Abstract
The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP), and North America (STP) to develop an internationally accepted nomenclature for proliferative and nonproliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying lesions observed in the soft tissues including skeletal muscle as well as the mesothelium of rats and mice. The standardized nomenclature of lesions presented in this document is also available electronically on the Internet (http://www.goreni.org/). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous developmental and aging lesions as well as those induced by exposure to test materials. A widely accepted and utilized international harmonization of nomenclature for lesions in soft tissues, skeletal muscle and mesothelium in laboratory animals will decrease confusion among regulatory and scientific research organizations in different countries and provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists. (DOI: 10.1293/tox.26.1S; J Toxicol Pathol 2013; 26: 1S–26S)
Keywords: ssoft tissues, skeletal muscle, mesotheium, preclinical safety—assessment/risk management, rat pathology, mouse pathology
Introduction
The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally-accepted nomenclature for proliferative and non-proliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying proliferative and non-proliferative lesions observed in soft tissues of laboratory rats and mice. Standardized nomenclature of proliferative and non-proliferative lesions of the soft tissues of both rats and mice has been previously published by the STP (50Greaves et al. 1992; 51Greaves and Seely 1996; 48Greaves et al. 2000). These were subject to extensive reviews and comments by members of STP and were used as the basis for the WHO - International Agency for Research on Cancer classification of tumors in mice and rats (16Carlton et al. 1992; 34Ernst et al. 2001). These STP publications have been used for this current classification. The standardized nomenclature of lesions presented here is also available electronically at the goRENI website (www.goreni.org). This document follows a similar approach to these previous publications.
The basic principles for recording microscopic changes in toxicity studies have been recently summarized (73Mann et al. 2012). In these studies microscopic observations should be recorded in a consistent and objective manner. Descriptive rather than diagnostic terminology is advisable in order to avoid misleading comparisons with particular diseases.
The conventional view of the soft tissues is one of a cellular component comprising fibroblasts, fat containing cells, specialized skeletal muscle fibers, a matrix of ground substance and various structural proteins including collagen and elastic fibers. These are accompanied by blood vessels and mobile bone-marrow derived cells such as lymphocytes, granular leukocytes and macrophages. This simple concept must now be modified since the discovery of mesenchymal stem cells and their important contribution to tissue homeostasis (114Valtieri and Sorrentino 2008). These cells are undifferentiated multi-potential cells which reside primarily in the bone marrow but also in other sites including adipose tissue and skeletal muscle (69Liu et al. 2009). They are now believed to be important in the functional repair of damaged tissues. Moreover, they have been found to have a central role in the pathogenesis and progression of neoplasia as part of the non-malignant supporting stroma (114Valtieri and Sorrentino 2008).
For convenience, mesothelial lesions are also reviewed in this document. Mesothelial cells are derived from the mesoderm but express many epithelial characteristics. They do not regenerate in the same way as epithelial cells although the exact mechanisms involved following injury remain uncertain (82Mutsaers et al. 2007). Lesions of the synovium are discussed under the INHAND classification of lesions of the skeletal system.
The pathological features of both spontaneous and induced conditions of soft tissues are similar among different laboratory rodent species as well as humans. Hence, the basic classification developed for mesenchymal lesions can be used for all species, although the range of soft tissue neoplasms described in rodents is far less than those characterized in humans. Nevertheless unusual types of mesenchymal neoplasms are encountered that do not fit into the usual classification scheme.
Careful recording of the nature, intensity and duration of the inflammatory response of the soft tissues to implanted or injected substances is important in the assessment of the local tolerability of agents intended for contact with human tissues. The chemical and physical properties of injected chemicals or vaccines and their adjuvants as well as size, shape and surface texture of implanted biomaterials may modify the histological features and temporal pattern of the inflammatory and reparative responses (123Williams 1987; 4Anderson and Langone 1999; 115Verdier et al. 2005; 27Dincer et al. 2006).
Morphology
Non-proliferative lesions
Soft tissues
• Infiltrate, inflammatory cell
• Inflammation
• Fibrosis
• Fibroplasia
• Necrosis
• Mineralization
• Metaplasia
• Amyloid
Adipose tissues
• Infiltrate, inflammatory cell
• Inflammation
• Inflammation, lipogranulomatous
• Necrosis
• Atrophy
• Hyperplasia
Smooth muscle
The distribution of lesions of smooth muscle generally parallels the normal distribution of smooth muscle in the body so that lesions occur mostly in the female genital tract, the gastrointestinal tract and skin but only rarely in deep soft tissue. However, it has been shown that adipose tissue may have an important paracrine function in smooth muscle cell proliferation in blood vessels (79Miao and Li 2012). In soft tissues, particularly in inflammatory processes, smooth muscle cells may be difficult to distinguish from myofibroblasts. They both express smooth muscle actin but smooth muscle cells usually contain desmin (62Kempson et al. 2001).
• Hyperplasia, smooth muscle
Skeletal muscle
Whilst skeletal muscle contains a similar range of connective tissue elements as the other soft tissues, its bulk is predominantly composed of highly specialized skeletal muscle cells or fibers (myocytes). These cells form a syncytium by fusion of fetal myoblasts to produce a muscle fiber whose length is often many thousand times greater than its diameter. Although the principles of pathological changes are similar in muscle to other tissues of the body, its unusual cellular structure and its contractile nature give rise to a constellation of pathological changes that need to be considered separately. The general system of nomenclature of rodent skeletal muscle fiber types is based on the adenosine triphosphatase (ATPase) reaction (12Brooke and Kaiser 1970; 13Brooke et al. 1971). When this reaction is performed at pH 9.4, type I or slow twitch fibers show low myofibrillar ATPase activity. Type II or fast twitch fibers show greater ATPase reactivity and can be divided into type IIA, IIB and IIC on the basis of inhibition of the reaction at lower values of pH. Thus, in the rat type IIA fibers are inhibited at pH 4.5, type IIB at pH 4.3 and type IIC at pH 3.9. Immunocytochemical techniques based on the fast and slow isoforms of myosin have also been used and correlate reasonably well with fiber type analysis from the standard ATPase method and are applicable to fixed material (6Behan et al. 2002; 120Westwood et al. 2005).
This heterogeneity as well as the fact that muscle represents nearly 40% of body mass has to be considered in the selection of muscle sampling for histopathological examination. Over recent years study of the muscle damage produced in rodents by lipid regulating drugs notably the fibrates and statins have shown that muscle fiber types vary in their sensitivity to xenobiotics (97Schaefer et al. 2004; 120Westwood et al. 2005; 26De Souza et al. 2006; 84Okada et al. 2007; 37Faiola et al. 2008; 122Westwood et al. 2008; 85Okada et al. 2009). It is important therefore to include both type 1 and type II fibers for histological assessment. For example the soleus represents a type I-predominant skeletal muscle whereas type II-predominant skeletal muscles are represented by the quadriceps and extensor digitorum longus.
• Infiltrate, inflammatory cell
• Necrosis
• Inflammation
• Hypertrophy
• Atrophy
• Degeneration
• Vacuolation
• Mineralization
Mesothelium
• Hyperplasia
Neoplastic proliferative lesions
One diagnostic challenge in the assessment of soft tissue pathology is making a distinction between reactive, but self-limiting conditions and neoplastic lesions of mesenchymal cells. Soft tissue tumors are classified according to the adult tissue they resemble. However, they most probably develop from pluripotential mesenchymal cells that are able to differentiate in one or more different directions. For this reason a close histogenetic relationship exists between soft tissue neoplasms and bone tumors (54Hajdu 1986). It explains why mixed differentiation patterns occur among soft tissue sarcomas such as reported in some transgenic mouse models (41Floyd et al. 2002).
Mesenchymal tumors are relatively uncommon spontaneous lesions in conventional strains of rats and mice, although their incidence in aged untreated animals may vary from less than 1% to over 5% (49Greaves and Faccini 1981; 91Poteracki and Walsh 1998; 32Eiben 2001; 53Haines et al. 2001; 28Dinse et al. 2010). The most common malignant phenotype that develops spontaneously is usually fibrosarcoma or its pleomorphic variant (malignant fibrous histiocytoma).
Sarcomas can also be induced in rodents by injection of oncogenic viruses and carcinogens such as 7,12-dimethylbenz[a]anthracene as well as repeated injection of a diverse range of non-genotoxic chemicals (19Chesterman et al. 1966; 44Grasso and Goldberg 1966a, 45b; 58Hooson et al. 1973; 107Taguchi et al. 2006). Implantation into rodents of impervious solid foreign bodies made from a wide variety of materials also induces sarcomas in the surrounding tissues. Materials include polystyrene, cellophane, polyethylene, polyurethane, polyvinylchloride, polymethylmethacrylate, silicone, glass, aluminium oxide and metals such as stainless steel, gold, platinum, titanium, nickel, chromium (88Oppenheimer et al. 1964; 11Brand et al. 1976; 63Kirkpatrick et al. 2000). Implantation of spent uranium fragments also induces sarcomas (52Hahn et al. 2002). There are species and strain differences in the sensitivity of rodents to the induction of sarcomas by foreign bodies, the heterozygous transgenic p53+/− being particularly sensitive (8Blanchard et al. 1999). Again the main induced type of neoplasm is fibrosarcoma or its pleomorphic variant although other types are also reported. Sarcomas developing around implants appear to be very rare in humans (80Morgan and Elcock 1995; 61Keegan et al. 2008).
Most of these rodent neoplasms can be diagnosed by the use of routine hematoxylin and eosin stained sections, although immunocytochemistry can be used to characterize cell types more accurately. In the diagnosis of human soft tissue sarcomas, immunocytochemistry now has an important place. It has been shown that this can assist in the sub-categorization of pleomorphic sarcomas and the assessment of prognosis and proper planning of therapy (21Coindre et al. 2001; 111Tos 2006). However, in rodents the diversity of tumor types is far more limited. A recent study of a large number of fibrosarcomas and liposarcomas induced in different studies by peroxisome proliferator-activated receptor agonists in rats immunohistochemistry was relatively unhelpful in the characterization and grouping of these tumors for the purpose of safety assessment (55Hardisty et al. 2007).
• Fibroma
• Fibrosarcoma
• Fibrosarcoma, pleomorphic
• Lipoma
• Hibernoma
• Liposarcoma
• Rhabdomyoma: There is no fully substantiated report of rhabdomyoma occurring in laboratory rats or mice.
• Rhabdomyosarcoma
• Leiomyoma
• Leiomyosarcoma
• Mesenchymoma, malignant
• Sarcoma, NOS (Not otherwise specified)
• Mesothelioma, malignant
Infiltrate, inflammatory cell (N): Soft Tissue
Species: Mouse, Rat
Synonym: Aggregate
Modifiers: Mononuclear cell, Lymphocyte, Neutrophil, Eosinophil, Plasma cell, Polymorphonuclear cell, Mixed cell.
Pathogenesis: May be a normal physiological appearance.
Diagnostic features
• Small foci of mononuclear cells, lymphocytes, plasma cells or polymorphonuclear cells.
• No evidence of tissue damage.
Comment: These descriptive terms are applied to localized accumulations of lymphocytic, mononuclear or polymorphonuclear (neutrophils or eosinophils) cells that do not constitute evidence of a significant inflammatory process. They may be considered within physiological limits.
Inflammation (N) : Soft Tissue
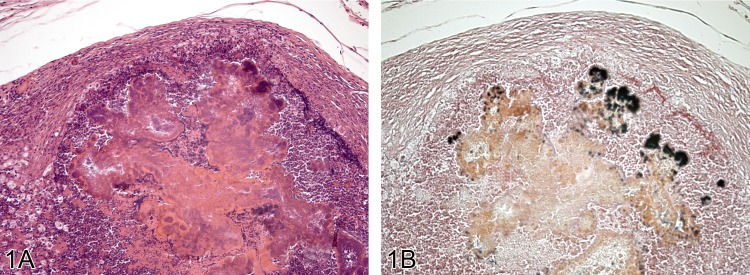
Figure 1.
A) Lesion from the face of a Wistar rats with marked inflammation and abscess formation and development of a fibrous capsule (H&E). The abscess contains much cellular debris including gram positive bacteria seen in B) (Gram stain).
Figure 2.
