnexal. EP4 transgenic mouse 11 days after topical DMBA application. Note the abnormal shape of hair follicles with loss of productive hair shaft formation. B) Rat skin: Dysplasia, adnexal. Abnormal enlargement of hair follicles and sebaceous glands (Courtesy of R. Herbert).
Synonyms: Abnormal development
Pathogenesis/cell of origin
• Hair follicle keratinocytes.
Diagnostic Features
• Abnormalities in the shape of the hair follicle and/or the hair shaft.
Differential diagnoses
• Atrophy, adnexal: Hair follicles and sebaceous glands are markedly reduced in size beyond a normal telogen stage.
• Necrosis, adnexal: Irreversible degeneration of hair follicle keratinocytes that may be associated with distortion of the hair follicle.
Comment: Adnexal dysplasia primarily affects hair follicles. It is not a preneoplastic hyperplastic lesion. Many genetically modified mice have been described that exhibit various forms of congenital hair follicle malformation. The loss of pigment from hair follicles, e.g. in coat color mutants, could also be classified as a dysplasia. (130Walsh and Gough, 1989; 110Sells and Gibson, 1987;86 Nakamura et al., 2001)
Inflammation, adnexal (Figures 12A and 12B)
Figure 12.
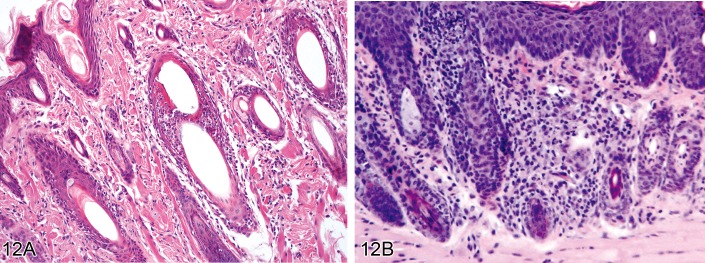
A) Rat skin: Inflammation, adnexal. Perifollicular and intrafollicular infiltration of neutrophils, lymphocytes, and plasma cells (Courtesy of S. Mueller). B) Mouse skin: Inflammation, adnexal. Perifollicular and intrafollicular infiltration of lymphocytes (Courtesy of D. Danilenko).
Modifiers: Lymphocytic, Neutrophilic, Eosinophilic, Granulomatous
Synonyms: Folliculitis, Perifolliculitis, Adenitis, Periadenitis, Furunculosis
Pathogenesis/cell of origin
• Unspecific inflammatory reaction.
Diagnostic features
• Infiltration of inflammatory cells (lymphocytes, plasma cells, macrophages, eosinophils, mast cells, basophils, granulocytes, or combinations of any of these) within or surrounding the adnexae.
• May be associated with necrosis of the adnexa.
• Edema, congestion, neovascularization or fibroplasia might be present.
Lymphocytic
• Lymphocytes predominate.
Neutrophilic
• Neutrophils predominate.
Eosinophilic
• Eosinophils predominate.
Granulomatous
• Histiocytes with epithelioid appearance predominate, giant cells may be present.
Differential diagnoses
• Dysplasia, adnexal: Abnormalities in the shape of the hair follicle and/or the hair shaft with no evident reduction in size.
• Atrophy, adnexal: Hair follicles and sebaceous glands are markedly reduced in size beyond a normal telogen stage.
• Necrosis, adnexal: Irreversible degeneration of hair follicle keratinocytes that may be associated with distortion of the hair follicle.
Comment: As is the case for the epidermis, infiltration of leukocytes into the cutaneous adnexa is always associated with dermal inflammation. Hair follicle inflammation can be further classified according to its precise localization, namely mural (anywhere within the hair follicle epithelium), bulbar (within the hair follicle bulb), and luminal (within the hair follicle lumen). In diagnostic pathology, the term 'interface folliculitis' is used when perifollicular and mural inflammation are associated with distinct necrosis of follicular keratinocytes. The term 'furunculosis' describes a penetrating and perforating follicular inflammation, meaning that the follicular wall is destroyed by the inflammatory process. Inflammation of the cutaneous adnexa in rodents can occur in association with dermatophytosis (see 'Common skin diseases of laboratory rodents') or as a sequel of topical or systemic treatment with chemicals. (80Mecklenburg, 2009; 16Brown et al., 2008)
Necrosis, adnexal (Figure 13)
Figure 13.
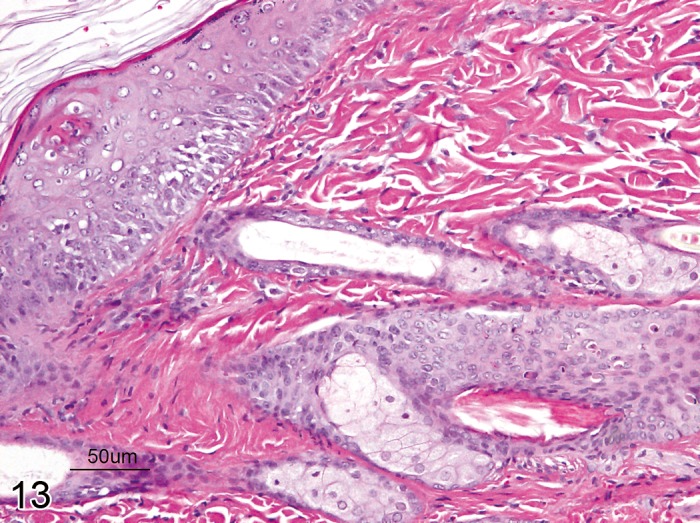
Rat skin, cutaneous adnexa: Necrosis, single cell type. Degeneration of single hair follicle keratinocytes characterized by hypereosinophilic cytoplasm and nuclear pyknosis (Courtesy of D. Danilenko).
Modifiers: Single cell type, Diffuse type
Synonyms: Vacuolar degeneration (see Comment)
Pathogenesis/cell of origin
• Non-specific, irreversible cell death of the follicular or glandular epithelium.
Diagnostic features
• Cell death of hair follicle keratinocytes, either as single cells (single cell type) or as multiple cells (diffuse type)
• Necrosis of keratinocytes may be associated with uneven distribution of melanin within the hair follicle and hair shaft, perifollicular melanophages, dilation of the follicular canal, or distortion of the entire hair follicle.
Single cell type:
• Individual keratinocytes show hyaline hypereosinophilic cytoplasm and nuclear pyknosis.
• Necrotic keratinocytes may be surrounded by lymphocytes (satellitosis).
Diffuse type:
• Complete loss of cellular detail from the adnexa.
Differential diagnoses
• Inflammation, adnexal: Infiltration of inflammatory cells predominates.
• Dysplasia, adnexal: Abnormalities in the shape of the hair follicle and/or the hair shaft with no evident reduction in size.
• Atrophy, adnexal: Hair follicles and sebaceous glands are markedly reduced in size beyond a normal telogen stage with no evidence of apoptosis, vacuolar degeneration or necrosis.
Comment: The term 'dystrophy' denotes a degenerative process that is due to 'malnutrition' of an organ. Morphological hallmarks of hair follicle dystrophy are uncoordinated vacuolar degeneration or apoptosis of keratinocytes. Since these are features of necrosis, hair follicle dystrophy can be grouped under 'necrosis'. The term 'anagen effluvium' is used in clinical medicine to emphasize that hair shafts are lost despite the fact that hair follicles are in the anagen stage of the hair cycle (opposed to 'telogen effluvium'). Chemotherapy-induced alopecia is a good example of hair follicle necrosis of the single cell type. (55Hendrix et al., 2005; 20Cerundolo and Mecklenburg, 2009)
Hyperkeratosis, adnexal (Figures 14A and 14B)
Figure 14.
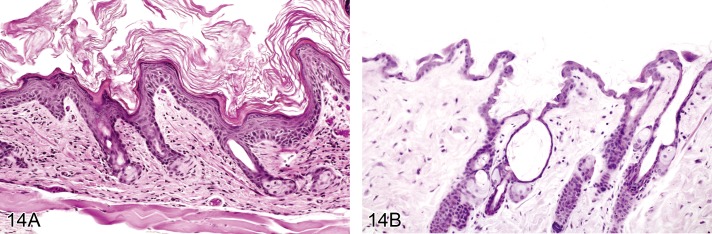
A) Mouse skin. Hyperkeratosis, adnexal. FVB mouse. Large amounts of keratin emerge from the follicles. B) Mouse skin: Hyperkeratosis, adnexal. Dilated follicular infundibulum (Courtesy of R. Herbert).
Synonyms: Chloracne, Hypercornification (see Comment)
Pathogenesis/cell of origin:
• Increased turnover of keratinocytes within the hair follicle infundibulum or sebaceous gland duct.
Diagnostic features
• The follicular infundibulum is dilated and filled with keratin that resembles epidermal differentiation.
• The hair follicle canal may be cystic.
• Ducts of adnexal glands may also become keratin-plugged.
• Keratin plugging can result in retained hairs or secretions.
Differential diagnoses
• Keratoacanthoma: Well demarcated mass with large central keratin-filled cavity surrounded by hyperplastic squamous epithelium.
Comment: Hyperkeratosis with dilation of the follicular infundibulum can be observed as a treatment-related lesion. The follicular hyperkeratosis manifested in dioxin toxicosis known as 'chloracne' is an example. (99Peckham and Heider 1999)
SKIN – DERMIS AND SUBCUTIS
Non-proliferative lesions in the dermis and subcutis such as inflammation (syn. dermatitis or panniculitis), necrosis, fibrosis (syn. sclerosis), metaplasia, mineralization and amyloid deposition, lesions in the hypodermal adipose tissue such as lipogranulomatous inflammation, necrosis, atrophy and hyperplasia, and lesions in the subcutaneous muscle such as necrosis and inflammation, hypertrophy, atrophy, degeneration, vacuolation and mineralization are all described in the manuscript on proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle and mesothelium (48Greaves et al., 2013). Amyloidosis in the dermis of mice is usually a manifestation of systemic amyloidosis which occurs frequently in some strains of mice, particularly CD-1 mice. Dermal mineralization is usually a sequel of generalized mineralization in the body and may lead to dermal inflammation and erosion/ulceration of the epidermis. In terms of dermal peripheral nerves and vasculature, lesions are described in the manuscript on the nervous system (66Kaufmann et al., 2012) and the cardiovascular system (12Berridge et al., in preparation).
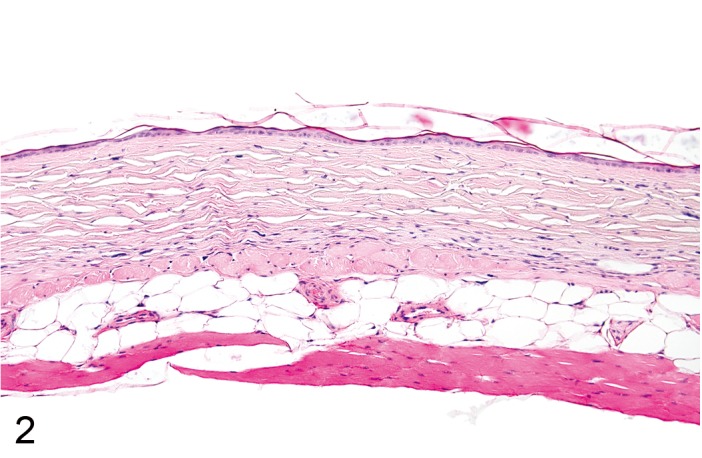
Atrophy, dermal (Figure 15)
Figure 15.