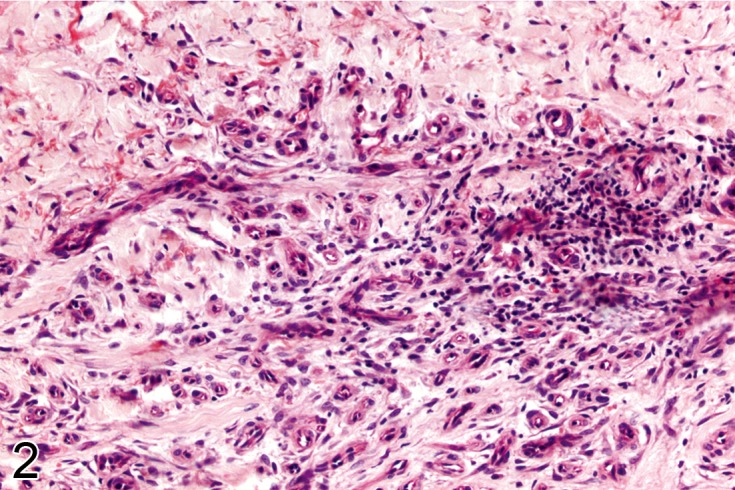
Other examples of chronic inflammation in connective tissue. In A) there is a dense chronic inflammatory infiltrate composed of mononuclear cells which occurred in response to injected pigment seen here as black fragments. In B) the tissues show no necrosis but contain a sparse scattering of chronic inflammatory cells with a vascular proliferative response (H&E).
Figure 3.
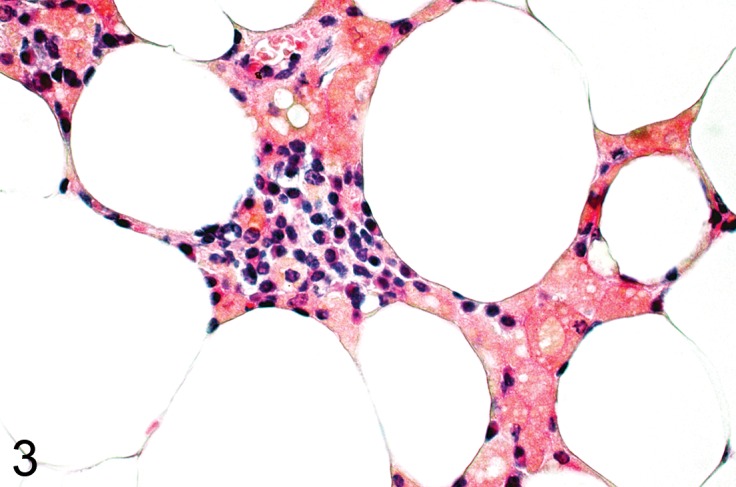
Mesenteric fat from a C67Bl mouse fed a high fat diet. There is a granulomatous inflammatory process in the fat with clusters of macrophages with lipid vacuoles and brown lipofuscin pigment (H&E).
Species: Mouse, Rat
Synonyms: Abscess, Granulation tissue, Granuloma
Modifiers: Neutrophil (purulent), Lymphocyte (non-purulent), Eosinophil, Plasma cell, Mononuclear cell, Mixed cell, Granulomatous.
Pathogenesis: Non-specific response to injury.
Diagnostic features
• Infiltration of leukocytes into the extracellular matrix.
• Often accompanied by edema and intravascular accumulation of leukocytes.
• Can be associated with prominent in-growth of fibroblasts and blood vessels (granulation tissue) typically in 2-3 days after injury.
Granuloma
• Represents a focal form of inflammation characterized by aggregates of histiocytes often with epithelioid appearance seen in isolation.
• May have a central zone of necrosis.
• Sparse infiltrate of other cells such mononuclear cells, polymorphonuclear leukocytes, blood vessels and fibroblasts.
Abscess
• Neutrophils accumulate focally and show central cell lysis with accumulation of cellular debris.
• Fibrous capsule formation
• Modifiers can be used to describe the most prominent character of the inflammatory process:
Neutrophil (purulent)
• Infiltration of neutrophils predominates.
Lymphocyte (non-purulent)
• Infiltration of lymphocytes and macrophages (mononuclear leukocytes) predominates.
• Plasma cells may be prominent.
Eosinophil
• Infiltration of eosinophilic granulocytes predominates.
Granulomatous
• An inflammatory process involving a significant proportion of macrophages and other mononuclear cells.
• Macrophages might adopt an epithelioid phenotype or form foreign body type giant cells (syncytia).
• There may be a central zone of necrosis.
• It may be surrounded by granulation tissue, blood vessels and fibroblasts.
• May be surrounded by a fibrous capsule
Differential diagnoses
Fibrosis and fibroplasia
• A very limited number of leukocytes, predominately mononuclear cells, extracellular collagen matrix not associated with active inflammation or a predominantly fibroblastic response.
Fibroma:
• Dense, poorly cellular fibrous mass of well-differentiated fibroblasts, compressing adjacent tissue; interwoven bands of mature collagen; rare mitotic figures; very little if any leukocyte infiltration.
Sarcoma, histiocytic:
• Comprised of fairly uniform but large histiocytic cells with vesicular nuclei and may show mitotic activity.
Comment: It should be underlined that the term 'inflammation' should be limited to those instances where there is evidence of a pathological inflammatory process. Descriptive terms such as lymphocyte or neutrophil aggregates or infiltration should be applied to localized accumulations of lymphocytic, monocytic or polymorphonuclear cells that may be considered within physiological limits (see Infiltrate, inflammatory cell).
Localized inflammatory processes in connective tissue usually develop as a consequence of local trauma, ulceration of the overlying epithelium, infarction, injection or implantation of foreign materials into soft tissues or their extravasation from blood vessels. Systemic effects of xenobiotics may also occasionally result in damage to the soft tissue without a local inciting cause being evident in the sections. Histological features may be modified by the nature and duration of the damage and type of tissue involved.
Initially, tissue damage or necrosis is accompanied by variable amounts of hemorrhage and edema and acute inflammatory cells, followed by mononuclear leukocytes. Within two or three days there is proliferation of fibroblasts and angiogenesis which is the basis for 'granulation tissue'. Subsequently, fibroblasts synthesize significant amounts of collagen usually reaching a peak by about the third week (fibroplasia). Remodeling of collagen ultimately occurs to produce mature scar tissue possessing high tensile strength (fibrosis).
If the inflammatory process is prolonged, chronic inflammation characterized predominantly by the presence of lymphocytes, plasma cells and macrophages develops. A fibroblastic response may also be prominent. Activated fibroblasts acquire -smooth muscle cell-like phenotype, often referred to as myofibroblasts. These not only synthesize and deposit extracellular matrix but also have contractile properties mediated by -smooth muscle actin organized in bundles of microfilament (57Hinz 2010).
A foreign body reaction with giant cells, abscess or cyst formation may also develop. Mineral can also be deposited at the site of injured tissue (dystrophic mineralization). This is characterized by fine or coarse granular deposits staining intensely with hematoxylin or positively with the von Kossa stain. Iron pigments (hemosiderin) may also be found particularly in macrophages when hemorrhage has occurred.
A granuloma represents a localized form of an inflammatory reaction in which the cells are similar to those in the reparative phase of the inflammatory process but in which the histiocytic component is predominant. The term granulomatous inflammation is used when there is a predominance of histiocytes and macrophages often showing epithelioid features or foreign body giant cell formation. They form as a local reaction to foreign materials but also more widely in soft tissue in response to infectious agents or as an immunological granulomatous inflammatory reaction. They may also develop as an expression of altered function of cells of the monocyte/macrophage series (121Westwood et al. 1995).
Whilst the basic inflammatory response is common to all forms of tissue injury, different injected or implanted materiel may modify certain histological features. For instance, smooth surfaced biomaterials may produce smaller fibrous capsules that textured implants (123Williams 1987; 90Picha et al. 1990). Pure aluminium metal implants are reported to induce a narrow zone of necrosis, copper a more vascular response and cobalt is associated with significant lymphoid infiltrates (78McNamara and Williams 1981).
Fibrosis (N): Soft Tissue
Species: Mouse, Rat
Synonyms: Scar formation, Sclerosis
Pathogenesis: Repair process after local tissue degeneration.
Diagnostic features
• Focal to focally extensive increase in extracellular collagen.
• Low number of fibroblasts.
• Collagen fibers are mostly arranged in long streams.
• Collagen fibers replace specialized normal cutaneous adnexae.
Differential diagnoses
Fibroma
• Dense, poorly cellular fibrous mass of well-differentiated fibroblasts, compressing adjacent tissue; interwoven bands of mature collagen; rare mitotic figures; very little if any leukocyte infiltration.
Fibrosarcoma
• Very cellular, composed of abnormal pleomorphic fibroblastic cells showing high mitotic activity.
Inflammation
• Infiltration of leukocytes predominates.
fibroplasia
• Cellular, containing numerous active fibroblasts which although may be plump, show no atypical features.
Comment: Fibrosis is usually but not always the end stage of a chronic inflammatory process. In contrast to active inflammatory processes or neoplasia it is usually poorly cellular.
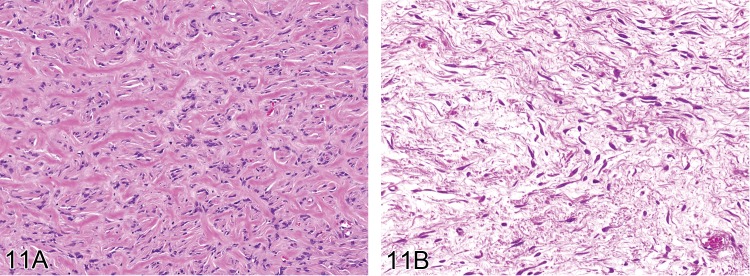
Fibroplasia (N): Soft Tissue
Species: Mouse, Rat
Pathogenesis: Active repair process after local tissue damage.
Diagnostic features
• Variable increase in extracellular stroma.
• High number of proliferating, active plump but normal fibroblasts.
Differential diagnoses
Fibrosis:
• Poorly cellular fibrous tissue containing few fibroblasts; very little if any leukocyte infiltration.
Fibroma:
• Dense, poorly cellular irregular fibrous mass of well-differentiated fibroblasts, compressing adjacent tissue; interwoven bands of mature collagen; rare mitotic figures; very little if any leukocyte infiltration.
Fibrosarcoma
• Very cellular, composed of abnormal pleomorphic fibroblastic cells showing high mitotic activity.
Inflammation:
• Infiltration of leukocytes predominates.
Comment: The term fibroplasia is used to denote the presence of abundant active, fibroblasts. A fibroblastic response without significant inflammation has been reported in the adipose tissues of rodents treated with peroxisome proliferator-activated receptor agonists (55Hardisty et al. 2007; 117Waites et al. 2007).
The differential diagnosis between an exaggerated fibroblastic response to tissue injury and a mesenchymal tumor (fibroma or fibrosarcoma) is usually made on the basis of size and growth pattern. Neoplasms are usually large and show evidence of nodularity with compression and infiltration of local tissues and adjacent organs. Careful studies of the biomaterial-induced sarcomas have shown a spectrum of change from non-neoplastic fibroblastic proliferation to incipient sarcoma. These premalignant zones are characterised by cellular and nuclear pleomorphism, hyperchromasia and prominent nucleoli that can be more easily identified by use of immunocytochemistry for proliferating cell nuclear antigen (63Kirkpatrick et al. 2000).
Necrosis (N): Soft Tissue
Species: Mouse, Rat
Pathogenesis: Cell death.
Diagnostic features
• Loss of cellular detail and loss of nuclei (coagulation necrosis).
• Replacement by cellular debris (lytic necrosis).
• Often associated with edema, hemorrhage, vascular congestion, infiltration of leukocytes, accumulation of fibrin, formation of granulation tissue, cyst formation, mineralization and deposition of iron pigment (hemosiderin).
Differential diagnoses
Inflammation:
• Infiltration of leukocytes predominates.
Mineralization (N): Soft Tissue
Species: Mouse, Rat
Synonym: Calcification
Pathogenesis: Local injury (dystrophic mineralization), or generalized mineral imbalance (metastatic mineralization).
Diagnostic features
• Intracellular and/or extracellular deposition of fine or coarse irregularly shaped granules staining intensely basophilic with hematoxylin.
• May be associated with infiltration by macrophages (including giant cells), lymphocytes and fibroblasts.
• Stains with histochemical reactions for minerals (e.g. calcium).
• Is mostly associated with other signs of injury (e.g. necrosis).
Differential diagnoses
Osseous Metaplasia:
• Bone tissue may be diagnosed histologically.
Comment: Mineral deposits can occur in the soft tissues (particularly the subcutis) of rodents, either following local tissue injury (dystrophic mineralization) or under circumstances that favour generalized mineralization. These include high dietary calcium:phosphate ratio, treatments that mobilize body calcium stores or raise blood calcium levels (metastatic mineralization) and chronic renal failure. When calcium balance is disturbed, soft tissues most commonly affected in the rodents appear to be natural localised trauma sites such as around the limbs and in breeding females' mammary tissues.
Metaplasia (N): Soft Tissue
Species: Mouse, Rat
Modifiers: Osseous, Cartilaginous type
Diagnostic features
• Cartilage or bone structure, abnormally located within collagenous connective tissue.
• Often surrounded by granulation tissue.
Osseous
• Osteoid, calcified bone tissue and even bone marrow.
Cartilaginous type
• Cartilage
Differential diagnoses
Mineralization
• Intracellular or extracellular deposits of mineral, no cartilage or bone formation.
Comment: Osseous metaplasia is a well-recognized phenomenon occurring in tissues affected by a variety of pathologic processes notably ischemia, hematoma and chronic inflammation, various degenerative and reparative changes as well as within neoplasms (68Liu et al. 2007).
Amyloid (N): Soft Tissue
Species: Mouse, Rat
Pathogenesis: Accumulation of an insoluble protein with β-pleated sheet secondary structure.
Diagnostic features
• Extracellular deposition of pale, amorphous, eosinophilic, hyaline material.
• Compression of adjacent tissue may be present.
• Can be associated with edema.
Special techniques for diagnosis
• Shows reddish metachromasia with crystal violet. Congo red stains amyloid orange-red and shows apple-green dichroism in polarizing light.
Differential diagnoses
Necrosis with deposition of fibrin
• Congo red staining is negative.
Deposition of other proteinacous material: Hyaline, Mature collagen
• Congo red staining is negative.
Comment: In mice, amyloid deposits only occur when there is significant systemic amyloid (36Faccini et al. 1990). In general, amyloidosis appears to be more common in aging CD-1 mice, compared with other conventional strains (72Majeed 1993).
Infiltrate, inflammatory cell (N): Adipose Tissue
Species: Mouse, Rat
Synonym: Aggregate
Modifiers: Mononuclear cell, Lymphocyte, Neutrophil, Eosinophil, Plasma cell, Polymorphonuclea cell, Mixed cell.
Pathogenesis: May be a normal physiological appearance.
Diagnostic features
• Small foci of mononuclear cells, lymphocytes, plasma cells or neutrophils.
• No evidence of tissue damage.
Comment: These descriptive terms are applied to localized accumulations of lymphocytic, mononcytic or polymorphonuclear cells that do not constitute evidence of a significant inflammatory process. They may be considered within physiological limits.
Inflammation (N): Adipose Tissue
Species: Mouse, Rat
Synonyms: Abscess, Granulation tissue, Granuloma.
Modifiers: Neutrophil (purulent), Lymphocyte (non-purulent), Eosinophil, Plasma cell, Mononuclear cell, Mixed cell, Granulomatous.
Pathogenesis: Non-specific response to injury.
Diagnostic features
• Infiltration of leukocytes among fat cells and extracellular matrix.
• Often accompanied by edema and intravascular accumulation of leukocytes.
• Can be associated with prominent in-growth of fibroblasts and blood vessels (granulation tissue) in 2-3 days after injury.
Comment: As in other tissue adipose tissue may be affected by a typical inflammatory process which shows similar histological features (see soft tissue Inflammation).
Inflammation, lipogranulomatous (N): Adipose Tissue
(Figure 3)
Species: Mouse, Rat
Synonyms: Steatitis, Obesity-associated inflammation
Pathogenesis: Response of adipose tissue to injury.
Diagnostic features
• Gross pathology: widespread small white or yellowish foci in adipose tissue.
• Clusters of macrophages often with small lipid vacuoles in the cytoplasm (foam cells).
• Cholesterol clefts, surrounded by giant cells may occur.
• Proliferation of blood vessels and connective tissue may occur.
Special techniques for diagnosis
• Presence of lipofuscin droplets (demonstrated by their autofluorescence): Lipofuscin is acid fast and shows yellow autofluorescence in fluorescent light.
Differential diagnoses
Liposarcoma:
• Shows histological features of malignancy such as pleomorphic spindle cells, mitotic activity, necrosis and the presence of lipoblasts.
Comment: Inflammatory processes may affect adipose tissue in a similar manner to the other soft tissues (see Inflammation). In addition, some more specific forms of inflammation may be seen. Obesity in rodents as well as humans has been associated with a widespread focal form of inflammation in the adipose tissue, characterized by the presence of macrophages (118Weisberg et al. 2003; 119Wellen and Hotamisligil 2003; 125Xu et al. 2003). Another form of generalized fat damage affecting adipose tissue in the rat termed 'steatitis' is believed to be the result of accumulation of pigment as a result of phagocytosis of blood-borne or interstitial reactive lipid molecules. It appears to develop as a result of vitamin E or antioxidant deficiency. This may develop as a consequence of excess dietary polyunsaturated fatty acids with a least three double bonds such as found in fish or linseed oil (25Danse 1989).
Focal damage to fat cells occurs under a variety of circumstances. Histologically, evidence of necrosis may be lacking but there is often a sparse accumulation of inflammatory cells including macrophages and giant cells. Clefts representing dissolved rhomboidal cholesterol crystals may also be seen.
Necrosis (N): Adipose Tissue
(Figure 4)
Figure 4.
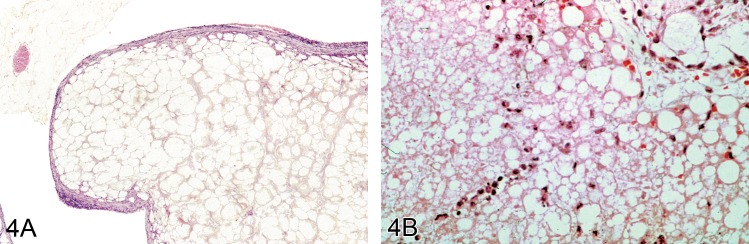
A) Mesenteric fat from a B6C3F1 mouse showing a large nodule of fat necrosis in which the acellular necrotic fat cells can be seen surrounded by a thin rim of poorly cellular connective tissues (H&E). Image by courtesy of the National Toxicology Program. B) Subcutaneous tissue from a Wistar rat treated with a novel drug that produced fat necrosis. This shows acute damage.
Species: Mouse, Rat
Synonym: Fat necrosis
Pathogenesis: Cell death.
Diagnostic features
• Loss of cellular detail and ghost-like appearance of fat cells.
• May be associated with edema, hemorrhage, vascular congestion, infiltration of leukocytes, accumulation of fibrin, formation of granulation tissue, cyst formation, fibrosis and deposition of iron pigment (hemosiderin) or calcium.
Differential diagnoses
Inflammation
• Infiltration of leukocytes predominates.
Atrophy (N): Adipose Tissue
Species: Mouse, Rat
Synonyms: Lipoatrophy, Fat atrophy
Modifiers: Serous, Mucoid
Diagnostic features
• Loss or reduction in size of fat cells.
• May be increase in connective tissue or fibrous septa.
• May show extracellular glycosaminoglycan (mucopolysaccharides)
Differential diagnoses
Lipoma
• Reduction in size of lipocytes is localised within the neoplasm and there is expansion and compression of surrounding tissues.
Comment: Loss of adipose tissue normally occurs under conditions where there is reduced caloric intake or increased energy expenditure. Other forms of atrophy may also occur. Loss of fat (lipoatrophy) in face, limbs, and abdominal subcutaneous region has been reported in people infected with the human immunodeficiency virus who are receiving highly active antiretroviral therapy (40Flint et al. 2009; 101Sevastianova et al. 2011). Analogous findings are reported in rodents (92Prot et al. 2006). Localized fat atrophy may also be related to injected drugs such as insulin and corticosteroids as well as recurrent pressure and inflammation of adipose tissue (42Garg 2004; 66Larade et al. 2008). In cachectic states pale staining amorphous extracellular glycosaminoglycan may become visible in atrophic adipose tissue and this can be termed serous atrophy (81Munfus and Menke 2009).
Hyperplasia, adipose tissue (H): Adipose Tissue
Species: Mouse, Rat
Synonym: Polymorphic adipose tissue
Diagnostic features
• Diffuse (or focal) increase of adipose tissue with macrovesiculation (white adipose tissue) or microvesiculation (brown adipose tissue).
• Can be associated with variable focal fibrosis or fibroblastic proliferation (fibroplasia).
• No compression of surrounding tissue.
Differential diagnoses
Lipoma
• Discrete rounded nodules of adipose tissue showing compression of the surrounding stroma.
Liposarcoma
• Shows histological features of malignancy such as pleomorphic spindle cells, mitotic activity, necrosis and the presence of lipoblasts.
Comment: Hyperplasia of adipose tissue has been described in rodents following administration of peroxisome proliferator-activated receptor agonists and has been attributed to a receptor-mediated pharmacodynamic process (55Hardisty et al. 2007; 117Waites et al. 2007).
Hyperplasia, smooth muscle (H): Soft Tissue
Species: Mouse, Rat
Diagnostic features
• Poorly circumscribed areas or bundles of irregular or parallel smooth muscle fibers.
• Well-differentiated smooth muscle cells with eosinophilic cytoplasm, distinct cell boundaries and cylindrical, blunt-ended or cigar-shaped nuclei.
• Longitudinal myofibrils can be demonstrated in the cytoplasm using the phosphotungstic acid-hematoxylin (PTAH) stain.
Differential diagnoses
Leiomyoma
• Is composed of interlacing bundles and whorls of smooth muscle cells showing a nodular growth pattern and compression of surrounding tissues.
Comment: Hyperplasia of smooth muscle is typically reported in the walls of the uterus, mesovarium, gastrointestinal tract or blood vessels in rodents rather than in soft tissues per se (43Gopinath and Gibson 1987;89 Parrott et al. 2001).
Infiltrate, inflammatory cell (N): Skeletal Muscle
Species: Mouse, Rat
Synonym: Aggregate
Modifiers: Mononuclear cell, Lymphocyte, Neutrophil, Eosinophil, Plasma cell, Polymorphonuclear cell, Mixed cell
Pathogenesis: May be a normal physiological appearance.
Diagnostic features
• Small foci of mononuclear cells, lymphocytes, plasma cells or neutrophils.
• No evidence of tissue damage.
Comment: These descriptive terms are applied to localized accumulations of mononuclear cells, polymorphonuclear cells, lymphocytes, plasma cells or mixed infiltrates that do not constitute evidence of a significant inflammatory process. They may be considered within physiological limits.
Necrosis (N)
(Figures 5 and 9): Skeletal Muscle
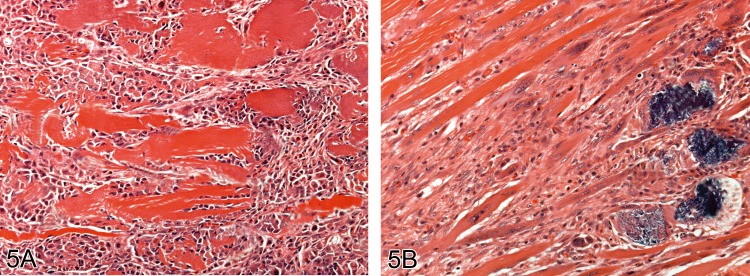
Figure 5.
Muscle showing the stereotypical necrosis and repair response four A) and 10 days B) following a single injection of the local anesthetic lignocaine. Mineralisation of muscle fibers is apparent at 10 days (H&E).
Figure 9.