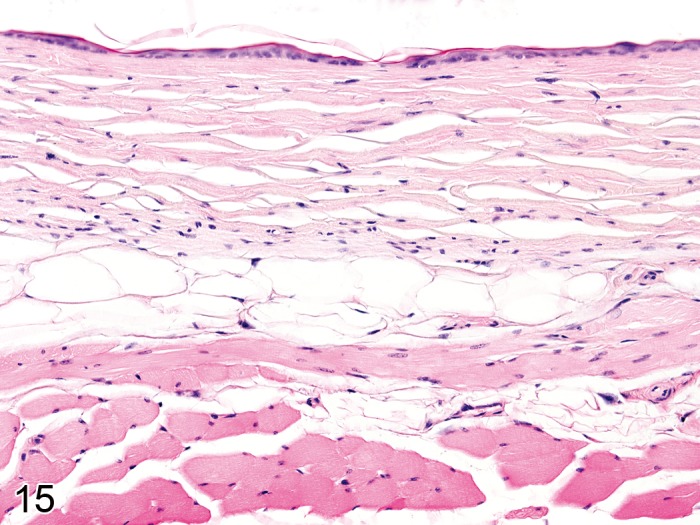
Mouse skin. Atrophy, dermal, associated with epidermal atrophy and atrophy of cutaneous adnexa (Courtesy of E.T. Adams, from NTP image database).
Pathogenesis/cell of origin
• Reduced metabolic activity of dermal fibroblasts.
Diagnostic Features
• Loss of collagen fibers and extracellular matrix.
• Fibroblasts are smaller and more ovoid.
• Mast cell numbers are decreased.
Differential diagnoses
• Edema, dermal: Separation of collagen fibers by a pale amorphous or slightly granular substance.
• Necrosis: Loss of cellular detail and loss of nuclei with or without replacement by cellular debris.
Comment: Atrophy of the dermis occurs typically with long-term administration of corticosteroids and is usually associated with atrophy of the epidermis and cutaneous adnexa. (70Lavker et al., 1986)
Edema, dermal (Figure 16)
Figure 16.
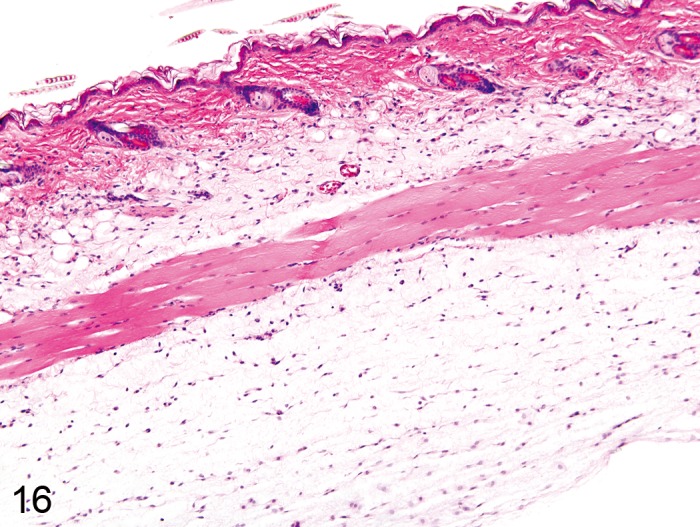
Mouse skin: Edema, dermal. Seperation of collagen fibers by a pale amorphous substance (Courtesy of E.T. Adams).
Pathogenesis/cell of origin
• Accumulation of interstitial fluid.
Diagnostic Features
• Separation of collagen fibers by a pale amorphous or slightly granular substance.
Differential diagnoses
• Atrophy, dermal: Loss of both collagen fibers and extracellular matrix.
Comment: Edema frequently accompanies dermal inflammation. It can also occur in the abdominal skin when there is poor blood flow and prolonged immobility. Edema can also occur spontaneously in the dermis or subcutis of mice. (99Peckham and Heider, 1999;21 Chan et al., 1982; 58Hirouchi et al., 1994)
Elastosis
Pathogenesis/cell of origin
• Dermal fibroblasts producing elastic fibers.
Diagnostic Features
• Accumulation of lightly basophilic, irregular, thickened elastic fibers in the upper dermis
• Elastic fibers form tangled masses, mostly oriented parallel to one another
Special techniques for diagnosis
• Orcein stain
Differential diagnoses
• Fibrosis: Increase in extracellular collagen, mostly arranged in long strands.
Comment: Elastosis is observed after excessive exposure to ultraviolet light. (108Sams et al., 1964; 85Nakamura and Johnson, 1968; 10Berger et al., 1980)
Non-neoplastic proliferative lesions
SKIN - EPIDERMIS
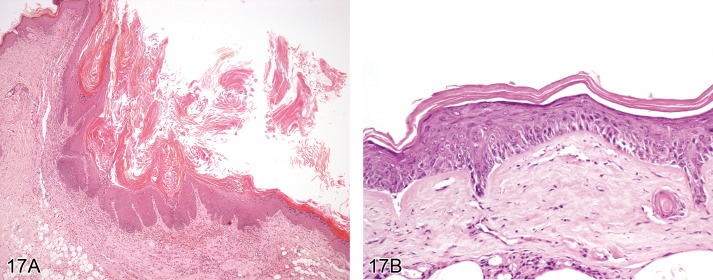
Hyperplasia, epidermal (Figures 17A and 17B)
Figure 17.
A) Rat skin: Hyplerplasia, epidermal. Increased thickness of non-keratinized layers of the epidermis with rete ridge formation and orthokeratotic hyperkeratosis (Courtesy of RITA). B) Mouse skin: Hyperplasia, epidermal, with cellular atypia. FVB mouse 5 days after topical DMBA application.Irregularly increased thickness of non-keratinized layers of the epidermis with partial loss of normal differentiation.
Modifiers: With cellular atypia
Synonyms: Acanthosis, Squamous hyperplasia, Epidermal hyperplasia
Pathogenesis/cell of origin
• Derives from epidermal keratinocytes.
Diagnostic features
• Increased thickness of non-keratinized layers of the epidermis, especially the stratum spinosum and granulosum.
• Increased number of epidermal cells, especially in stratum spinosum.
• Rete ridge formation is often present.
• Hyperkeratosis (orthokeratotic or hyperkeratotic) is frequently also present.
• It might include the epithelial lining of the hair follicle infundibulum.
• The underlying basement membrane is intact.
With cellular atypia:
• Irregularly increased thickness of non-keratinized layers of the epidermis, especially the stratum spinosum and granulosum.
• Differentiation into stratum basale, stratum spinosum and stratum granulosum is lost.
• Atypical keratinocytes with large hyperchromatic nuclei are found in the stratum basale and the lower stratum spinosum.
Differential diagnoses
• Papilloma, squamous cell: Thickened epidermis with exophytic or papilliform growth; regular squamous differentiation
• Carcinoma, squamous cell (keratinizing type): Invasive growth into basement membrane and surrounding tissue is present; mitotic figures are numerous with nuclear atypia; epithelial cells have variable squamous differentiation and keratinization.
Comment: Squamous cell hyperplasia is observed as a response to a variety of insults including spontaneous or induced inflammation, toxic irritation, repeated abrasion of the superficial stratum corneum, or prolonged exposure to ultraviolet light. Rarely, chemicals directly induce proliferation of epidermal keratinocytes. Direct induction of proliferation by chemicals such as tetradecanoyl phorbol acetate (TPA) is used for tumor initiation/promotion studies. Treatment-related epidermal hyperplasia was reported from mouse studies with xylene sulfate.
Hyperplasia of basal keratinocytes only is not considered to represent a separate morphological entity. It has been described to occur within papillomas or keratoacanthomas. Hyperplasia of basal epidermal keratinocytes is not considered a precursor lesion of basal cell tumor, since the latter is supposed to be of follicular origin (see 'Basal cell tumor').
Squamous cell hyperplasia with cellular atypia is frequently found in transgenic mice which show an increase in keratinocyte proliferation or in mice that were treated with topical carcinogens. Cellular atypia occurring in a squamous cell papilloma should be diagnosed as Papilloma, squamous cell, with cellular atypia (see 'Papilloma, squamous cell'). (33Evans et al., 1997; 7Bader et al., 1993; 46Greaves, 1990; 43Gopinath et al., 1987; 53Hasegawa et al., 1989; 17Bruner et al., 2001; 45Greaves, 2000; 117Stenbäck et al., 1986)
Cyst, squamous (Figures 18A and 18B)
Figure 18.
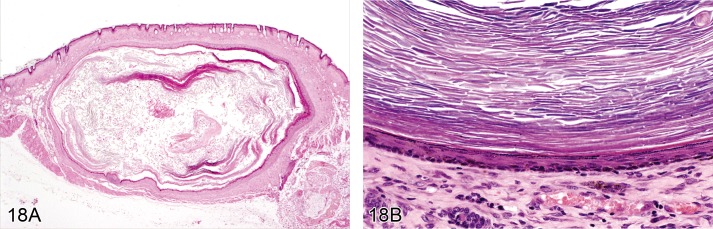
A) Mouse skin. Cyst, squamous. Cyst within the dermis with large lumen containing concentrically arranged lamellar keratin (Courtesy of J.Yamate). B) Mouse skin. Cyst, squamous. The cyst wall is composed of stratified keratinizing epithelium (Courtesy of R. Herbert).
Synonyms: Epidermal cyst, Horn cyst, Keratin cyst
Pathogenesis/cell of origin
• Keratinocyte.
Diagnostic features
• Cyst within the upper dermis.
• Cyst wall is composed of stratified keratinizing epithelium.
• The cyst lumen contains concentrically arranged lamellar keratin.
Differential diagnoses
• Hyperplasia, epidermal: No cyst formation.
• Papilloma, squamous cell: Irregular papilliform growth of the epidermis with no discernible lumen.
• Keratoacanthoma: central cavity surrounded by well differentiated, hyperplastic squamous epithelium, occasionally with papillary projections into the lumen; there may be intraepithelial whorls with central keratinisation, frequently containing cholesterol crystals intermingled with the keratin; edges of the wall may have prominent foci of basaloid cell.
Comment: Squamous cysts may occur as 'horn pearls' within squamous cell carcinoma (see 'Carcinoma, squamous cell'). Squamous cysts are not considered a simple hyperplastic lesion. Their cause is generally unknown. Most likely they arise from injured pilosebaceous units in which squamous epithelial cells producing keratin are trapped in the dermis. The lesion is comparable with 'infundibular cyst' or 'isthmus cyst' as described for dogs. Squamous cell cysts can spontaneously occur in mice, particularly the B6C3F1 strain. (99Peckham and Heider, 1999)
SKIN – CUTANEOUS ADNEXA
Hyperplasia, adnexal (Figure 19)
Figure 19.
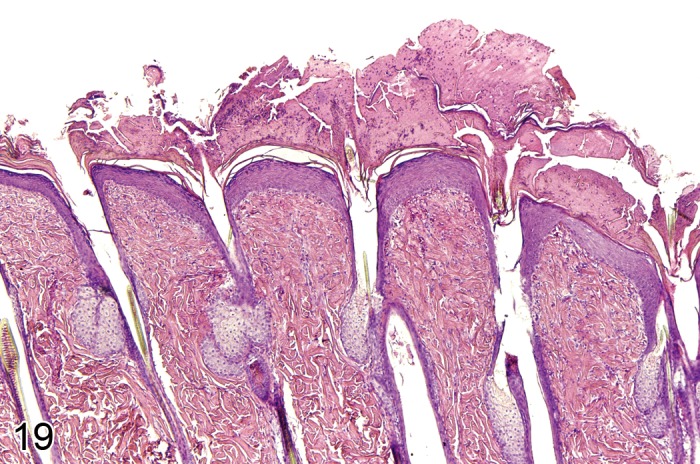
Mouse skin: Hyperplasia, adnexal. Enlarged sebaceous glands with increased number of sebaceous cells in individual acini and regular gland architecture (Courtesy of R. Herbert).
Modifiers: Hair follicle, Sebaceous gland
Synonyms: Sebaceous hyperplasia
Pathogenesis/cell of origin
• Derives from hair follicle or sebaceous gland epithelium.
Diagnostic features
• Hair follicle: Enlarged hair follicles with otherwise normal architecture
Sebaceous gland
• Sebaceous glands are enlarged and show an increased number of sebaceous cells in individual acini with few immature germinative cells and many mature glandular cells arranged around prominent central ducts.
Differential diagnoses
• Adenoma, sebaceous cell: Regular sebaceous gland architecture is distorted; growth pattern may be exophytic; large numbers of immature germinative basaloid cells are present at the periphery.