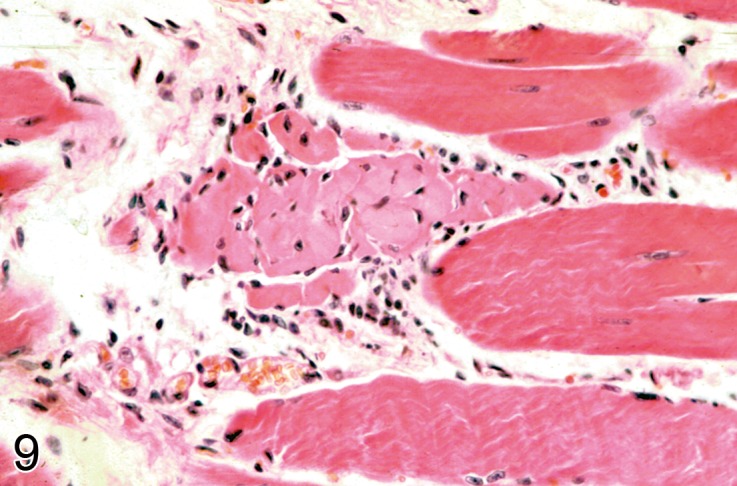
Spontaneous focal muscle fiber necrosis in a 2 year old C57Bl mouse. An intact muscle fiber is seen adjacent to a necrotic fiber with an early cellular response (H&E).
Species: Mouse, Rat
Pathogenesis: Response to injury.
Diagnostic features
• Necrosis, edema and hemorrhage.
• Rounded, hyalinized fibers with pyknotic nuclei (first few hours),.
• Loss of cross striations.
• Fragmentation of muscle fibers (24 hours).
• Polymorphonuclear leukocyte infiltrate (peak at 1 to 2 days).
• Infiltration of macrophages and proliferation of myoblasts, may form myotubules with long chains of nuclei (day 3).
• Regeneration of basophilic muscle fibers (day 5).
• Repair complete (3 weeks).
Differential diagnoses
Myopathy (see Degeneration, Vacuolation)
• Vacuolation of fibers or basophilic droplets or targetoid fibers with no or very little inflammation.
Comment: The characteristic process of muscle degeneration which leads to necrosis, inflammation and repair is observed following the intramuscular injection of myotoxic agents. Small amounts of localised fiber necrosis may even occur following injection of innocuous substances including saline (109Thuilliez et al. 2009).
In the first few hours following injection fibers become rounded and hyalinized with pyknotic nuclei. Myofibers often exhibit focal or segmental necrosis because of their length and multiple nuclei. Necrotic fibers are surrounded by variable degrees of hemorrhage and edema. Within 24 hours affected fibers become overtly fragmented and macrophages can be seen. By day three, lesions are composed of numerous infiltrating macrophages accompanied by proliferating myoblasts which may form myotubules with long chains of nuclei. After five days there are numerous regenerating muscle fibers characterized by basophilic cytoplasm and vesicular nuclei and prominent nucleoli.
Satellite cells around intact muscle fibers in the region of necrotic muscle may also show activation. Affected satellite cells show increased cytoplasmic volume and mitotic activity and are separated from skeletal muscle fibers by an unusually wide space which may contain prominent basal lamina.
Finally, regenerating fibers subsequently enlarge and by three weeks after injury, the muscle tissue becomes essentially normal although fibers may retain some central nuclei (74Manor and Sadeh 1989). Although regeneration following local damage is surprisingly complete, muscle fibers may not regenerate after severe, extensive or repeated damage and as a consequence fibrous scarring may follow.
Skeletal muscle degeneration and necrosis can occur after systemic exposure to a variety of xenobiotic and different fiber types may be affected (46Greaves 2012). Type II or fast twitch fibers appear to be the most sensitive to inhibitors of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase (statins) (120Westwood et al. 2005) whereas type I fibers appear to be generally more sensitive to the adverse effects of peroxisome proliferator–activated receptor (PPAR) agonists (26De Souza et al. 2006; 37Faiola et al. 2008).
Inflammation (N): Skeletal Muscle
(Figure 5)
Species: Mouse, Rat
Synonyms: Abscess, Granulation tissue, Granuloma
Modifiers: Neutrophil (purulent), Lymphocyte (non-purulent), Eosinophil, Plasma cell, Mononuclear cell, Mixed cell, Granulomatous.
Pathogenesis: Non-specific response to injury.
Diagnostic features
• Infiltration of leukocytes among muscle fibers and extracellular matrix.
• Often accompanied by edema and intravascular accumulation of leukocytes.
• Can be associated with prominent in-growth of fibroblasts and blood vessels (granulation tissue) in 2-3 days after injury.
Comment: As in other tissue skeletal muscle may be affected by a primary inflammatory process associated with little or no overt necrosis.
Hypertrophy (N): Skeletal Muscle
Species: Mouse, Rat
Diagnostic features
• Enlargement of muscle fibers.
Comment: Hypertrophy of muscle fibers may be focal or diffuse. Focal compensatory hypertrophy may occur in muscle showing atrophic alterations. Diffuse hypertrophy can develop in response to increased exercise or as a response to prolonged excessive growth factors such as growth hormone or growth-promoting xenobiotics (93Prysor-Jones and Jenkins 1980; 76McClung et al. 2005). Growth hormone has been shown to affect mainly type I fibers. Hypertrophy is characterized histologically by an increase in muscle fiber diameter but this may be difficult to assess histologically without morphometric analysis.
Atrophy (N): Skeletal Muscle
Species: Mouse, Rat
Synonyms: Denervation atrophy, disuse atrophy, nutritional atrophy, age-related atrophy.
Diagnostic features
• Decreased muscle fiber size, presence of angulated fibers.
• Replacement by adipose tissue.
Differential diagnoses
Myopathy (see Degeneration, Vacuolation):
• Vacuolation, targetoid fibers or hyaline change.
Comment: Atrophy of skeletal muscle may result from inactivity or cachexia as well as primary degenerative processes originating in muscle fibers. It also occurs secondary to denervation (denervation atrophy). These types of atrophy can be induced experimentally in rodents. Moreover, atrophy of skeletal muscle occurs spontaneously in rats in advancing age although whether the changes result from primary muscle degeneration or changes in the nerve supply is disputed.
The nature of an individual muscle fiber is determined by its innervation and as a consequence all fibers supplied by an individual neuron are of the same type. The distribution of fiber type varies between muscles. For instance the soleus muscle in the F344 rat comprises over 80% of type I fibers whereas the extensor digitorum longus contains mainly type II fibers (31Eddinger et al. 1985). Normally, different fibers are intermingled randomly within a muscle. Consequently if a neurone supplying a muscle motor unit dies, the muscle fibers supplied by that unit undergo atrophy and take on a characteristic histological feature of denervation atrophy that of a compressed angular profile when seen in cross section.
Ultimately, surviving intramuscular axons form collateral branches which connect with denervated fibers which then display histochemical characteristics of the type determined by the new innervations (6Behan et al. 2002). A characteristic grouping of the fibers into homogeneous groups of fiber type rather than the mosaic pattern of normal muscle develops following re-innervation (13Brooke et al. 1971). Furthermore, if a neuron supplying the enlarged motor unit degenerates, atrophic fibers are more likely to be grouped together and surviving fibers may undergo compensatory hypertrophy to give a characteristic histological pattern of both atrophy and hypertrophy.
Atrophy from non-neurogenic causes such as those induced by the administration of myotoxic xenobiotics is usually characterized by the lack of angulated fibers and the presence of myopathic changes such as necrotic or rounded hyaline fibers, centrally nucleated cells and divided or so called 'split fibers' (see Degeneration). The muscle atrophy and increased protein catabolism that follows chronic administration of corticosteroids in the rat is characterized by uncomplicated reduction in the size of type II fast twitch fibers (70Livingstone et al. 1981). This is most readily observed in type II fiber-rich muscles such as the biceps femoris.
In aging rats, atrophy of muscle fiber affects predominantly the hind limbs. There is atrophy of muscle fibers, increased variation in muscle fiber size and accumulation of degenerative inclusion bodies, lipofuscin and lipid droplets and increased connective tissue (35Everitt et al. 1985). The presence of angular fibers suggests that the changes may result from spinal degeneration.
Degeneration (N)
(Figures 6, 7, 8, 9): Skeletal Muscle
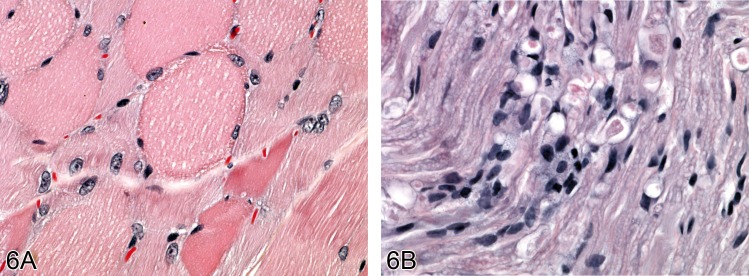
Figure 6.
In A) there is muscle degeneration due to spinal nerve compromise in an aged rat. B) shows section of spinal nerve showing degenerative alterations to nerve fibres (H&E).
Figure 7.
Skeletal muscle from thigh of a Sprague Dawley rat treated for 3 months with a peroxisomal proliferator (WY-14643) showing a degenerative form of myopathy. In A) there focal inflammation associated with muscle fiber necrosis characterised by cytoplasmic eosinophilia and loss of cytoplasmic structure. In B) typical reparative response is seen with numerous central nuclei being visible in fibers that are variable in thickness (H&E). Images by courtesy of the National Toxicology Program.
Figure 8.
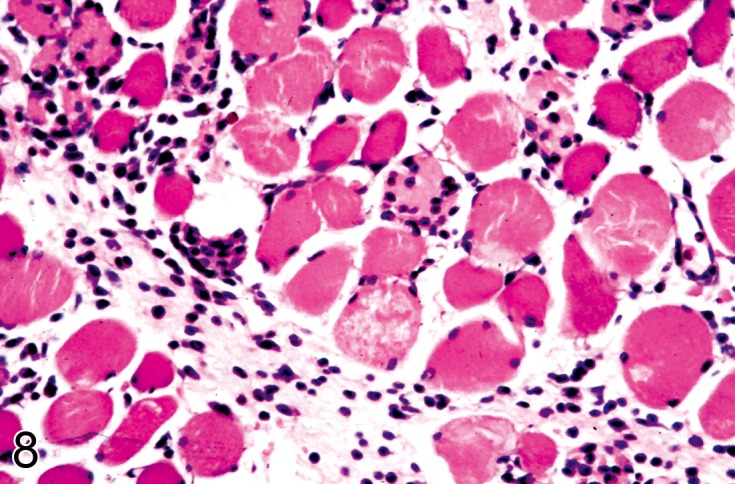
Quadriceps muscle from a Wistar rat treated with a novel therapeutic agent drug that produce mild muscle degeneration associated with a chronic inflammatory infiltrate seen in this view (H&E).
Species: Mouse, Rat
Synonyms: Myopathy, Myopathic changes, Cytopathic changes
Diagnostic features
• Vacuolation of fibers.
• Basophilic cytoplasmic droplets (phospholipid).
• Targetoid fibers.
• Split fibers.
• Hyaline changes.
Differential diagnoses
A variety of different pathological processes are embraced under this term. Clear distinction often requires special staining techniques to clarify the precise nature of the degenerative alterations. In view of the overlap between degeneration and other cytological changes that can be seen in skeletal muscle some prefer the more general term myopathy to embrace all such alterations including simple vacuolation (see Vacuolation).
Comment: Pathologic insults to muscle may lead to a variety of cytopathic changes in muscle fibers. The precise nature of the changes is dependent on the nature of the damage. Following systemic administration of agents which have a direct myotoxic effect such as 6-mercaptopurine, vincristine and emetine, muscle fibers exhibit rounding, vacuolation, hyalinization with loss of myofibrils, pale staining central zones (targetoids) and split fibers (59Jaweed et al. 1985).
Vacuolation (N): Skeletal Muscle
Species: Mouse, Rat
Synonyms: Myopathy, Myopathic changes, Cytopathic changes
Diagnostic features
• Fibers contain clear, pale or basophilic cytoplasmic vacuoles.