Comments: Hyperplasia of cutaneous adnexa mostly affects sebaceous glands. Sebaceous cell hyperplasia can be found in cases of chronic inflammatory irritation of the skin. It can also occur simultaneously with squamous cell hyperplasia. An increase in the overall size of hair follicles associated with an increased number of hair follicle keratinocytes can occur in genetically engineered mice. As an example, an increased size of anagen hair follicles has been described in p27Kip1 knockout mice. The increased size of hair follicles is associated with an increase in the diameter of the follicular dermal papilla. (33Evans et al., 1997; 99Peckham and Heider, 1999; 17Bruner et al., 2001; 111Sharov et al. 2006)
SKIN – DERMIS AND SUBCUTIS
Hyperplasia of the subcutaneous adipose tissue is covered by the manuscript on proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle and mesothelium (48Greaves et al., 2013).
Hyperplasia, melanocyte (Figure 20)
Figure 20.
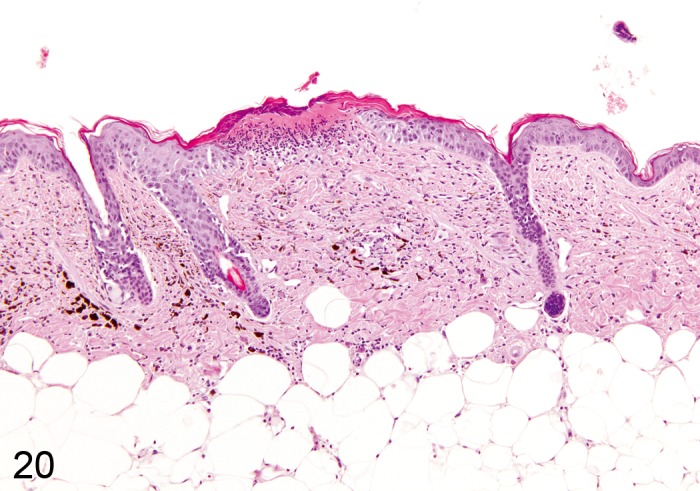
Mouse skin, dermis and subcutis: Hyperplasia, pigment cell. Accumulation of pigmented cells within the dermis (Courtesy of E.T. Adams, from NTP image database).
Pathogenesis/cell of origin
• Melanocyte.
Diagnostic features
• Accumulation of pigmented cells within the dermis, located between hair follicles and sebaceous glands.
Differential diagnoses
• Melanoma, benign: Dense nodular proliferation in the dermis with or without association towards the epidermis.
Comment: Melanocyte hyperplasia has been observed in some initiation-promotion and skin painting studies in mice. Melanocyte hyperplasia should be differentiated from infiltration of macrophages with phagocytozed pigment. (99Peckham and Heider, 1999)
Neoplastic proliferative lesions
SKIN - EPIDERMIS
Papilloma, squamous cell (Figure 21)
Figure 21.
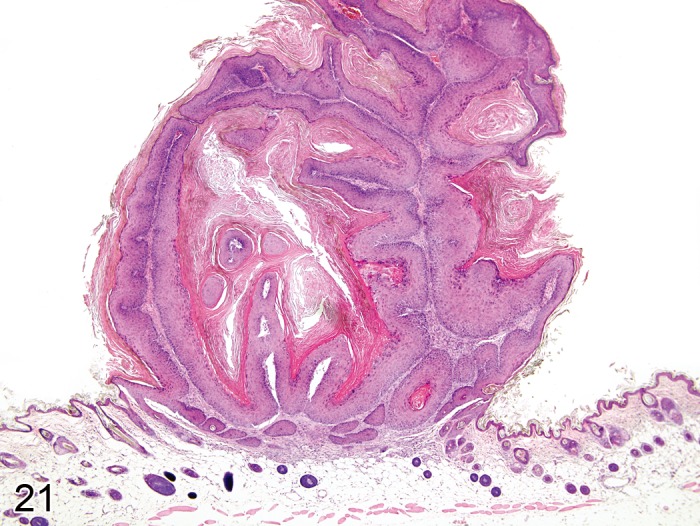
Mouse skin: Papilloma, squamous cell, exophytic type. Well circumscribed papilliform exophytic mass composed of keratinizing squamous epithelium overlying a stroma (Courtesy of R. Herbert).
Modifiers: Exophytic, Endophytic, With cellular atypia, Non-keratinizing
Pathogenesis/cell of origin
• Derives from epidermal keratinocytes.
Diagnostic features
• Well circumscribed papilliform exophytic or endophytic mass with no capsule.
• The mass is composed of keratinizing squamous cells overlying a well vascularised stroma.
• Basal cells are fusiform or columnar with distinct cell borders and little basophilic cytoplasm; they show few mitotic figures.
• Suprabasal cells show gradual squamous differentiation and keratinization with a thickened granular cell layer and irregularly enlarged keratohyalin granules.
• Mitotic figures are common.
• Individual suprabasal cells may show premature keratinization (dyskeratosis).
• There is a variable degree of parakeratotic hyperkeratosis.
• Ulceration and inflammation may be seen.
Exophytic
• A more or less distinct stalk is present at the base of the mass (also known as 'pedunculated' papilloma).
Endophytic
• There is no evidence of a stalk; instead the mass is continuous with the adjacent hyperplastic epidermis, and a crater is produced by invagination; the tumor stroma is indistinctly demarcated from the underlying dermis.
With cellular atypia
• Atypical squamous cells with large hyperchromatic nuclei are found primarily in the basal and suprabasal layer of the epidermis. Mitotic figures might be found in suprabasal layers.
Non-keratinizing
• The epithelium lacks typical keratinization.
Differential diagnoses
• Hyperplasia, epidermal: Thickened epidermis without formation of a well circumscribed papilliform mass.
• Keratoacanthoma: A large central cavity or multiple smaller cavities filled with concentrically arranged keratin material.
• Carcinoma, squamous cell: Invasive growth into basement membrane and surrounding tissues is present; mitotic figures are numerous with nuclear atypia; epithelial cells have variable squamous differentiation and keratinization.
Comment: Spontaneous squamous cell papilloma in rats and mice is not associated with papilloma virus and its occurrence is rare except for in aged animals. In Tg.AC (v-Ha-ras) transgenic mice, squamous cell papillomas occur at typical sites of chronic grooming (e.g. ears, nose, lips, paws, ano-urogenital area) as early as 8 weeks of age (125Usui et al., 2001). The overall incidence is low (0–2%) in hemizygous mice but up to 17% in homozygous animals (123Tennant et al., 2001). Chemical carcinogens readily induce squamous cell papillomas in mice. Papillomas can give rise to squamous cell carcinomas and may show prominent foci of basal cells (see 'Hyperplasia, squamous cell'). Fibropapillomas, i.e. papillomas with an increased amount of fibrous tissue at their base, as they occur in turtle and some domestic animal species (e.g. cattle, horse, cat), have not been described in rodents. (7Bader et al., 1993; 14Bogovski, 1994; 28Deerberg et al., 1986; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 38Frith and Ward, 1988; 47Greaves and Faccini, 1984; 68Kovatch, 1990; 75Maita et al., 1988; 81Mohr and Hunt, 1989; 99Peckham and Heider, 1999; 102Poteracki and Walsh, 1998; 124Thomas and Rohrbach, 1975; 141Zackheim et al., 1990; 144Zwicker et al., 1992; 53Hasegawa et al., 1989; 105Rehm et al., 1989; 39Fukuda et al., 1981; 17Bruner et al., 2001; 2Anver et al., 1982; 131Ward et al., 1979; 41Ghadially, 1961; 125Usui et al., 2001)
Carcinoma, squamous cell (Figures 22A and 22B)
Figure 22.
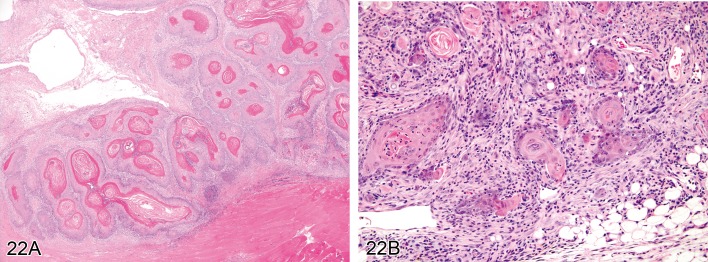
A) Rat skin: Carcinoma, squamous cell, well differentiated type. Coalescing islands with centrally located concentric layers of keratin (Courtesy of RITA). B) Mouse skin: Carcinoma, squamous cell, poorly differentiated type. Keratinization is restricted to single cells, and cells show marked anisocytosis and anisokaryosis (Courtesy of R. Herbert).
Modifiers: Well differentiated type, Moderately differentiated type, Poorly differentiated type
Synonyms: Epidermoid carcinoma
Pathogenesis/cell of origin
• Derives from epidermal keratinocytes.
Diagnostic features
• Poorly demarcated mostly endophytic (rarely exophytic) mass.
• There is no compression of the surrounding mesenchyme, but some tumors show a desmoplastic reaction in the surrounding dermis.
• The mass is composed of islands or cords of cells that penetrate the basal lamina and invade the dermis; tumor cells might reach the subcutis or subcutaneous muscle.
• There is some evidence of squamous differentiation, although its extent is variable.
• Tumor cells are polygonal with mostly distinct cell borders and eosinophilic cytoplasm with varying degrees of keratinization; nuclei are variable in size, with prominent nucleoli and numerous mitotic figures.
• Ulceration and inflammation are frequently present.
• Some tumor lobules may show a central lumen containing individualized (acantholytic) keratinocytes that are surrounded by several layers of neoplastic epithelial cells ('pseudo-glandular pattern').
Well differentiated type
• Coalescing islands with gradual squamous differentiation and centrally located concentric layers of keratin ('keratin pearls', 'cancer pearls', 'horn pearls')
• Abnormal keratinization (dyskeratosis) of single cells occurs sporadically.
• Intercellular bridges are easily found.
• Nuclear atypia is mild.
Moderately differentiated type
• Coalescing islands with gradual squamous differentiation and centrally located concentric layers of keratin ('keratin pearls', 'cancer pearls', 'horn pearls')
• Abnormal keratinization (dyskeratosis) of single cells occurs frequently.
• Intercellular bridges are less common.
• The nucleus to cytoplasm ratio is increased.
• There is considerable nuclear atypia.
Poorly differentiated type
• Keratinization is restricted to single cells.
• Cells are mostly fusiform to spindle-shaped with marked anisocytosis and anisokaryosis; tumor cells are not readily identifiable as squamous cells.
• Intercellular bridges are difficult to discern.
• The nucleus to cytoplasm ratio is quite high and nuclei show severe atypia.
Differential diagnoses
• Papilloma, squamous cell: There is no evidence of invasion and squamous differentiation is regular.
• Keratoacanthoma: There is no evidence of invasion and the tumor is well demarcated.
• Adenosquamous carcinoma (mammary gland): The tumor shows both glandular and squamous differentiation.
• Tumor, basal cell, malignant: There is no evidence of squamous differentiation; nests and cords of basaloid cells are well demarcated; no intercellular bridges are present.
Comment: Subdividing squamous cell carcinoma into well, moderately and poorly differentiated type is traditionally performed but does not appear to add value in most experimental studies. Metastasis of squamous cell carcinomas to lungs and regional lymph nodes has been described. Spindle cell tumors in Tg.AC mice are poorly differentiated squamous cell carcinomas. Cases of squamous cell carcinoma in situ (i.e. without penetration of the basal membrane) have not been reported in rodents. (7Bader et al., 1993; 14Bogovski, 1994; 28Deerberg et al., 1986; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 38Frith and Ward, 1988; 47Greaves and Faccini, 1984; 53Hasegawa et al., 1989; 57Hirose, 1989; 75Maita et al., 1988; 97Okum et al., 1988; 99Peckham and Heider, 1999; 105Rehm et al., 1989; 116Squire et al., 1978; 132Weiss and Frese, 1974; 141Zackheim et al., 1990; 144Zwicker et al., 1992 ; 17Bruner et al., 2001; 4Asano et al., 1998)
SKIN – CUTANEOUS ADNEXA
Tumor, basal cell, benign (Figures 23A–23C)
Figure 23.