Comment: Round cytoplasmic basophilic bodies of up to 3µm in diameter composed ultrastructurally of laminated membranous material (spheromembranous bodies) have been described in the muscles of rats treated with agents such as vincristine, colchicine and chloroquine (103Slotwiner et al. 1966; 20Clarke et al. 1972; 100Seiden 1973; 10Bradley et al. 1976).
Some agents inducing systemic phospholipidosis produce vacuolation of skeletal muscle cells. Muscle vacuoles usually appear empty in routinely processed sections stained with hematoxylin and eosin but in toluidine blue stained plastic embedded sections they stain dark blue. Ultrastructural examination shows that they are composed of lamellated membranous inclusions associated with lysosomes. Glycogen, neutral lipid may also be present in vacuoles in skeletal muscle. Swollen or giant mitochondria may also appear as pale vacuoles in conventional histological sections.
Mineralization (N): Skeletal Muscle
Species: Mouse, Rat
Comment: Mineralization of dystrophic or metastatic type may be observed within skeletal muscle. It shows histological characteristic which are similar to mineral in other tissue (see Mineralization).
Hyperplasia (H): Mesothelium
Species: Mouse, Rat
Diagnostic features
• Usually localized but may be diffuse.
• Focal thickening or villous projections covered by relatively uniform cuboidal cells with little or no stratification, usually limited to 1-2 cells in thickness.
• Lacks evidence of significant mitotic activity.
• Little or no cellular atypia.
• May possess a small fibrovascular core or stalk.
• Fibrosis or inflammation may accompany the changes.
Differential diagnoses
Mesothelioma, malignant
• Is highly cellular and pleomorphic and shows extensive spread within the body cavities or infiltrates adjacent tissues.
Epithelioid Mesothelioma, malignant
• Exhibits poorly formed glandular structures or ill-formed glands.
Sarcomatoid mesothelioma, malignant
• Consists of spindle-shaped cells with elongated nuclei.
Comment: These are often small incidental lesions seen in the pleura, pericardium, peritoneum or tunica vaginalis (77McConnell et al. 1992).
In inhalation studies fiber-associated lesions most frequently are found at the parietal pleura.
Fibroma (B): Soft Tissue
(Figures 10 and 11)
Figure 10.
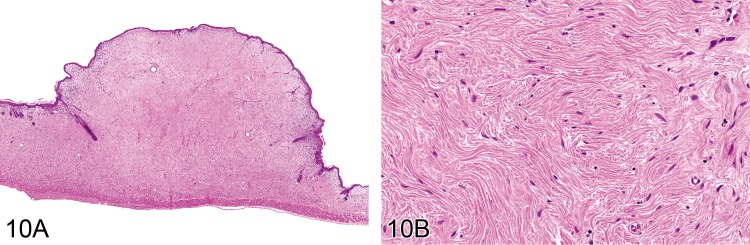
A) Subcutaneous fibroma from a mouse showing homogenous fibrous structure with overlying epidermis. B) Higher power view of fibroma seen in A) shows paucity of fibroblastic cells (H&E).
Figure 11.
Two examples of rat fibromas. A) poorly cellular tumour with abundant dense eosinophilic bands of collagen (H&E, bar = 100 µm). B) also poorly cellular but with a loose pale-staining myxoid stroma (H&E).
Species: Mouse, Rat
Synonym: Benign fibrous histiocytoma
Diagnostic features
• Dense, poorly cellular fibrous mass, compressing adjacent tissue.
• Interwoven bands of mature collagen.
• Interlacing bundles of well-differentiated fibroblasts.
• Fusiform cells with elongated, tapered, hyperchromatic or vesicular nuclei with one or more nucleoli.
• Rare mitotic figures.
• May have pale blue staining myxomatous areas and stellate cells.
• May show well-differentiated fibroblasts and collagen bundles showing infiltration of the musculature but without cytological features of malignancy.
Differential diagnoses
Fibrosis and fibroplasia
• No neoplastic features, associated with wounds, ulcers or inflammation.
Fibroadenoma of mammary glands
• Glandular elements within fibrous tumor tissue.
Fibrosarcoma
• Shows marked cellularity, cellular pleomorphism, increased mitotic activity, necrosis, tissue invasion and metastases.
Comment: Tumor cells can be arranged in a storiform or cartwheel pattern producing short thin bundles of collagen. This pattern was formerly classified as 'benign fibrous histiocytoma'. This term is now not usually used.
Fibromas are occasionally seen in untreated rats but they are rarer in untreated aged mice (9Boorman et al. 1989; 56Heider and Eustis 1994). In rats, a fibroma of desmoid type (also known as fibromatosis-type) has been described as postoperative lesion in Bhd gene mutant rats (65Kouchi et al. 2008). This form of fibroma may occasionally be seen in conventional strains. Fibromas have also been reported to develop in Wistar rats at the site of repeated injection of low doses of iron dextran solution (95Roe and Carter 1967). Myxomatous areas sometimes contain star-shaped fibroblasts (stellate cells).
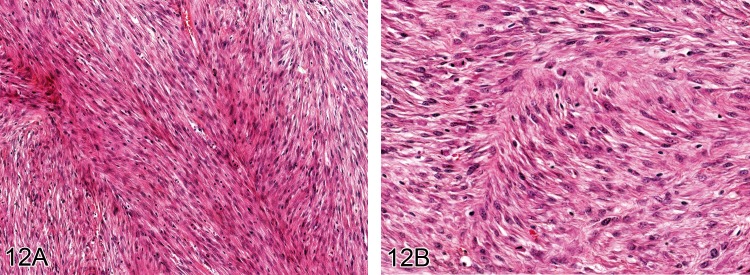
Fibrosarcoma (M): Soft Tissue
Figure 12.
A) A well differentiated fibrosarcoma from a rat. (H&E, bar = 100 µm). In B) the fairly cellular appearance composed of fine spindle cells exhibiting relatively little nuclear pleomorphism can be seen (H&E).
Species: Mouse, Rat
Diagnostic features
• Solid fibrous mass.
• Monomorphic interwoven fascicles of spindle cells with oval nuclei and basophilic cytoplasm.
• Cells often arranged in a 'herring-bone' pattern.
• Variable collagen content depending on the degree of differentiation.
• Extensive local invasion.
• Areas of hemorrhage and necrosis.
• May have metaplastic bone and cartilage (non-neoplastic).
• May have plentiful mitotic figures.
• Can predominantly consist of pale blue staining myxomatous areas with stellate cells.
Differential diagnoses
Fibroma and fibroplasia
• No cellular atypia, low mitotic rate.
Fibrosarcoma, pleomorphic
• Storiform pattern of uniform plump spindle cells to a highly pleomorphic pattern of bizarre spindle cells and tumour giant cells.
Liposarcoma (myxoid type)
• Fat forming cells within myxomatous tissue.
Comment: There is no sharp division between monomorphic fibrosarcoma and more pleomorphic variants. Along with the pleomorphic sarcoma these are the most common sarcoma type induced by injection of chemicals or implantation of solid materials (see pleomorphic fibrosarcoma below). Myxomatous areas sometimes contain star-shaped fibroblasts (stellate cells).The presence of neoplastic bone, cartilage, muscle or other differentiated features are diagnostic of other sarcoma types or mesenchymoma where there are two or more types of differentiation (see Sarcoma, NOS (Not otherwise specified), Fibrosarcoma, pleomorphic, Liposarcoma, Rhabdomyosarcoma, Leiomyosarcoma).
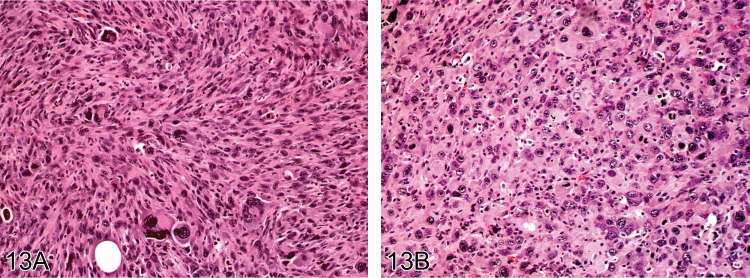
Fibrosarcoma, pleomorphic (M): Soft Tissue
Figure 13.
Pleomorphic fibrosarcoma induced by implanted foreign bodies in Sprague Dawley rats. A) and B) show the range of histological features of this type of neoplasm: spindle cells, plump cells, multinucleated cells and bizarre cells (H&E).
Species: Mouse, Rat
Synonym: Malignant fibrous histiocytoma.
Diagnostic features
• Solid fibrous mass.
• Variable histology ranging from storiform pattern of uniform plump spindle cells, small rounded cells, to a highly pleomorphic pattern of bizarre spindle cells and tumor giant cells.
• Abundant but variable amount of interstitial collagen.
• Ground substance variable but may show myxomatous zones.
• Widespread infiltration and invasion of local tissues.
• Areas with hemorrhage and necrosis.
• Variable mitotic activity.
Differential diagnoses
Fibroma and fibroplasia
• No cellular atypia, low mitotic rate.
Fibrosarcoma
• Monomorphic spindle cells
• Lacks storiform pattern and shows no cellular pleomorphism or tumor giant cells.
Sarcoma, histiocytic
• Contains rounded histiocyte-like cells, Langhans type giant cells and shows immunoreactivity or CD-68 (rats) or F4/80 (mice).
Rhabdomyosarcoma
• Cells contain PTAH-positive cross-striations and immune-reactivity for myoglobin.
Sarcoma, NOS (Not otherwise specified)
• No histological characteristics that enable categorization.
Comment: In both laboratory animals and humans tumors of this type have been frequently termed malignant fibrous histiocytomas. They are considered to be a group of largely undifferentiated or primitive sarcomas. In humans they are now often subdivided according to their immunohistochemical and electron microscopic characteristics (38Fletcher 1987; 39Fletcher 2006). Similar sarcomas have been well characterized in rodents where they develop spontaneously. They are the most common type induced in rats by carcinogens and non-carcinogenic chemicals, subcutaneous implants and viruses (19Chesterman et al. 1966; 64Konishi et al. 1982; 124Wright et al. 1991; 113Tsuchiya et al. 1993; 98Schneider et al. 1999; 63Kirkpatrick et al. 2000). Identical neoplasms occur in the mouse under similar circumstances where they have been variously known as malignant fibrohistiocytomas or pleomorphic fibrosarcomas (11Brand et al. 1976; 105Stewart 1979; 36Faccini et al. 1990). A mononuclear or polymorphonuclear infiltrate is sometimes seen and blood vessels can be prominent. Collagen formation usually is marked in spindle cell zones and myxoid change can develop. Large lesions may show necrosis and ulceration of overlying skin. Metastases may occasionally occur in lung, liver and other organs. Those that develop in the peritoneal cavity may spread along the visceral and parietal peritoneum and mesentery surrounding abdominal organs (102Shoieb et al. 2012).
These sarcomas are the most common type induced in rodents by powerful carcinogenic chemicals such as polycyclic hydrocarbons as well as the repeated subcutaneous injection of agents not considered carcinogens. Substances among the latter class include concentrated solutions of glucose and other sugars, sodium chloride, certain water-soluble food colourings and surfactants, carboxymethycellulose and macromolecular dextrans (44Grasso and Goldberg 1966a; 45Grasso and Goldberg 1966b; 17Carter 1970; 18Carter et al. 1971;58 Hooson et al. 1973).
Some of these materials such as macromolecular iron dextrans have been used therapeutically in humans by the parenteral route for many years without evidence of tumour induction (17Carter 1970).
Subcutaneous implantation of inert plastics, metals and other materials of certain dimensions can likewise give rise to sarcomas around implantation sites in rodents, the so called 'Oppenheimer effect' or 'solid state' carcinogenesis (86Oppenheimer et al. 1953;5 Autian 1973; 11Brand et al. 1976; 63Kirkpatrick et al. 2000; 52Hahn et al. 2002). These sarcomas also develop around small glass and polypropylene covered microchips implanted into the subcutaneous tissues of heterozygous transgenic p53+/− mice within periods as little as 15 weeks (8Blanchard et al. 1999). However, sarcomas have been induced by implanted microchips in Fischer 344 rats and various conventional mouse strains. Reported incidences in implanted Fischer rats is about 1% and mice they range between about 2 and 4% in B6C3F1 mice, 1.2% in CBA/J female mice, 0.5% in CBA/J male mice whereas CD-1 mice are more resistant(110Tillmann et al. 1997; 33Elcock et al. 2001; 67Le Calvez et al. 2006).