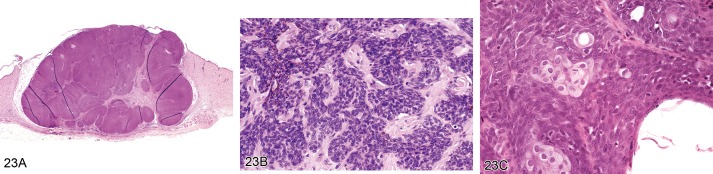
A) Mouse skin. Tumor, basal cell, benign. Well circumscribed mass composed of uniform lobules of closely packed basaloid cells (Courtesy of R. Herbert). B) Mouse skin. Tumor, basal cell, benign. Cords of closely packed basaloid cells supported by a fibrovascular stroma (Courtesy of R. Herbert). C) Rat skin. Tumor, basal cell, benign, trichoblastoma type. Uniform lobules of closely packed basaloid cells with small foci of sebaceous cells (Courtesy of RITA).
Modifiers: Basosquamous type, Trichoblastoma type, Granular type
Synonyms: Basalioma, Basal cell adenoma, Trichoblastoma, Basosquamous tumor
Pathogenesis/cell of origin
• Most likely derives from stem cells within the hair follicle bulge.
Diagnostic features
• Well circumscribed multilobulated mass with some association to the epidermis.
• There is no invasion of the basement membrane and no desmoplasia of the surrounding dermal mesenchyme.
• The mass is composed of uniform lobules, islands or cords of closely packed basal cells that might be arranged in cords or fine ribbons. They are supported by a variable degree of fibrovascular stroma and may show central cystic degeneration.
• Tumor cells are round to columnar with scant cytoplasm (resembling normal basal cells) and palisade at the periphery of lobules; tumor cells can also show spindle-shape.
• Neoplastic cells lack intercellular bridges.
• Nuclei are hyperchromatic and round to oval; mitotic figures are rare.
• Foci of squamous and sebaceous differentiation may also be present.
• Melanin pigment may be present.
Basosquamous type
• Several foci of keratinization are present.
Trichoblastoma type
• Small foci of sebaceous cells and/or trichogenesis are present.
Granular type
• Cells containing PAS-positive granules are present.
Differential diagnoses:
• Carcinoma, basal cell: Invasion into basement membrane and surrounding tissue is present with evidence of indistinct demarcation; cytological and growth pattern is heterogeneous, and mitotic figures are numerous.
• Tumor, hair follicle, benign: Mass located in the dermis, composed of lobules that show a distinct stage of trichogenic differentiation.
Comment: The term 'adenoma' is avoided, since basal cell tumors do not form glandular structures. Basal cell tumors are traditionally considered epidermal neoplasms although it appears much more likely that many of them originate from hair follicle stem cells, which is the reason why many of these tumors show some differentiation into sebaceous cells. Spontaneous basal cell tumors in the mouse are rare. (13Bogovski, 1979; 23Courtney et al., 1992; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 53Hasegawa et al., 1989; 61Jones et al., 1989 ; 68Kovatch, 1990; 99Peckham and Heider, 1999; 124Thomas and Rohrbach, 1975; 137Yoshitomi and Boorman, 1994; 144Zwicker et al., 1992; 17Bruner et al. 2001; 44Grachtchouk et al. 2011)
Carcinoma, basal cell (Figures 24A and 24B)
Figure 24.
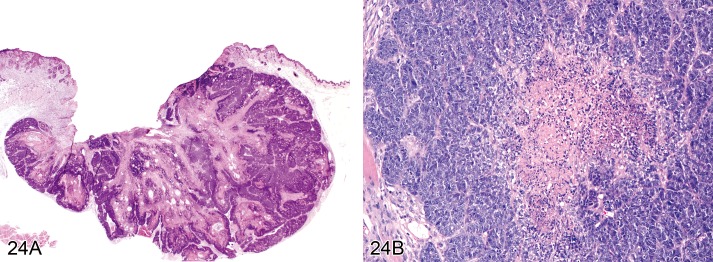
A) Mouse skin: Carcinoma, basal cell, basosquamous type. Dermal mass composed of lobules and cords of closely packed cells that are supported by a variable degree of fibrovascular stroma (Courtesy of R. Herbert). B) Mouse skin: Carcinoma, basal cell, solid type. Tumor cells form circumscribed nests with central necrosis (Courtesy of RITA).
Modifiers: Basoquamous type, Solid type.
Synonyms: Tumor, Basal cell, Malignant
Pathogenesis/cell of origin
• Most likely derives from stem cells within the hair follicle bulge.
Diagnostic features:
• Poorly circumscribed dermal mass with some association to the epidermal adnexa and local invasion.
• The mass is composed of lobules and cords of closely packed cells that are supported by a variable degree of fibrovascular stroma.
• Tumor cells are round to polyhedral with scant cytoplasm (resembling normal basal cells) and palisade at the periphery of lobules; tumor cells can show spindle-shape.
• Most cells are small with dark blue, round to oval nuclei and scanty cytoplasm.
• Mitotic figures are numerous.
• There might be central necrosis in tumor lobules.
• In pigmented strains, melanin pigmentation is commonly found.
• Desmoplasia in the surrounding mesenchyme is common.
• Extensive local invasion may be present.
Basosquamous type
• There is a significant proportion of keratinizing squamous cells (resembling sebaceous gland ducts).
Solid type
• Tumor cells form circumscribed islands and lobules.
Differential diagnoses
• Tumor, basal cell, benign: Well demarcated mass with no evidence of invasion and uniform cytological pattern; mitotic figures are rare.
• Tumor, hair follicle, benign: Mass with distinct stages of trichogenic differentiation.
• Carcinoma, sebaceous cell: Predominant growth pattern is glandular with sebaceous differentiation.
Comment: Basal cell tumors are traditionally considered epidermal neoplasms although it appears much more likely that most of them originate from hair germ epithelium, which is the reason why many of these tumors show some differentiation into sebaceous cells. Basal cell carcinomas are usually of low grade malignancy and metastases are rare. (13Bogovski, 1979; 28Deerberg et al., 1986; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 47Greaves and Faccini, 1984; 53Hasegawa et al., 1989;68 Kovatch, 1990; 99Peckham and Heider, 1999; 121Szabo and Sugar, 1989; 124Thomas and Rohrbach, 1975; 140Zackheim, 1992; 141Zackheim et al., 1990; 99Peckham and Heider, 1999; 17Bruner et al. 2001; 44Grachtchouk et al. 2011)
Adenoma, sebaceous cell (Figure 25)
Figure 25.
Mouse skin: Adenoma, sebaceous cell. Well demarcated dermal mass, composed of lobules with basaloid cells at the periphery and all stages of sebocyte maturation towards the center (Courtesy of R. Herbert).
Synonyms: Sebaceous gland adenoma
Pathogenesis/cell of origin: Derives from reserve sebaceous cells
Diagnostic features
• Regular sebaceous gland architecture is disrupted.
• Well demarcated exophytic or endophytic dermal mass, composed of acini forming lobules supported by a fibrovascular stroma.
• Lobules show large numbers of basaloid cells at the periphery with all stages of sebocyte maturation (foamy to clear cytoplasm and nuclear pyknosis) towards the center.
• Cystic areas within lobules are common.
• Differentiation into keratinizing squamous cells (sebaceous gland ducts) may be present.
• Mitotic figures may be present in the periphery of lobules (germinative basaloid cells).
Differential diagnoses
• Hyperplasia, adnexal: Regular sebaceous gland architecture is maintained; most cells are mature glandular cells with only a few immature germinative cells being present.
• Carcinoma, sebaceous cell: Invasive growth or metastasis is present and cells are poorly differentiated; cellular and nuclear atypia are present.
• Tumor, basal cell, benign: The growth pattern is non-glandular with basal cells predominating and only rare areas of sebaceous cell differentiation.
Comment: Spontaneous sebaceous cell adenoma is a rare tumor in mice and rats. (13Bogovski, 1979; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 68Kovatch, 1990; 97Okum et al., 1988; 99Peckham and Heider, 1999; 141Zackheim et al., 1990;144Zwicker et al., 1992; 61Jones et al., 1989; 38Frith and Ward, 1988; 17Bruner et al., 2001; 2Anver et al., 1982; 115Sommer, 1997)
Carcinoma, sebaceous cell (Figure 26)
Figure 26.
Rat skin: Carcinoma, sebaceous cell. Coalescing lobules composed of polygonal cells with anisocytosis and variable intracytoplasmatic lipid vacuoles (Courtesy of RITA).
Pathogenesis/cell of origin
• Derives from reserve sebaceous cells.
Diagnostic features
• Dermal nodule with invasive growth.
• Coalescing lobules and acini are composed of poorly differentiated basaloid cells with anisocytosis and variable intracytoplasmatic lipid vacuoles.
• Tumor lobules may also contain individual well differentiated sebocytes.
• There is high mitotic activity with numerous atypical mitotic figures.
• Squamous differentiation and individual cell necrosis may be present.
• Cystic spaces filled with amorphous cellular debris may be present.
Differential diagnoses
• Adenoma, sebaceous cell: No evidence of invasive growth or metastasis; cells are well differentiated with no evidence of nuclear atypia.
• Carcinoma, basal cell: Areas of sebaceous cell differentiation are rare; cellular and nuclear atypia is infrequent.
Comments: Spontaneous sebaceous cell carcinoma is a very rare tumor in mice. Most sebaceous gland carcinomas represent low grade malignant neoplasms (in domestic animals, these tumors are mostly classified as 'sebaceous gland epitheliomas'). (13Bogovski, 1979; 28Deerberg et al., 1986; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 47Greaves and Faccini, 1984; 68Kovatch, 1990; 99Peckham and Heider, 1999; 141Zackheim et al., 1990; 144Zwicker et al., 1992 ; 61Jones et al., 1989; 38Frith and Ward, 1988; 99Peckham and Heider, 1999; 17Bruner et al. 2001)
Keratoacanthoma (Figures 27A and 27B)
Figure 27.
A) Mouse skin: Keratoacanthoma. Well demarcated mass in the superficial dermis with intraepithelial whorls of central keratinisation (Courtesy of RITA). B) Rat skin: Keratoacanthoma. A central cavity filled with concentric layers of keratin is surrounded by a well differentiated, hyperplastic squamous epithelium (Courtesy of R. Herbert).
Synonyms: Infundibular keratinizing acanthoma, Intracutaneous cornifying epithelioma
Pathogenesis/cell of origin
• Derives from the squamous epithelium of the hair follicle infundibulum.
Diagnostic features
• Well demarcated mass in the superficial dermis with direct association to the epidermis and the hair follicle infundibulum; a pore (opening in the epidermis) may be present.
• There is a large central cavity filled with concentric layers of keratin, surrounded by well differentiated, hyperplastic squamous epithelium, occasionally with papillary projections into the lumen.
• There may be intraepithelial whorls with central keratinisation, frequently containing cholesterol crystals intermingled with the keratin.
• The surrounding dermal stroma is frequently compressed, but no capsule is formed; the surrounding mesenchyme may show inflammation.
• The nucleus to cytoplasm ratio of the proliferating epithelial cells is low.
• Neoplastic epithelial lining may have prominent basal cell layer.
Differential diagnoses
• Squamous cyst, Epidermis: Intradermal cyst wall is composed of regular keratinizing epithelium, and cyst lumen contains concentrically arranged lamellar keratin.
• Hyperkeratosis, Cutaneous adnexa: Hair follicle is dilated and filled with keratin.
• Tumor, hair follice, benign: There are distinct stages of trichogenic differentiation.
• Papilloma, squamous cell, endophytic: Mass is continuous with the adjacent hyperplastic epidermis and a crater is produced by invagination.
• Carcinoma, squamous cell: Downward invasion is irregular with penetration through the basal lamina; nuclear atypia is present.
Comments: Experimental keratoacanthomas in mice can undergo spontaneous regression, most-likely caused by a non-immunological process that is correlated with the hair growth cycle. On the other hand, some keratoacanthomas in mice can transform into squamous cell carcinomas, with invasive growth deep into the dermis and subcutis. (7Bader et al., 1993; 14Bogovski, 1994; 19Canfield et al., 1985; 28Deerberg et al., 1986; 32Elwell et al., 1990; 33Evans et al., 1997; 34Faccini et al., 1990; 41Ghadially, 1961; 47Greaves and Faccini, 1984; 68Kovatch, 1990; 75Maita et al., 1988; 97Okum et al., 1988; 99Peckham and Heider, 1999; 104Ramselaar et al., 1980; 144Zwicker et al., 1992; 17Bruner et al. 2001)
Tumor, hair follicle, benign (Figures 28A-28E )
Figure 28.
A) Mouse skin: Tumor, hair follicle, benign, trichofolliculoma type. Well demarcated dermal mass with large central lumen that is lined by squamous epithelium which gives rise to numerous radiating fairly well differentiated hair follicles (Courtesy of RITA). B) Mouse skin: Tumor, hair follicle, benign, trichoepithelioma type. Well demarcated dermal mass with irregular islands of basaloid cells that show differentiation into outer root sheath, inner root sheath, and hair matrix (Courtesy of R. Herbert). C) Mouse skin: Tumor, Hair follicle, benign, trichoepithelioma type. Basaloid cells show abrupt keratinization and differentiation into outer root sheath, inner root sheath, and hair matrix (Courtesy of R. Herbert). D) Rat skin: Tumor, hair follicle, benign, tricholemmoma type. Small islands of epithelial cells arranged in a circular pattern with pallisading of peripheral cells, surrounded by a prominent basal membrane (Courtesy of RITA). E) Rat skin: Tumor, Hair follicle, benign, pilomatricoma type. Multilayered epithelial cells that surround a central lumen filled with keratin and ghost cells (Courtesy of D. Danilenko).
Modifiers: Trichofolliculoma type, Trichoepithelioma type, Tricholemmoma type, Pilomatricoma type
Synonyms: Trichoepithelioma, Tricholemmoma, Pilomatricoma, Trichofolliculoma
Pathogenesis/cell of origin
• Derives from hair follicle epithelium.
Diagnostic features
• Well demarcated dermal mass with no capsule and no invasion.
• The mass is composed of lobules that are supported by fibrovascular stroma.
• Within lobules there are different stages of trichogenic differentiation (see modifiers) and a single or multiple cyst(s).
• Mitoses are rare.
Trichofolliculoma type
• Large central lumen (cyst) is present that contains keratin and hair shafts.
• The central lumen is lined by squamous epithelium which gives rise to numerous radiating fairly well differentiated hair follicles with hair shaft formation.
• Small groups of sebocytes may be present.
Trichoepithelioma type
• Irregular islands of basaloid cells with abrupt keratinization (without keratohyalin granules) and centrally located horn cysts.
• Basaloid epithelial cells show distinct but irregular differentiation into outer root sheath, inner root sheath, and hair matrix (with highly eosinophilic trichohyalin granules).
• There is no differentiation into sebocytes.
• In the periphery of lobules with basaloid cells, the basal membrane is often invaginated, resembling hair dermal papillae.
Tricholemmoma type
• Small islands of epithelial cells are present that are arranged in a circular pattern.
• Peripheral cells are pallisading and basaloid, suprabasal cells are vacuolated (glycogen).
• Central cells show tricholemmal keratinisation (highly eosinophilic amorphous keratin); islands of epithelial cells are surrounded by a prominent basal membrane.
Pilomatricoma type
• Nodules of multilayered epithelial cells are present that surround a central lumen filled with keratin and ghost cells;
• Keratinization is abrupt (without keratohyalin granules).
Differential diagnoses
• Cyst, squamous: Cyst wall is composed of regular keratinizing epithelium, and cyst lumen contains concentrically arranged lamellar keratin.
• Hyperkeratosis, adnexal: Hair follicle is dilated and filled with keratin.
• Papilloma, squamous cell, endophytic: Mass is continuous with the adjacent hyperplastic epidermis and a crater is produced by invagination.
• Carcinoma, squamous cell: Downward invasion is irregular with penetration through the basal lamina; nuclear atypia is present.
• Tumor, basal cell, benign: Mass with some association to the epidermis; primarily composed of basal cells with no trichogenic differentiation but evidence of sebocyte differentiation.
• Carcinoma, basal cell: Mass composed of closely packed cells with numerous mitotic figures, and frequent desmoplasia; no evidence of trichogenic differentiation.
• Keratoacanthoma: Well demarcated mass with direct association to the epidermis and large central cavity filled with keratin; no evidence of trichogenic differentiation.
Comment: The benign neoplasms summarized under this term all derive from hair follicle epithelium. They vary with regard to the potency of tumor cells to form distinct anatomical structures of the hair follicle. The relevance of subclassifying these tumors into the 4 distinct modifiers is still unknown. Trichoepitheliomas are neoplasms of the hair follicle matrix epithelium which can give rise to the inner root sheath and hair shafts. They have a subtle mesenchymal component resembling the hair follicle dermal papilla, which becomes evident by focal invaginations of the basement membrane. As opposed to basal cell neoplasms, the epithelium of trichoepitheliomas does not differentiate into sebaceous cells. Compared to trichoepitheliomas, trichofolliculomas are large cystic neoplasms with a more mature development into hair follicles that radiate from the cystic center. Pilomatricomas are still an enigma with regard to their histogenesis. They can differentiate into hair matrix and hair cortex cells and are characterized by ghost cells which are entrapped by an abruptly keratinizing epithelium. Tricholemmomas are neoplasms of the outer root sheath epithelium. More differentiated cells contain PAS-positive glycogen granules similar to those in the external outer root sheath epithelium of normal anagen hair follicles. Malignant hair follicle tumors as they occur in man (e.g. malignant pilomatricoma, malignant trichilemmoma) have not been described in rodents. (13Bogovski, 1979; 28Deerberg et al., 1986; 33Evans et al., 1997; 34 Faccini et al., 1990; 99Peckham and Heider, 1999; 141Zackheim et al., 1990; 144Zwicker et al., 1992 ; 61Jones et al., 1989; 73Maekawa, 1989; 32Elwell et al., 1990; 17Bruner et al., 2001; 2Anver et al., 1982; 131Ward et al., 1979; 42Goerttler et al., 1984)
Carcinoma, eccrine gland
Synonyms: Primary adenoid cystic carcinoma
Pathogenesis/cell of origin
• Derives from the epithelium of eccrine glands.
Diagnostic features
• Cystic lesions on distal extremities.
• The cyst wall is lined by 1 to 2 layers of basaloid cells with papillary projections protruding into the cyst cavity.
• Cytologic atypia and rare mitoses are present.
Differential diagnoses
• Carcinoma, sebaceous cell: Invasive growth or metastasis is present and cells are poorly differentiated; cellular and nuclear atypia are present.
• Carcinoma, basal cell: Lobules and cords of basaloid cells with pallisading at the periphery.
• Carcinoma, squamous cell: Polygonal tumor cells with some evidence of squamous differentiation.
Comment: Eccrine glands are present in rodent foot pads only and spontaneous neoplasms have not been reported. Genetically engineered mice, however, might display tumors deriving from the eccrine glands resembling sweat gland tumors in man. The occurrence of a benign tumor originating from eccrine glands in rodents has not been reported so far. (76Matthias et al., 2012)
SKIN – DERMIS AND SUBCUTIS
Neoplastic lesions in the dermis and subcutis that derive from mesenchymal cells are covered in the manuscript on proliferative and non-proliferative lesions of the rat and mose soft tissue, skeletal muscle and mesothelium (48Greaves et al., 2013). Neoplasms in the skin that derive from mesenchymal stem cells are classified as fibroma, fibrosarcoma, pleomorphic fibrosarcoma (syn. malignant fibrous histiocytoma), lipoma (including angiomatous type), hibernoma, liposarcoma, rhabdomyosarcoma, leiomyoma, leiomyosarcoma, malignant mesenchymoma, and sarcoma, not otherwise specified.
In addition, neoplasms originating from nervous tissue (schwannoma, neural crest tumor), vascular tissue (haemangioma, haemangiosarcoma, haemangiopericytoma) and lymphoid tissue (lymphosarcoma, mast cell tumor, plasma cell tumor, histiocytic sarcoma) exist in the skin and are described in the according manuscripts (66Kaufmann et al., 2012; 12Berridge et al., in preparation; 133Willard-Mack et al., in preparation).
Melanocytes in the skin of mice only briefly reside in the epidermis of the trunk (88Noonan et al., 2000). Melanoblasts, however, may also reside in the dermis where they can proliferate and cause pigmented neoplasms (64Kanno, 1989).
Melanoma, benign (Figure 29)
Figure 29.
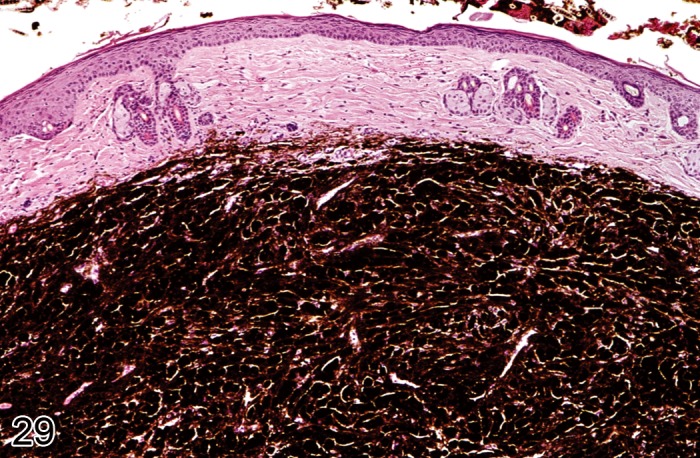
Mouse skin. Melanoma, benign. Polygonal tumor cells with large amount of intracytoplasmic dark brown pigment granules (Courtesy of R. Herbert).
Synonyms: Melanocytoma.
Pathogenesis/cell of origin
• Melanocytes, i.e. neuroectodermal cells that have migrated into the dermis and epidermal adnexa.
Diagnostic features
• Dense nodular proliferation in the dermis with or without association towards the epidermis.
• Tumor cells are polygonal, epitheloid or spindle shaped with variable degree of pigmentation.
• There are large amounts of intracytoplasmic dark brown pigment granules.
Differential diagnoses
• Melanoma, malignant: There is invasive growth, less pigmentation and a higher mitotic activity.
Comment: Benign melanomas are exceedingly rare incidental tumors in rats and mice. (7Bader et al., 1993; 14Bogovski, 1994; 28Deerberg et al., 1986; 33Evans et al., 1997; 64Kanno, 1989; 67Kort et al., 1984; 99Peckham and Heider, 1999; 103Ramon y Cajal et al., 1991; 132Weiss and Frese, 1974; 136Yoshitomi and Boorman, 1993; 138Yoshitomi et al., 1995; 141Zackheim et al., 1990; 143Zurcher and Roholl, 1989; 144Zwicker et al., 1992; 17Bruner et al., 2001; 18Burek, 1978; 131Ward et al., 1979 ; 115Sommer, 1997)
Melanoma, malignant (Figures 30A and 30B)
Figure 30.
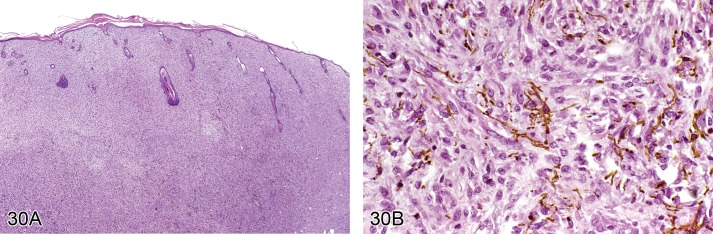
A) Mouse skin. Melanoma, malignant. Dense nodular proliferation in the dermis with low degree of pigmentation (Courtesy of R. Herbert). B) Mouse skin. Melanoma, malignant. Polygonal to spindle shaped tumor cells with small amounts of intracytoplasmic dark brown pigment granules (Courtesy of R. Herbert).