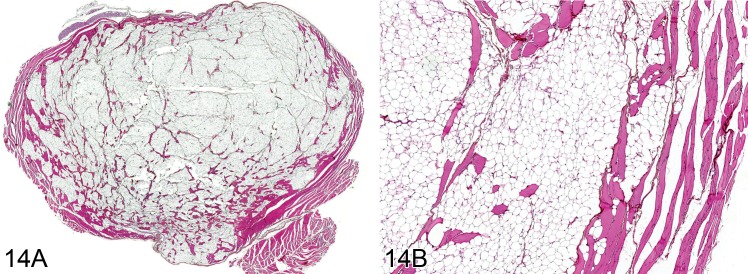
Lipoma (B): Soft Tissue
(Figures 14 and 15)
Figure 14.
Lipoma from a rat. A) Low power view of a round nodule of near normal fat cells partially surrounded by a thin fibrous capsule. B) Higher power view showing cellular detail (H&E).
Figure 15.
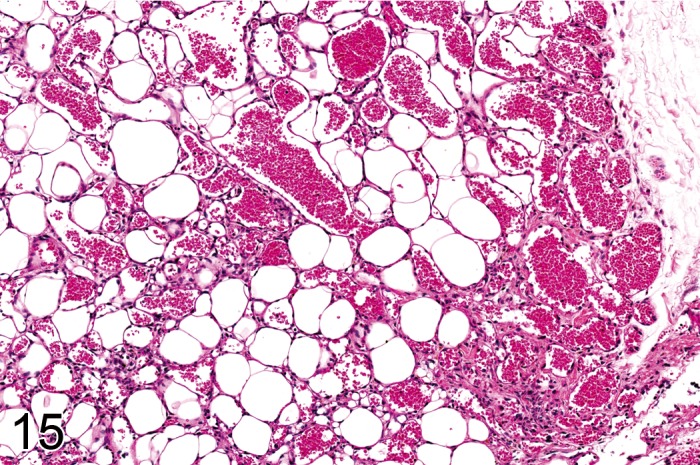
Lipoma of angiomatous type from a rat containing numerous thin walled blood vessels (H&E).
Species: Mouse, Rat
Modifier: Angiomatous Type
Diagnostic features
• Soft nodular, well-demarcated, lobulated fatty mass displacing surrounding tissue.
• Mature fat cells each typically containing a single fat vacuole with eccentrically located nucleus.
• Cells are usually separated into lobules by fibrous septa.
• Devoid of significant mitotic activity, cellular pleomorphism, necrosis or myxoid change.
• Can have brown discoloration and central necrosis if pedunculated.
• Fibrous component can be very prominent.
Angiomatous type
• Contains many thin-walled blood vessels.
• Typically found in mice.
Differential diagnoses
Hyperplasia, adipose tissue
• Is a diffuse change with little or no nodularity.
Hibernoma
• Typically contains multivesicular brown fat cells with centrally located nuclei.
Liposarcoma
• Shows histological features of malignancy such as pleomorphic spindle cells, mitotic activity and the presence of malignant lipoblasts.
Angioma
• Typically devoid of intercellular fat cells
Comment: Subcutaneous lipoma is a rare tumor in mice although lipomas of the brain are more common. These may represent lipomatous hamartomas rather than true neoplasms. Intracranial lipomas may my present in the interstitium of the choroid plexus. Lipomas have been induced in the subcutaneous tissues of rats treated with peroxisome proliferator-activated receptor (PPAR) agonists (55Hardisty et al. 2007).
Hibernoma (B): Soft Tissue
(Figures 16, 17, 18)
Figure 16.
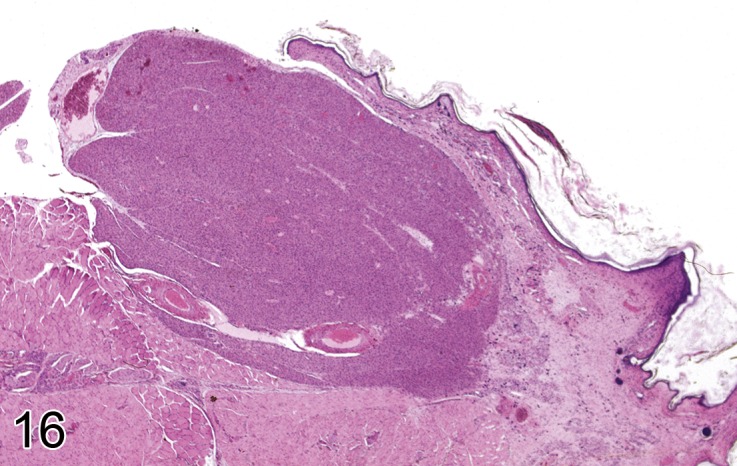
Subcutaneous hibernoma from a B6C3F1 mouse (H&E). Image by courtesy of the National Toxicology Program.
Figure 17.
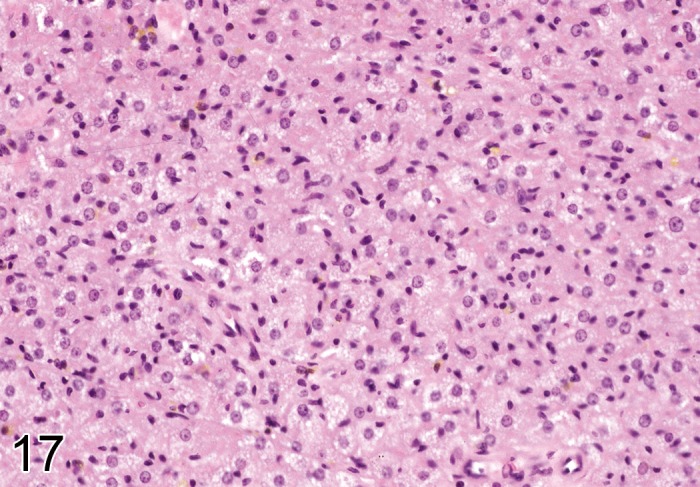
Higher power view of tumor in Figure 16 showing cellular detail, notably the dense eosinophilic staining cytoplasm (H&E). Image by courtesy of the National Toxicology Program.
Figure 18.
A) and B) low and high power view of hibernoma from the thorax from an aged Wistar rat showing pale staining foamy cytoplasm (H&E).
Species: Mouse, Rat
Synonym: Brown fat tumor
Diagnostic features
• Lobulated pale yellowish mass in posterior thoracic or abdominal region or within the mediastinum.
• Fairly uniform rounded cells with abundant fine cytoplasmic lipid droplets and central, round nuclei.
• Mitotic activity and cellular pleomorphism usually low.
Differential diagnoses
Liposarcoma
• Histological features of malignancy such as pleomorphic spindle cells, mitotic activity and the presence of malignant lipoblasts.
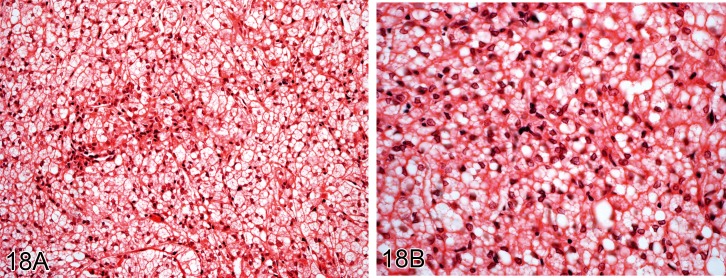
Comment: These are very characteristic tumors showing brown fat differentiation. Tumor cells are round oval or polygonal with dense rounded central nuclei. The foamy cytoplasm stains with oil red O. Electron microscopic study shows numerous mitochondria and small lipid droplets. They are rare tumors in all species including humans but they appear to be more commonly reported in rats (22Coleman 1980; 1Al Zubaidy and Finn 1983; 104Stefanski et al. 1987; 23Coleman 1989; 14Bruner et al. 2009). A recent report has shown an increase in incidence in a colony of Sprague-Dawley rats (14Bruner et al. 2009). These tumors can be stained immunocytochemically using antisera to mitochondrial uncoupling protein (UCP-1), a protein essential for the thermogenic properties of brown fat in mammals (14Bruner et al. 2009).
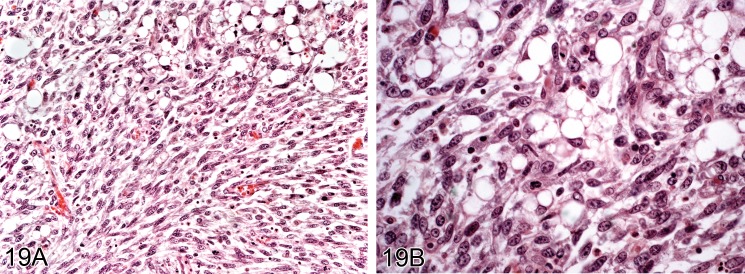
Liposarcoma (M): Soft Tissue
Figure 19.
Liposarcoma from an aged Wistar rat. A) Shows spindle cells and fat containing cells. B) Higher power view showing atypical fat forming cells (lipoblasts) (H&E).
Species: Mouse, Rat
Diagnostic features
• Fatty mass of variable appearance.
• Rounded or oval fat-forming cells with single large or multiple small cytoplasmic fat vacuoles, spindle cells, primitive undifferentiated cells
• Cellularity and mitotic activity variable
• Myxoid change may be prominent in stroma.
• Necrosis may be marked.
Differential diagnoses
Lipoma
• Shows no evidence of pleomorphic spindle cells, malignant lipoblasts or significant mitotic activity.
Comment: Liposarcoma is characterized by the presence of fat forming cells, termed lipoblasts. These can be primitive mesenchymal cells with fine lipid droplets, larger rounded or oval cells with large central or eccentric nuclei and large cytoplasmic fat vacuoles. Various other cell types occur including 'brown' fat cells, foam cells, giant cells, myxoid cells and spindle and stellate cells. The stroma is usually well vascularized and a myxoid appearance may be evident and sometimes extensive (47Greaves and Barsoum 1990). Undifferentiated cells also can be found and mitotic activity can be intense. Rapidly growing liposarcomas frequently have zones of necrosis. These are uncommon spontaneous neoplasms in rats and mice but have been induced along with fibrosarcomas in rats treated with peroxisome proliferator-activated receptor (PPAR) agonists (55Hardisty et al. 2007; 71Long et al. 2009).
Rhabdomyosarcoma (M): Soft Tissue
(Figures 20 and 21)
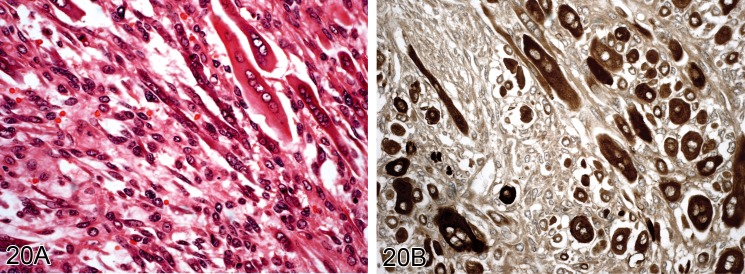
Figure 20.
Well-differentiated rhabdomysarcoma from a Wistar rat. A) Tumor cells showing good muscle differentiation (H&E). B) Same tumor stained for myoglobin (Immunoperoxidase).
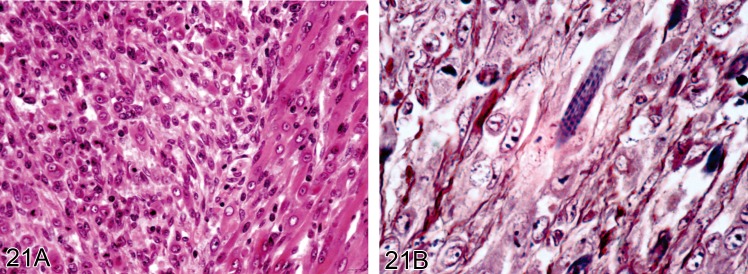
Figure 21.
Rhabdomyosarcoma from a CD1 mouse (H&E). A) Well differentiated tumor cells. B) Shows cross striations confirming muscle differentiation (PTAH).