Modifiers: Amelanotic type
Synonyms: Melanosarcoma, Melanocytic tumor
Pathogenesis/cell of origin
• Melanocytes, i.e. neuroectodermal cells that have migrated into the epidermis and epidermal adnexa.
Diagnostic features
• Dense nodular proliferation in the dermis with or without association towards the epidermis.
• Tumor cells are polygonal, epitheloid or spindle shaped with variable degree of pigmentation.
• There is invasive growth.
• There are variable amounts of intracytoplasmic dark brown pigment granules.
• May show frequent mitotic figures and nuclear atypia.
Amelanotic type
• Tumor cells do not contain pigment granules.
Special techniques for diagnosis
• Histochemistry: Masson-Fontana stain, demonstration of melanosomes
• Transmission electron microscopy: demonstration of melanosomes
• Immunohistochemistry: staining for tyrosinase-related protein 2 (TRP-2)
Differential diagnoses
• Carcinoma, basal cell: Palisading cells are present at the periphery; there is no evidence of introcytoplasmic pigment.
• Schwannoma, malignant: Cells are not oriented perivascularly; there is no evidence of introcytoplasmatic pigment.
Comment: Malignant melanomas are exceedingly rare incidental tumors in rats and mice, but there are some reports about induced malignant lesions. Predilection sites for amelanotic melanomas in rats are ear pinna, eyelid, scrotum and perianal area. (7Bader et al., 1993; 14Bogovski, 1994; 28Deerberg et al., 1986; 33Evans et al., 1997; 64Kanno, 1989; 67Kort et al., 1984; 99Peckham and Heider, 1999; 103Ramon y Cajal et al., 1991; 132Weiss and Frese, 1974; 136Yoshitomi and Boorman, 1993; 138Yoshitomi et al., 1995; 141Zackheim et al., 1990; 143Zurcher and Roholl, 1989; 144Zwicker et al., 1992; 18Burek, 1978; 131Ward et al., 1979; 112Sher, 1982; 115Sommer, 1997; 42Goerttler et al., 1984; 11Berkelhammer and Oxenhandler, 1987)
Conflict of Interest
Financial disclosure: No money was paid for the preparation of this manuscript. During preparation of this manuscript, salaries of contributors were paid by their respective employer. None of the content of the manuscript contains any information that could be patentable or claimed as intellectual property of the contributors or their respective companies.
Acknowledgements
The assistance of Emily Singletary and the contribution of images from Dr. Susan M. Fischer, Dr. R. Herbert, and the Registry of Industrial Toxicology Animal Data (RITA) are greatly acknowledged.
References
- 1.Ackerman AB.(1978). Histologic diagnosis of inflammatory skin disease. A method by pattern analysis. Lea & Febiger, Philadelphia. [Google Scholar]
- 2.Anver MR, Cohen BJ, Lattuada CP, Foster SJ.Age-associated lesions in barrier-reared male Sprague-Dawley rats: a comparison between Hap: (SD) and Crl:COBS[R]CD[R](SD) stocks. Exp Aging Res. 8: 3–24 1982 [DOI] [PubMed] [Google Scholar]
- 3.Argyris TS.Hair growth cycles and skin neoplasia. Conference on Biology of Cutaneous Cancer. Natl Cancer Inst Monogr. 10: 33–41 1963 [Google Scholar]
- 4.Asano S, Trempus CS, Spalding JW, Tennant RW, Battalora MJ.Morphological characterization of spindle cell tumors induced in transgenic Tg.AC mouse skin. Toxicol Pathol. 26: 512–519 1998 [DOI] [PubMed] [Google Scholar]
- 5.Ash GW.An epidemic of chronic skin ulceration in rats. Lab Anim. 5: 115–122 1971 [DOI] [PubMed] [Google Scholar]
- 6.Azzi L, El-Alfy M, Martel C, Labrie F.Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 124: 22–27 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bader R, Gembardt C, Kaufmann W, Kuettler K, Mann PC, van Zwieten MJ, Zurcher C. (1993). 5. Integumentary System. In: Mohr U, Capen CC, Dungworth DL, Griesemer RA, Ito N, Turusov VS (eds) International classification of rodent tumours, Part I: The Rat. IARC Scientific Publications No. 122, Lyon, pp 1–21. [Google Scholar]
- 8.Balsari A, Bianchi C, Cocilovo A, Dragoni I, Poli G, Ponti W.Dermatophytes in clinically healthy laboratory animals. Lab Anim. 15: 75–77 1981 [DOI] [PubMed] [Google Scholar]
- 9.Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF.The hairless mouse in skin research. J Dermatol Sci. 53: 10–18 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger H, Tsambaos D, Mahrle G.Experimental elastosis induced by chronic ultraviolet exposure. Arch Dermatol Res. 269: 39–49 1980 [DOI] [PubMed] [Google Scholar]
- 11.Berkelhammer J, Oxenhandler RW.Evaluation of premalignant and malignant lesions during the induction of mouse melanomas. Cancer Res. 47: 1251–1254 1987 [PubMed] [Google Scholar]
- 12.Berridge, et al. Proliferative and Non-proliferative Lesions of the Rat and Mouse Vascular System In preparation (INHAND organ working group manuscript). [Google Scholar]
- 13.Bogovski P.(1979). Tumours of the skin. In: Turusov VS (ed) Pathology of tumours in laboratory animals. Vol II. Tumours of the mouse. IARC Scientific Publications No. 23, Lyon, pp 1–41. [PubMed] [Google Scholar]
- 14.Bogovski P.(1994). Tumours of the skin. In: Turusov VS, Mohr U (eds) Pathology of tumours in laboratory animals. Vol 2. Tumours of the mouse, 2nd edition. IARC Scientific Publications No. 111, Lyon, pp 1–45. [Google Scholar]
- 15.Brix AE, Hardisty JF, McConnell EE. (2010). Combining neoplasms for evaluation of rodent carcinogenesis studies. In: Hsu CH, Stedeford T (eds) Cancer Risk Assessment. Wiley, Hoboken, NJ, pp 699-715. [Google Scholar]
- 16.Brown AP, Dunstan RW, Courtney CL, Criswell KA, Graziano MJ.Cutaneous lesions in the rat following administration of an irreversible inhibitor of erbB receptors, including the epidermal growth factor receptor. Toxicol Pathol. 36: 410–419 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bruner R, Kuettler K, Baer R, Kaufmann W, Boothe A, Enomoto M, Holland JM, Parish WE. (2001). Integumentary system. In: International Classification of Rodent Tumors: The Mouse. Mohr U (ed), Springer-Verlag, Berlin, pp 2-22. [Google Scholar]
- 18.Burek JD. (1978). Age-associated pathology. In: Pathology of Aging Rats, Chapter 4. CRC Press, West Palm Beach, FL. Pp 29-167. [Google Scholar]
- 19.Canfield PJ, Greenoak GE, Reeve VE, Gallagher CH.Characterization of UV induced keratoacanthoma-like lesions in HRA/Skh-1 mice and their comparison with keratoacanthomas in man. Pathology. 17: 613–616 1985 [DOI] [PubMed] [Google Scholar]
- 20.Cerundolo R, Mecklenburg L.(2009). Hair follicle dystrophy and atrophy. In: Mecklenburg L, Linek M, Tobin DJ (eds). Haior loss disorders in domestic animals. Wiley-Blackwell, Ames, IA, pp 177-184. [Google Scholar]
- 21.Chan PK, O'Hara GP, Hayes AW. (1982). Principles and methods for acute and subchronic toxicity. In: Principles and Methods of Toxicology. Hayes AW (ed.) Raven Press, New York. Pp. 1-51. [Google Scholar]
- 22.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL.Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci. 45: 131–139 1995 [PubMed] [Google Scholar]
- 23.Courtney CL, Hawkins KL, Graziano MJ.Granular basal cell tumor in a Wistar rat. Toxicol Pathol. 20: 122–124 1992 [DOI] [PubMed] [Google Scholar]
- 24.CPMP/SWP 398/01. ( 2002). Note for guidance on photosafety testing. The European Agency for the Evaluation of Medicinal Products, London, 27. June 2002. [Google Scholar]
- 25.Crippa L, Gobbi A, Ceruti RM, Clifford CB, Remuzzi A, Scanziani E.Ringtail in suckling Munich Wistar Fromter rats: a histopathologic study. Comp Med. 50: 536–539 2000 [PubMed] [Google Scholar]
- 26.Csiza CK, McMartin DN.Apparent acaridal dermatitis in a C57BL/6 Nya mouse colony. Lab Anim Sci. 26: 781–787 1976 [PubMed] [Google Scholar]
- 27.Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, Pierce GF.Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 147: 145–154 1995 [PMC free article] [PubMed] [Google Scholar]
- 28.Deerberg F, Knüp F, Rehm S.Spontaneous epithelial tumours of the skin in Han: WIST- and DA/Han rats. Z Versuchstierkd. 28: 45–57 1986 [PubMed] [Google Scholar]
- 29.Dick EJ, Kittell CL, Meyer H, Farrar PL, Ropp SL, Esposito JJ, Buller RM, Neubauer H, Kang YH, McKee AE.Mousepox outbreak in a laboratory mouse colony. Lab Anim Sci. 46: 602–611 1996 [PubMed] [Google Scholar]
- 30.Dry FW.The coat of the mouse (Mus musculus). J Genet. 16: 287–340 1926 [Google Scholar]
- 31.Dunson DB, Haseman JK, van Birgelen APJM, Stasiewicz S, Tennant RW.Statistical analysis of skin tumor data from Tg.AC mouse bioassay. Toxicol Sci. 55: 293–302 2000 [DOI] [PubMed] [Google Scholar]
- 32.Elwell MR, Stedham MA, Kovatch RM.(1990). Skin and subcutis. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, Jr, MacKenzie WF (eds) Pathology of the Fischer rat. Reference and atlas. Academic Press, San Diego New York London, pp 261–277. [Google Scholar]
- 33.Evans MG, Cartwright ME, Sahota PS, Clifford CB.(1997). Proliferative lesions of the skin and adnexa of rats. ISI In: Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington, CD. [Google Scholar]
- 34.Faccini JM, Abbott DP, Paulus GJJ.(1990). Mouse histopathology. A glossary for use in toxicity and carcinogenicity studies. I. Integumentary System. Elsevier, Amsterdam New York Oxford, pp 1–17. [Google Scholar]
- 35.FDA/CDER ( 2003). Guidance for Industry: Photosafety testing. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, May 2003. [Google Scholar]
- 36.Forbes PD.Relevance of animal models of photocarcinogenesis to humans. Photochem Photobiol. 63: 357–362 1996 [DOI] [PubMed] [Google Scholar]
- 37.Fox JG, Niemi SM, Murphy JC, Quimby FW.Ulcerative dermatitis in the rat. Lab Anim Sci. 27: 671–678 1977 [PubMed] [Google Scholar]
- 38.Frith CH, Ward JM.(1988). Color atlas of neoplastic and non-neoplastic lesions in aging mice. Elsevier, Amsterdam New York Tokyo. [Google Scholar]
- 39.Fukuda K, Matsushita H, Sakabe H, Takemoto K.Carcinogenicity of benzyl chloride, benzal chloride, benzotrichloride and benzoyl chloride in mice by skin application. Gann. 72: 655–664 1981 [PubMed] [Google Scholar]
- 40.Galvin S, Loomis C, Manabe M, Dhouailly D, Sun TT.The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 4: 277–299, discussion 300 1989 [PubMed] [Google Scholar]
- 41.Ghadially FN.The role of the hair follicle in the origin and evolution of some cutaneous neoplasms of man and experimental animals. Cancer. 14: 801–816 1961 [DOI] [PubMed] [Google Scholar]
- 42.Goerttler K, Loehrke H, Hesse B, Schweizer J.Skin tumor formation in the European hamster (Cricetus cricetus L.) after topical initiation with 7,12-dimethylbenz[a]anthracene (DMBA) and promotion with 12-O-tetradecanoylphorbol-13-acetate (TPA). Carcinogenesis. 5: 521–524 1984 [DOI] [PubMed] [Google Scholar]
- 43.Gopinath C, Prentice DE, Lewis DT. (1987). Atlas of Experimental Toxicological pathology. MTP Press Limited, Boston, MA. pp 159–166. [Google Scholar]
- 44.Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, Wilbert D, Patel RM, Ferris J, Diener J, Allen M, Lim S, Syu LJ, Verhaegen M, Dlugosz AA.Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 121: 1768–1781 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greaves P. (2000). Histopathology of Preclinical Toxicity Studies: Interpretation and relevance in drug safety Evaluation. 2nd ed. Elsevier, Amsterdam. pp1–54. [Google Scholar]
- 46.Greaves P. (1990). Histopathology of preclinical toxicity studies: interpretation and relevance in drug safety evaluation. Elsevier, New York, pp 1–47. [Google Scholar]
- 47.Greaves P, Faccini JM. (1984). Rat histopathology. A glossary for use in toxicity and carcinogenicity studies. Elsevier, Amsterdam New York Oxford, pp 1–3. [Google Scholar]
- 48.Greaves P, Chouinard L, Ernst H, Mecklenburg L, Pruimboom-Brees IM, Rinke M, Rittinghausen S, Thibault S, von Erichsen J, Yoshida T.Proliferative and Non-proliferative Lesions of the Rat and Mouse Soft Tissue, Skeletal Muscle and mesothelium. J Toxicol Pathol. 26(3 Suppl): 1S–26S 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gross TL, Ihrke PJ, Walder EJ, Affolter VK.(2005). Skin Diseases of the Dog and Cat. Blackwell Science Ltd. Oxford, UK pp 105-115. [Google Scholar]
- 50.Hansen LS, Coggle JE, Wells J, Charles MW.The influence of the hair cycle on the thickness of mouse skin. Anat Rec. 210: 569–573 1984 [DOI] [PubMed] [Google Scholar]
- 51.Hargis AM, Ginn PE.(2007). The Integument. In McGavin MD and Zachary JF (eds) Pathologic Basis of Veterinary Disease. Mosby Elsevier, St. Louis, pp. 1107-1261. [Google Scholar]
- 52.Haschek WM, Rousseaux CG, Wallig MA.(2010). Fundamentals of Toxicologic Pathology. Elsevier, Amsterdam, 2nd ed. pp 135–159. [Google Scholar]
- 53.Hasegawa R, Miyakawa Y, Sato H.(1989). Basal cell tumors, skin, mouse. In: Integument and Mammary Glands. Jones TC, Mohr U, Hunt RD (eds.). Springer-Verlag. New York. pp 52–55. [Google Scholar]
- 54.Krinke GJ.(2004). Normative histology of organs: Skin. In: Hedrich H, Bullock GR (eds). The laboratory mouse. Elsevier, Amsterdam, p. 156. [Google Scholar]
- 55.Hendrix S, Handjiski B, Peters EM, Paus R.A guide to assessing damage response pathways of the hair follicle: lessons from cyclophosphamide-induced alopecia in mice. J Invest Dermatol. 125: 42–51 2005 [DOI] [PubMed] [Google Scholar]
- 56.Hill LR, Kille PS, Weiss DA, Craig TM, Coghlan LG.Demodex musculi in the skin of transgenic mice. Contemp Top Lab Anim Sci. 38: 13–18 1999 [PubMed] [Google Scholar]
- 57.Hirose M. (1989). Squamous cell carcinoma, skin, rat. In: Jones TC, Mohr U, Hunt RD (eds.) Monographs on pathology of laboratory animals. Integument and Mammary Glands. Springer, Berlin Heidelberg New York Tokyo, pp 25–30. [Google Scholar]
- 58.Hirouchi Y, Iwata H, Yamakawa S, Kato M, Kobayashi K, Yamamoto T, Inoue H, Enomoto M, Shiga A, Koike Y.Historical data of neoplastic and non-neoplastic lesions in B6C3F1 (C57BL/6CrSlc x C3H/HeSlc) mice. J Toxicol Pathol. 7: 153–177 1994 [Google Scholar]
- 59.Hofstetter J, Suckow MA, Hickman DL.(2006). Morphophysiology. In: Suckow MA, Weisbroth SH, Franklin CL (eds.) The laboratory rat. Elsevier, Amsterdam. pp 94–120. [Google Scholar]
- 60.Jeong MS, Lee CM, Jeong WJ, Kim SJ, Lee KY.Significant damage of the skin and hair following hair bleaching. J Dermatol. 37: 882–887 2010 [DOI] [PubMed] [Google Scholar]
- 61.Jones TC, Mohr U, Hunt RD. (1989). Monographs on Pathology of Laboratory Animals. Integument and Mammary glands. Springer Verlag. [Google Scholar]
- 62.Jungmann P, Guénet JL, Cazenave PA, Coutinho A, Huerre M.Murine acariasis: I. Pathological and clinical evidence suggesting cutaneous allergy and wasting syndrome in BALB/c mouse. Res Immunol. 147: 27–38 1996 [DOI] [PubMed] [Google Scholar]
- 63.Kalueff AV, Minasyan A, Keisala T, Shah ZH, Tuohimaa P.Hair barbering in mice: implications for neurobehavioural research. Behav Processes. 71: 8–15 2006. [DOI] [PubMed] [Google Scholar]
- 64.Kanno J. (1989). Melanocytic tumours, skin, mouse. In: Jones TC, Mohr U, Hunt RD (eds) Monographs on pathology of laboratory animals. Integument and mammary glands. Springer, Berlin Heidelberg New York Tokyo, pp 63–70. [Google Scholar]
- 65.Kastenmayer RJ, Fain MA, Perdue KA.A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci. 45: 8–12 2006 [PubMed] [Google Scholar]
- 66.Kaufmann W, Bolon B, Bradley A, Butt M, Czasch S, Garman RH, George C, Gröters S, Krinke G, Little P, McKay J, Narama I, Rao D, Shibutani M, Sills R.Proliferative and nonproliferative lesions of the rat and mouse central nervous system. Toxicol Pathol. 40: 87–157 2012 [DOI] [PubMed] [Google Scholar]
- 67.Kort WJ, Zondervan PE, Hulsman LOM, Weijma IM, Westbroek DL.Incidence of spontaneous tumors in a group of retired breeder female Brown Norway rats. J Natl Cancer Inst. 72: 709–713 1984 [PubMed] [Google Scholar]
- 68.Kovatch R.(1990). Neoplasms of the integument. In: Stinson SF, Schuller HM, Reznik GK (eds) Atlas of tumour pathology of the Fischer rat. CRC Press, Boca Raton, pp 19–32. [Google Scholar]
- 69.Kurien BT, Gross T, Scofield RH.Barbering in mice: a model for trichotillomania. BMJ. 331: 1503–1505 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavker RM, Schechter NM, Lazarus GS.Effects of topical corticosteroids on human dermis. Br J Dermatol. 115 (Suppl 31): 101–107 1986 [DOI] [PubMed] [Google Scholar]
- 71.Lawson PT, Churchman PR.Ringtail in a breeding colony of rats due to malfunction of the heating, ventilating, and air conditioning system. Contemp Top. 32: 22 1993 [Google Scholar]
- 72.Loser K, Beissert S.Dendritic cells and T cells in the regulation of cutaneous immunity. Adv Dermatol. 23: 307–333 2007 [DOI] [PubMed] [Google Scholar]
- 73.Maekawa A. (1989). Trichopeithelioma, skin, rat. In: Integument and Mammary Glands. Jones TC, Mohr U, Hunt RD (eds.). Springer-Verlag, New York. Pp 56-63. [Google Scholar]
- 74.Mahl JA, Vogel BE, Court M, Kolopp M, Roman D, Nogués V.The minipig in dermatotoxicology: Methods and challenges. Exp Toxicol Pathol. 57: 341–345 2006 [DOI] [PubMed] [Google Scholar]
- 75.Maita K, Hirano M, Harada T, Mitsumori K, Yoshida A, Takahashi K, Nakashima N, Kitazawa T, Enomoto A, Inui K, Shirasu Y.Mortality, major cause of moribundity, and spontaneous tumors in CD-1 mice. Toxicol Pathol. 16: 340–349 1988 [DOI] [PubMed] [Google Scholar]
- 76.Matthias N, Lockworth CR, Zhang F, Lee MH, Yeung SC, Tsai KY, Hamir AN.Multiple cystic sweat gland tumors in transgenic mice. Comp Med. 62: 27–30 2012 [PMC free article] [PubMed] [Google Scholar]
- 77.McElwee KJ, Freyschmift-Paul P, Sundberg JP, Hoffmann R. (2003). The pathogenesis of alopecia areata in rodent models. J Invest dermatol Symp Proc 8: 6-11. [DOI] [PubMed]
- 78.McEwen BJ, Barsoum NJ.Auricular chondritis in Wistar rats. Lab Anim. 24: 280–283 1990. [DOI] [PubMed] [Google Scholar]
- 79.Mecklenburg L, Nakamura M, Sundberg JP, Paus R.The nude mouse skin phenotype: the role of Foxn1 in hair follicle development and cycling. Exp Mol Pathol. 71: 171–178 2001 [DOI] [PubMed] [Google Scholar]
- 80.Mecklenburg L. (2009). How to approach alopecia diseases: Histopathological aspects. In: Mecklenburg L, Linek M, Tobin DJ (eds). Hair loss disorders in domestic animals. Wiley-Blackwell, Ames, IA, pp 77-92. [Google Scholar]
- 81.Mohr U, Hunt RD. (1989). (eds) Monographs on pathology of laboratory animals. Integument and mammary glands. Springer, Berlin Heidelberg New York Tokyo, pp 19–24. [Google Scholar]
- 82.Morrow DT, Robinette LR, Saubert CW, 4th , and Van Hoosier GL., JrPoditis in the rat as a complication of experiments in exercise physiology. Lab Anim Sci. 27: 679–681 1977 [PubMed] [Google Scholar]
- 83.Meyer W.(2009). Hair follicles in domesticated mammals with comparison to laboratory animals and humans. In: Mecklenburg L, Linek M, Tobin DJ (eds). Hair loss disorders in domestic animals. Wiley-Blackwell, Ames, IA, pp 43–64. [Google Scholar]
- 84.Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, Stenn KS, Paus R.A comprehensive guide fort he accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 117: 3–15 2001 [DOI] [PubMed] [Google Scholar]
- 85.Nakamura K, Johnson WC.Ultraviolet light induced connective tissue changes in rat skin: a histopathologic and histochemical study. J Invest Dermatol. 51: 253–258 1968 [DOI] [PubMed] [Google Scholar]
- 86.Nakamura M, Sundberg JP, Paus R.Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: annotated tables. Exp Dermatol. 10: 369–390 2001. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura M, Tobin DJ, Richards-Smith B, Sundberg JP, Paus R.Mutant laboratory mice with abnormalities in pigmentation: annotated tables. J Dermatol Sci. 28: 1–33 2002 [DOI] [PubMed] [Google Scholar]
- 88.Noonan FP, Otsuka T, Bang S, Anver MR, Merlino G.Accelerated ultraviolet radiation-induced carcinogenesis in hepatocyte growth factor/scatter factor transgenic mice. Cancer Res. 60: 3738–3743 2000 [PubMed] [Google Scholar]
- 89.OECD ( 1987). OECD guideline for testing of chemicals no. 402: Acute Dermal Toxicity. 24 Feb 1987.
- 90.OECD (1981a). OECD guideline for testing of chemicals no. 410: Repeated Dose Dermal Toxicity. 1/28-day Study. 12 May 1981.
- 91.OECD (1981b). OECD guideline for testing of chemicals no. 411: Subchronic Dermal Toxicity: 90-day Study. 12 May 1981.