Species: Mouse, Rat
Diagnostic features
• Mass frequently showing necrosis and hemorrhage.
• Highly pleomorphic with rhabdomyoblasts, immature spindle and strap-like cells, mononucleated, rounded and polygonal cells.
• Rhabdomyoblasts characterized by eosinophilic fibrillar cytoplasm with myofilaments, glycogen inclusions, cross striations, immunocytochemical staining for myoglobin and ultrastructural evidence of Z bands.
• High mitotic activity with abnormal mitotic figures.
• Locally infiltrative with frequent distant metastases.
Differential diagnoses
Fibrosarcoma, pleomorphic
• Shows no evidence of skeletal muscle differentiation.
Leiomyosarcoma:
• Generally less pleomorphic and show immune reactivity for desmin and smooth muscle actin.
Comment: The diagnosis of rhabdomyosarcoma depends on the histological identification of rhabdomyoblasts. These are characterized by the presence of eosinophilic cytoplasm, perinuclear fibrillar material, cross striations, glycogen, myofilaments well-stained with PTAH, immunoreactivity for desmin and myoglobin. At the ultrastructural level, they show cytoplasmic Z lines. They are uncommon spontaneously developing neoplasms in rodents. In the National Toxicology Program less that 0.2% of Fischer 344 rats developed this neoplasm (83NTP 2006). Only 14 rhabdomyosarcomas were diagnosed at necropsy in 10,000 mice in one study, most often originating in the quadriceps muscles with a mean age of 4 months (106Sundberg et al. 1991). They have been induced in rodents by injection nickel sulphide, monocrotaline or its major metabolite, dehydnonetronecine (2Allen et al. 1975; 3Altmannsberger et al. 1985). Rhabdomyosarcomas have also been reported to develop around inert implants or repeated subcutaneous injection of iron dextran or other poorly absorbed substances (87Oppenheimer et al. 1958; 17Carter 1970).
Leiomyoma (B): Soft Tissue
Species: Mouse, Rat
Diagnostic features
• Well circumscribed, nodular mass.
• Interlacing bundles and whorls of uniform spindle cells arranged in criss-cross patterns of bundles.
• Nuclei typically blunt-ended or cigar shaped.
• Eosinophilic cytoplasm contains longitudinal myofilaments and perinuclear clear space and show immunoreactivity for desmin.
• Minimal mitotic activity and nuclear pleomorphism.
Differential diagnoses
Leiomyosarcoma
• Shows mitotic activity and cellular pleomorphism.
Fibrosarcoma
• Shows mitotic activity and cellular pleomorphism but not discernible smooth muscle differentiation.
Comment: Benign tumors of smooth muscle are uncommonly found in the soft tissues of rodents. Most have been described in the uterus. They are well-circumscribed, nodular tumors characterized histologically by interlacing bundles of uniform spindle cells with typically blunt ended or cigar shaped nuclei. The cell cytoplasm may contain perinuclear clear spaces. Myofibrils are characteristically demonstrable as linear streaks which stain blue with phosphotungstic acid hematoxylin (PTAH) and red by Masson trichrome stain. Mitotic activity and nuclear pleomorphism are minimal. These neoplasms show immunocytochemical staining for desmin and smooth muscle actin. However, desmin may be also present in sarcomas of other subtypes (55Hardisty et al. 2007).
Ultrastructural features include a cytoplasm packed with thin filaments with focal densities, some mitochondria, sparse profiles of endoplasmic reticulum and a Golgi apparatus situated adjacent to nuclear poles, micropinocytic vesicles and dense plaques at the points of attachment of myofilaments to the cell membrane. A poorly developed basal lamina may be present.
Leiomyosarcoma (M): Soft Tissue
Figure 22.
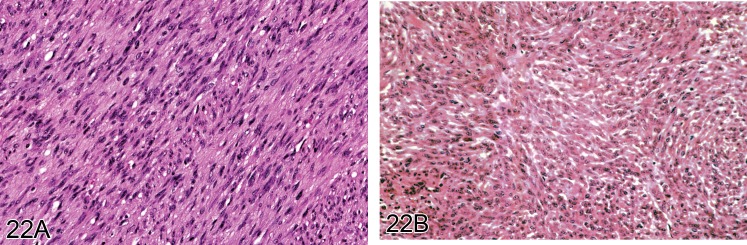
A) Well differentiated leiomyosarcoma from a rat showing interwoven bundles of spindle cells with typical oval shaped nuclei of smooth muscle. B) Another example of a leiomyosarcoma but showing clear muscle differentiation (H&E).
Species: Mouse, Rat
Diagnostic features
• Similar to leiomyoma but more pleomorphic and with mitotic activity.
• Interlacing bundles and whorls of spindle cells arranged in criss-cross patterns of bundles.
• Nuclei typically blunt-ended or cigar shaped
• Bizarre polygonal cells with double or multiple nuclei may be present
• Necrosis, cystic change, mineralization common features.
• Distant metastases.
Differential diagnoses
Leiomyoma
• Shows little or no cellular pleomorphism or mitotic activity.
Fibrosarcoma
• Devoid of smooth muscle differentiation.
Rhabdomyosarcoma
• Shows skeletal muscle differentiation.
Comment: This neoplasm is similar to the leiomyoma but it is invasive and shows mitotic activity and marked cellular pleomorphism. Bizarre polygonal cells with double or multiple nuclei as well as vacuolated cytoplasm may be present. Longitudinal myofibrils are demonstrable using the phosphotungstic acid hematoxylin (PTAH) stain. There are varying degrees of vascularity and abundant reticulin fibers run parallel to the tumour cells. Cellular pleomorphism, necrosis, cystic degeneration, mineralization and distant metastases are also typical features. However, the distinction between fibrosarcoma and leiomyosarcoma is not always clear cut. These are uncommon tumors in the soft tissues but are reported to develop occasionally in rodents around implanted biomaterials (63Kirkpatrick et al. 2000).
Mesenchymoma, malignant (M): Soft Tissue
Species: Mouse, Rat
Synonym: Mixed sarcoma.
Diagnostic features
• Mesenchymal tumor showing two or more types of mesenchymal differentiation in addition to fibrosarcomatous elements.
Differential diagnoses
• Sarcomas showing only one type of mesenchymal differentiation (Fibrosarcoma, Fibrosarcoma, pleomorphic, Liposarcoma, Rhabdomyosarcoma, Leiomyosarcoma).
Comment: This term is used for those rare tumors characterized by two or more differentiated mesenchymal tissue types such as fat, muscle, bone or cartilage in addition to undifferentiated or fibrosarcomatous elements. Electron microscopy or immunocytochemistry may be required to establish the presence of skeletal muscle differentiation in such tumors.
Sarcoma, NOS (Not otherwise specified) (M): Soft Tissue
Species: Mouse, Rat
Diagnostic features
• Mesenchymal tumor composed of sheets of undifferentiated round, spindle or pleomorphic cells.
• No recognizable light microscopic differentiation.
• Electron microscopy and immunocytochemistry can sometimes lead to accurate diagnosis.
• Not all sarcomas have distinguishing features even with ultrastructural or immunohistochemical study.
Differential diagnoses
• Sarcomas showing focal differentiation of a specific cell type type (Fibrosarcoma, Fibrosarcoma, pleomorphic, Liposarcoma, Rhabdomyosarcoma, Leiomyosarcoma).
Comment: In view of the potential variety of histological appearances of neoplasms of mesenchymal cells, it may not always be possible to make a precise diagnosis of all soft tissue tumors, particularly without ultrastructural examination or a battery of appropriate antisera for immunocytochemical demonstration of antigens. It is appropriate to classify such neoplasms as sarcoma, NOS.
Mesothelioma, malignant (M): Mesothelium
(Figures 23 and 24)
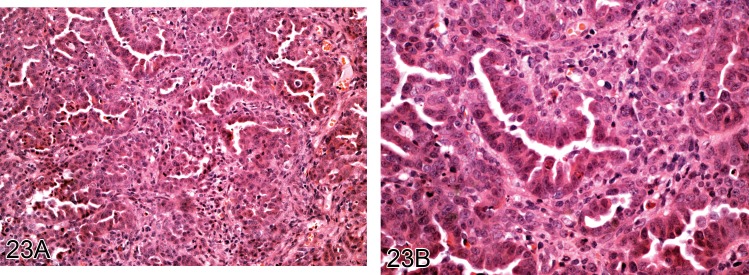
Figure 23.
A) Low and B) high power view of mesothelioma of epithelioid type which developed in the pleura of a rat in response to the administration of fine fibers (H&E).
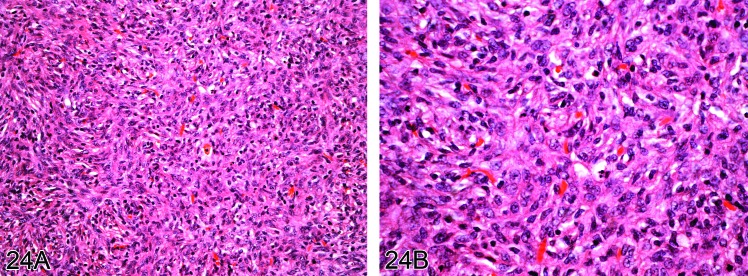
Figure 24.
A) Low and B) high power view of mesothelioma of sarcomatoid type which developed in the pleura of a rat in response to the administration of fine fibers (H&E).
Species: Mouse, Rat
Modifier: Epithelioid type, Sarcomatoid type, Biphasic type.
Diagnostic features
• Predominantly superficial growth of tumor on the serosal surface where cells usually show considerable cellular pleomorphism, mitotic activity, necrosis and infiltration of adjacent tissues.
Epithelioid type
• Fronds or papillary structures.
• Poorly formed glandular structures or ill-formed glands.
• Rounded cells with round vesicular nuclei.
• Infiltration into adjacent connective tissue.
Sarcomatoid type
• Relatively uniform spindle-shaped cells with elongated nuclei.
• Interlacing bundles similar to fibrosarcomas or leiomyosarcomas or forming whorls suggestive of pleomorphic sarcomas (malignant fibrous histiocytomas).
• Areas resembling osteosarcoma, chondrosarcoma or other sarcomas may be present (94Rittinghausen et al. 1992; 15Cardesa et al. 1994).
Biphasic type
• Contains both epithelioid and sarcomatoid patterns.
• Any patterns noted above may be present. Each component should represent at least 10% of the tumor
Differential diagnoses
Metastatic tumors:
• Presence of primary tumor with similar morphologic characteristics.
The differentiation between sarcomatoid forms and other sarcoma types may be difficult without special techniques.
Fibrosarcoma
• May show a similar spindle cell appearance. Without special stains it may be difficult to distinguish spindle cell fibrosarcomas arising in soft tissues from sarcomatoid mesotheliomas particularly when they large and the site of origin unclear.
Thymoma
• Localized thoracic mass with highly variable epithelial patterns usually interspersed with lymphoid tissue.
Extension of primary lung malignancies into thoracic cavity
• Presence of microscopic evidence of tumor extension into pleura, and similar histological patterns in extended tumor mass.
Hyperplasia, mesothelium
• Lack of evidence of mitotic activity, cellular atypia, or extension into adjacent tissues. Usually focal thickening or villous projections with cellular stratification. It may be accompanied by inflammation.
Comment: Desmoplastic areas characterized by dense collagenous tissue separated by atypical cells may occur in all types of mesothelioma.
Pleural and peritoneal cavity mesotheliomas are rare spontaneous lesions in rodents but have been induced in rats, mice and hamsters by a number of persistent fibrous materials or by other xenobiotics like potassium-bromate (99Schwartz et al. 1994; 24Crosby et al. 2000; 60Kane 2006; 108Takagi et al. 2008; 75Maronpot et al. 2009; 96Sakamoto et al. 2009; 7Bernstein et al. 2010; 30Donaldson et al. 2010). Although a body of evidence suggests that SV40 might be one causative agent of malignant mesothelioma in humans this remains a contentious issue (116von Ruhland et al. 2004).