- 92.OECD (1992). OECD guideline for testing of chemicals no.406: Skin Sensitisation. 17. Jul 1992.
- 93.OECD (2002). OECD guideline for testing of chemicals no.404: Acute Dermal Irritation/Corrosion. 24. Apr. 2002.
- 94.OECD (2010a). OECD guideline for testing of chemicals no. 429: Skin Sensitization: Local Lymph Node Assay. 22 Jul 2010.
- 95.OECD (2010b). OECD guideline for testing of chemicals no.442A: Skin Sensitization: Local Lymph Node Assay: DA. 22 Oct 2010.
- 96.OECD (2010c). OECD guideline for testing of chemicals no.442A: Skin Sensitization: Local Lymph Node Assay: BrdU-ELISA. 22. Oct 2010.
- 97.Okum MR, Edelstein LM, Fisher BK. (1988). Gross and microscopic pathology of the skin, Vol. II. Dermatopathology Foundation Press, Canton, MA, pp 804–818. [Google Scholar]
- 98.Paus R, Müller-Röver S, Van Der Veen C, Maurer M, Eichmüller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B.A comprehensive guide fort he recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 113: 523–532 1999 [DOI] [PubMed] [Google Scholar]
- 99.Peckham JC, Heider K. (1999). Skin and subcutis. In: Maronpot RR, Boorman GA, Gaul BW (eds) Pathology of the mouse. Reference and atlas. Cache River Press, Vienna, pp 555–612. [Google Scholar]
- 100.Peper RL.Diagnostic exercise: mite infestation in a laboratory rat colony. Lab Anim Sci. 44: 172–174 1994 [PubMed] [Google Scholar]
- 101.Percy DH, Barthold SW. (2007). Pathology of Laboratory Rodents and Rabbits. 3rd edition. Blackwell Publishing. [Google Scholar]
- 102.Poteracki J, Walsh KM.Spontaneous neoplasms in control Wistar rats: a comparison of reviews. Toxicol Sci. 45: 1–8 1998 [DOI] [PubMed] [Google Scholar]
- 103.Ramon y Cajal S, Suster S, Halaban R, Filvaroff E, Dotto GP.Induction of different morphologic features of malignant melanoma and pigmented lesions after transformation of murine melanocytes with bFGF-cDNA and H-ras, myc, neu, and E1a oncogenes. Am J Pathol. 138: 349–358 1991 [PMC free article] [PubMed] [Google Scholar]
- 104.Ramselaar CG, Ruitenberg EJ, Kruizinga W.Regression of induced keratoacanthomas in anagen (hair growth phase) skin grafts in mice. Cancer Res. 40: 1668–1673 1980 [PubMed] [Google Scholar]
- 105.Rehm S, Ward JM, Devor DE. (1989). Squamous cell carcinoma arising in induced papilloma, skin, mouse. In: Jones TC, Mohr U, Hunt RD (eds) Monographs on pathology of laboratory animals. Integument and mammary glands. Springer, Berlin Heidelberg New York Tokyo, pp 38–42. [Google Scholar]
- 106.Rudmann D, Cardiff R, Chouinard L, Goodman D, Küttler K, Marxfeld H, Molinolo A, Treumann S, Yoshizawa K.INHAND Mammary, Zymbal’s, Preputial, and Clitoral Gland Organ Working Group Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal’s, preputial, and clitoral glands Toxicol Pathol. 40 (Suppl): 7S–39S 2012. [DOI] [PubMed] [Google Scholar]
- 107.Salerno A, Dieli F.Role of gamma delta T lymphocytes in immune response in humans and mice. Crit Rev Immunol. 18: 327–357 1998 [DOI] [PubMed] [Google Scholar]
- 108.Sams WM, Smith JG, Burk PG.The experimental production of elastosis with ultraviolet light. J Invest Dermatol. 43: 467–471 1964. [PubMed] [Google Scholar]
- 109.Sawada M, Terada N, Taniguchi H, Tateishi R, Mori Y.Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 56: 684–686 1987. [PubMed] [Google Scholar]
- 110.Sells DM, Gibson JP.Carcinogtenicity studies with medroxalol hydrochloride in rats and mice. Toxicol Pathol. 15: 457–467 1987 [DOI] [PubMed] [Google Scholar]
- 111.Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, Yang S, Brissette JL, Dotto GP, Botchkarev VA.Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci USA. 103: 18166–18171 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sher SP.Tumors in control hamsters, rats, and mice: literature tabulation. Crit Rev Toxicol. 10: 49–79 1982 [DOI] [PubMed] [Google Scholar]
- 113.Slaga TJ, Thompson S, Smuckler EA.Prolonged inhibition of mouse epidermal DNA synthesis by dexamethasone. J Natl Cancer Inst. 54: 931–936 1975. [PubMed] [Google Scholar]
- 114.Smith KR.The structure and function of the Harscheibe. J Comp Neurol. 131: 459–474 1967. [DOI] [PubMed] [Google Scholar]
- 115.Sommer MM.Spontaneous skin neoplasms in Long-Evans rats. Toxicol Pathol. 25: 506–510 1997 [DOI] [PubMed] [Google Scholar]
- 116.Squire RA, Goodman DG, Valerio MG, Fredrickson T, Strandberg JD, Levitt MH, Lingeman CH, Harshbarger JC, Dawe CJ.(1978). Tumours. In: Benirschke K, Garner FM, Jones TC (eds) Pathology of laboratory animals Vol II. Springer, Berlin Heidelberg New York Tokyo, pp 1051–1283. [Google Scholar]
- 117.Stenbäck F, Wasenius VM, Kallioinen M.Basement membranes in experimentally induced skin tumors. J Invest Dermatol. 87: 185–189 1986 [DOI] [PubMed] [Google Scholar]
- 118.Sundberg JP, Peters EM, Paus R.Analysis of hair follicles in mutant laboratory mice. J Investig Dermatol Symp Proc. 10: 264–270 2005 [DOI] [PubMed] [Google Scholar]
- 119.Sundberg JP, Taylor D, Lorch G, Miller J, Silva KA, Sundberg BA, Roopenian D, Sperling L, Ong D, King LE, Everts H.Primary follicular dystrophy with scarring dermatitis in C57BL/6 mouse substrains resembles central centrifugal cicatricial alopecia in humans. Vet Pathol. 48: 513–524 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sundberg JP. (1994). Handbook of Mouse Mutations with Skin and Hair Abnormalities: Animal Models and Biomedical Tools. CRC Press Inc. [Google Scholar]
- 121.Szabo E, Sugar J. (1989). Basal cell carcinoma, skin, rat. In: Jones TC, Mohr U, Hunt RT (eds) Monographs on Pathology of Laboratory Animals. Integument and mammary gland. Springer, Berlin Heidelberg New York Tokyo, pp 43–51. [Google Scholar]
- 122.Taylor DK, Bubier JA, Silva KA, Sundberg JP.Development, structure, and keratin expression in C57BL/6 mouse eccrine glands. Vet Pathol. 49: 146–154 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tennant RW, Stasiewicz S, Eastin WC, Mennear JH, Spalding JW.The Tg.AC (v-Ha-ras) transgenic mouse: nature of the model. Toxicol Pathol. 29 (Suppl): 51–59 2001 [DOI] [PubMed] [Google Scholar]
- 124.Thomas C, Rohrbach R.(1975). Pathogenese und Morphologie der epithelialen Hauttumoren bei der Ratte. In: Altmann HW, Büchner F, Cottier H, Grundmann E, Holle G, Letterer E, Masshoff W, Meesen H, Roulet F, Seifert G, Siebert G (eds) Handbuch der allgemeinen Pathologie Vol 6, part 7, Tumours III, pp 335–389. [Google Scholar]
- 125.Usui T, Mutai M, Hisada S, Takoaka M, Soper KA, McCullough B, Alden C.CB6F1-rasH2 mouse: overview of available data. Toxicol Pathol. 29(Suppl): 90–108 2001 [DOI] [PubMed] [Google Scholar]
- 126.Veldman C, Feliciani C.Pemphigus: a complex T cell-dependent autoimmune disorder leading to acantholysis. Clin Rev Allergy Immunol. 34: 313–320 2008. [DOI] [PubMed] [Google Scholar]
- 127.Wagner JE, Owens DR, LaRegina MC, Vogler GA.Self trauma and Staphylococcus aureus in ulcerative dermatitis of rats. J Am Vet Med Assoc. 171: 839–841 1977 [PubMed] [Google Scholar]
- 128.Walberg JA, Stark DM, Desch C.Demodicosis in laboratory rats (Rattus norvegicus). Lab Anim Sci. 31: 60–62 1981. [PubMed] [Google Scholar]
- 129.Wallace GD, Buller RML.Kinetics of ectromelia virus (mousepox) transmission and clinical response in C57BL/6J, BALB/cByJ and AKR/J inbred mice. Lab Anim Sci. 35: 41–46 1985. [PubMed] [Google Scholar]
- 130.Walsh KM, Gough AW.Hypopigmentation in dogs treated with an inhibitor of platelet aggregation. Toxicol Pathol. 17: 549–553 1989. [DOI] [PubMed] [Google Scholar]
- 131.Ward JM, Goodman DG, Squire RA, Chu KC, Linhart MS.Neoplastic and nonneoplastic lesions in aging (C57BL/6N x C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 63: 849–854 1979 [DOI] [PubMed] [Google Scholar]
- 132.Weiss E, Frese K.Tumours of the skin. Bull World Health Organ. 50: 79–100 1974. [PMC free article] [PubMed] [Google Scholar]
- 133.Willard-Mack Proliferative and nonproliferative lesions of the rat and mouse lymphatic system, in preparation.
- 134.Wuepper KD, Dimond RL, Knutson DD.Studies of the mechanism of epidermal injury by a Staphylococcal epidermolytic toxin. J Invest Dermatol. 65: 191–200 1975 [DOI] [PubMed] [Google Scholar]
- 135.Yamanishi K.Gene-knockout mice with abnormal epidermal and hair follicular development. J Dermatol Sci. 18: 75–89 1998. [DOI] [PubMed] [Google Scholar]
- 136.Yoshitomi K, Boorman GA.Palpebral amelanotic melanomas in F344 rats. Vet Pathol. 30: 280–286 1993 [DOI] [PubMed] [Google Scholar]
- 137.Yoshitomi K, Boorman GA.Granular cell basal cell tumor of the eyelid in an F344 rat. Vet Pathol. 31: 106–108 1994 [DOI] [PubMed] [Google Scholar]
- 138.Yoshitomi K, Elwell MR, Boorman GA.Pathology and incidence of amelanotic melanomas of the skin in F-344/N rats. Toxicol Pathol. 23: 16–25 1995 [DOI] [PubMed] [Google Scholar]
- 139.Young AR.The sunburn cell. Photodermatol. 4: 127–134 1987 [PubMed] [Google Scholar]
- 140.Zackheim HS.Cutaneous basal cell carcinomas in the rat. J Natl Cancer Inst. 84: 811–812 1992 [DOI] [PubMed] [Google Scholar]
- 141.Zackheim HS, Zurcher C, Krutovskikh VA, Troyanovsky SM.(1990). Tumours of the skin. In: Turusov VS, Mohr U (eds) Pathology of tumours in laboratory animals. Vol I. Tumours of the rat, 2nd edition. IARC Scientific Publications No. 99, Lyon, pp 1–35. [PubMed] [Google Scholar]
- 142.Zeiss CJ, Ward JM, Allore HG.Designing phenotyping studies for genetically engineered mice. Vet Pathol. 49: 24–31 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zurcher C, Roholl PJM. (1989). Melanocytic tumours, rat. In: Jones TC, Mohr U, Hunt RD (eds) Monographs on pathology of laboratory animals. Integument and mammary glands. Springer, Berlin Heidelberg New York London Tokyo, pp 76–86. [Google Scholar]
- 144.Zwicker GM, Eyster RC, Sells DM, Gass JH.Spontaneous skin neoplasms in aged Sprague-Dawley rats. Toxicol Pathol. 20: 327–340 1992. [DOI] [PubMed] [Google Scholar]