The morphology of this tumor is similar in rats and humans (112Travis et al. 2004). Differentiation of pleural mesotheliomas from primary lung carcinomas can be difficult. They not only occur in the pleural cavity or pericardium but also the peritoneum. One of the most frequent sites in the rat is the tunica vaginalis of the testis from which they may spread to the peritoneal cavity (77McConnell et al. 1992). Spontaneous, as well as several, xenobiotic-associated tunica vaginalis mesotheliomas are causally associated with Leydig-cell tumors that lead to an autocrine growth factor-induced mesothelial mitogenesis (75Maronpot et al. 2009).
For epithelioid mesothelioma, the most useful markers are broad spectrum cytokeratin, cytokeratin 5/6, cytokeratin 18, Wilm's Tumor-gene 1 WT1 and calretinin (60Kane 2006; 75Maronpot et al. 2009). Mesothelin is also considered to be a reliable marker (29Doi et al. 2010). Most sarcomatoid mesotheliomas typically stain positively for cytokeratin when a broad-spectrum antibody is used. They may stain positively for vimentin, smooth muscle actin, desmin, or S-100.
Mesotheliomas uncommonly exhibit positive mucin staining, when combined diastase and periodic acid-Schiff (D-PAS) is used. A diffuse strongly positive D-PAS in an epithelioid pleural tumor is strongly suggestive of pulmonary bronchiolo-alveolar carcinoma, whilst weak or focal staining is of less diagnostic value.
References
- 1.Al Zubaidy AJ, Finn JP.Brown fat tumours (hibernomas) in rats: histopathological and ultrastructural study. Lab Anim. 17: 13–17 1983 [DOI] [PubMed] [Google Scholar]
- 2.Allen JR, Hsu IC, Carstens LA.Dehydroretronecine-induced rhabdomyosarcomas in rats. Cancer Res. 35: 997–1002 1975 [PubMed] [Google Scholar]
- 3.Altmannsberger M, Weber K, Droste R, Osborn M.Desmin is a specific marker for rhabdomyosarcomas of human and rat origin. Am J Pathol. 118: 85–95 1985 [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JM, Langone JJ.Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J Control Release. 57: 107–113 1999 [DOI] [PubMed] [Google Scholar]
- 5.Autian J.The new field of plastics toxicology—methods and results. CRC Crit Rev Toxicol. 2: 1–40 1973 [DOI] [PubMed] [Google Scholar]
- 6.Behan WMH, Cossar DW, Madden HA, McKay IC.Validation of a simple, rapid, and economical technique for distinguishing type 1 and 2 fibres in fixed and frozen skeletal muscle. J Clin Pathol. 55: 375–380 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein DM, Rogers RA, Sepulveda R, Donaldson K, Schuler D, Gaering S, Kunzendorf P, Chevalier J, Holm SE.The pathological response and fate in the lung and pleura of chrysotile in combination with fine particles compared to amosite asbestos following short-term inhalation exposure: interim results. Inhal Toxicol. 22: 937–962 2010 [DOI] [PubMed] [Google Scholar]
- 8.Blanchard KT, Barthel C, French JE, Holden HE, Moretz R, Pack FD, Tennant RW, Stoll RE.Transponder-induced sarcoma in the heterozygous p53+/− mouse. Toxicol Pathol. 27: 519–527 1999 [DOI] [PubMed] [Google Scholar]
- 9.Boorman GA, Eustis SL, Elwell MR.(1989). Fibrosarcoma, dermis and subcutis, mouse. Monographs on Pathology of Laboratory Animals, Integument and Mammary Glands. T. C. Jones, U. Mohr and R. D. Hunt. Berlin, Springer: 95-100. [Google Scholar]
- 10.Bradley WG, Fewings JD, Harris JB, Johnson MA.Emetine myopathy in the rat. Br J Pharmacol. 57: 29–41 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand KG, Johnson KH, Buoen LC.Foreign body tumorigenesis. CRC Crit Rev Toxicol. 4: 353–394 1976 [DOI] [PubMed] [Google Scholar]
- 12.Brooke MH, Kaiser KK.Muscle fiber types: how many and what kind? Arch Neurol. 23: 369–379 1970. [DOI] [PubMed] [Google Scholar]
- 13.Brooke MH, Williamson E, Kaiser KK.The behavior of four fiber types in developing and reinnervated muscle. Arch Neurol. 25: 360–366 1971 [DOI] [PubMed] [Google Scholar]
- 14.Bruner RH, Novilla MN, Picut CA, Kirkpatrick JB, O'Neill TP, Scully KL, Lawrence WB, Goodman DG, Saladino BH, Peters DG, Parker GA.Spontaneous hibernomas in Sprague-Dawley rats. Toxicol Pathol. 37: 547–552 2009 [DOI] [PubMed] [Google Scholar]
- 15.Cardesa A, Carlton WW, Dungworth DL, Enomoto Y, Halm S, Koestner A, Krinke GJ, Render JA, Rittinghausen S, Ruben Z, Solleveld H, Turusov VS, Weisse I, Yoshitomi K. Central nervous system; heart; eye; mesothelium. International Classification of Rodent Tumours. Part 1 The Rat. U. Mohr. Lyon, International Agency for Research on Cancer. IARC Sci Pub. 7(122): 61–65. 1994 [Google Scholar]
- 16.Carlton W. W., Ernst H., Faccini J. M., Greaves P., Krinke G. J., Long P. H., Maekawa A., Newsholme S. J., G W.(1992). Soft tissue and musculoskeletal system, 2. Part 1. International Classification of Rodent Tumours. U. Mohr. Lyon, International Agency for Research on Cancer.
- 17.Carter RL. (1970). Induced subcutaneous sarcomata: Their development and critical appraisal. Metabolic Aspects of Food Safety. F. J. C. Roe. Oxford, Blackwell: 569–591. [Google Scholar]
- 18.Carter RL, Roe FJC, Peto R.Tumor induction by plastic films: attempt to correlate carcinogenic activity with certain physicochemical properties of the implant. J Natl Cancer Inst. 46: 1277–1289 1971 [PubMed] [Google Scholar]
- 19.Chesterman FC, Harvey JJ, Dourmashkin RR, Salaman MH.The pathology of tumors and other lesions induced in rodents by virus derived from a rat with Moloney leukemia. Cancer Res. 26: 1759–1768 1966 [PubMed] [Google Scholar]
- 20.Clarke JTR, Karpati G, Carpenter S, Wolfe LS.The effect of vincristine on skeletal muscle in the rat. A correlative histochemical, ultrastructural and chemical study. J Neuropathol Exp Neurol. 31: 247–266 1972. [DOI] [PubMed] [Google Scholar]
- 21.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchère D, Sastre X, Vilain MO, Bonichon F, N'Guyen Bui B.Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 91: 1914–1926 2001 [DOI] [PubMed] [Google Scholar]
- 22.Coleman GL.Four intrathoracic hibernomas in rats. Vet Pathol. 17: 634–637 1980 [DOI] [PubMed] [Google Scholar]
- 23.Coleman GL. (1989). Hibernoma, rat. Integument and Mammary Glands, Monographs on Pathology of Laboratory Animals. T. C. Jones, U. Mohr and R. D. Hunt. Berlin, Springer-Verlag: 126-129. [Google Scholar]
- 24.Crosby LM, Morgan KT, Gaskill B, Wolf DC, DeAngelo AB.Origin and distribution of potassium bromate-induced testicular and peritoneal mesotheliomas in rats. Toxicol Pathol. 28: 253–266 2000. > [DOI] [PubMed] [Google Scholar]
- 25.Danse BHJC.(1989). Steatitis, subcutaneous tissue and generalised, rat. Monographs on Pathology of Laboratory Animals. Integument and Mammary Glands. T. C. Jones, U. Mohr and R. D. Hunt. Berlin, Springer-Verlag: 146-152. [Google Scholar]
- 26.De Souza AT, Cornwell PD, Dai X, Caguyong MJ, Ulrich RG.Agonists of the peroxisome proliferator-activated receptor alpha induce a fiber-type-selective transcriptional response in rat skeletal muscle. Toxicol Sci. 92: 578–586 2006 [DOI] [PubMed] [Google Scholar]
- 27.Dincer Z, Jones S, Haworth R.Preclinical safety assessment of a DNA vaccine using particle-mediated epidermal delivery in domestic pig, minipig and mouse. Exp Toxicol Pathol. 57: 351–357 2006 [DOI] [PubMed] [Google Scholar]
- 28.Dinse GE, Peddada SD, Harris SF, Elmore SA.Comparison of NTP historical control tumor incidence rates in female Harlan Sprague Dawley and Fischer 344/N Rats. Toxicol Pathol. 38: 765–775 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi T, Kotani Y, Takahashi K, Hashimoto S, Yamada N, Kokoshima H, Tomonari Y, Wako Y, Tsuchitani M.Malignant mesothelioma in the thoracic cavity of a Crj:CD(SD) rat characterized by round hyalinous stroma. J Toxicol Pathol. 23: 103–106 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaldson K., Murphy F. A., Duffin R., Poland C. A. (2010). Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Particle and Fibre Toxicology 7: Article No.: 5. [DOI] [PMC free article] [PubMed]
- 31.Eddinger TJ, Moss RL, Cassens RG.Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J Histochem Cytochem. 33: 1033–1041 1985 [DOI] [PubMed] [Google Scholar]
- 32.Eiben R.Frequency and time trends of spontaneous tumors found in B6C3F1 mice oncogenicity studies over 10 years. Exp Toxicol Pathol. 53: 399–408 2001 [DOI] [PubMed] [Google Scholar]
- 33.Elcock LE, Stuart BP, Wahle BS, Hoss HE, Crabb K, Millard DM, Mueller RE, Hastings TF, Lake SG.Tumors in long-term rat studies associated with microchip animal identification devices. Exp Toxicol Pathol. 52: 483–491 2001 [DOI] [PubMed] [Google Scholar]
- 34.Ernst H, Carlton WW, Courtney C, Rinke M, Greaves P, Isaacs KR, Krinke G, Konishi Y, Mesfin GM, Sandusky G. (2001). Soft tissue and skeletal muscle. Heidelberg, Springer Verlag. [Google Scholar]
- 35.Everitt AV, Shorey CD, Ficarra MA.Skeletal muscle aging in the hind limb of the old male Wistar rat: inhibitory effect of hypophysectomy and food restriction. Arch Gerontol Geriatr. 4: 101–115 1985 [DOI] [PubMed] [Google Scholar]
- 36.Faccini JM, Abbott DP, Paulus GJJ. (1990). Mouse Histopathology. A glossary for use in toxicity and carcinogenicity studies. Amsterdam, Elsevier. [Google Scholar]
- 37.Faiola B, Falls JG, Peterson RA, Bordelon NR, Brodie TA, Cummings CA, Romach EH, Miller RT.PPAR alpha, more than PPAR delta, mediates the hepatic and skeletal muscle alterations induced by the PPAR agonist GW0742. Toxicol Sci. 105: 384–394 2008 [DOI] [PubMed] [Google Scholar]
- 38.Fletcher CDM.Malignant fibrous histiocytoma? Histopathology. 11: 433–437 1987 [DOI] [PubMed] [Google Scholar]
- 39.Fletcher CDM.The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 48: 3–12 2006 [DOI] [PubMed] [Google Scholar]
- 40.Flint OP, Noor MA, Hruz PW, Hylemon PB, Yarasheski K, Kotler DP, Parker RA, Bellamine A.The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 37: 65–77 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Floyd E, Mann P, Long G, Ochoa R.The Trp53 hemizygous mouse in pharmaceutical development: points to consider for pathologists. Toxicol Pathol. 30: 147–156 2002 [DOI] [PubMed] [Google Scholar]
- 42.Garg A.Acquired and inherited lipodystrophies. N Engl J Med. 350: 1220–1234 2004 [DOI] [PubMed] [Google Scholar]
- 43.Gopinath C, Gibson WA.Mesovarian leiomyomas in the rat. Environ Health Perspect. 73: 107–113 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grasso P, Golberg L.Early changes at the site of repeated subcutaneous injection of food colourings. Food Cosmet Toxicol. 4: 269–282 1966a [DOI] [PubMed] [Google Scholar]
- 45.Grasso P, Golberg L.Subcutaneous sarcoma as an index of carcinogenic potency. Food Cosmet Toxicol. 4: 297–320 1966b [DOI] [PubMed] [Google Scholar]
- 46.Greaves P.(2012). Skeletal Muscle. Histopathology of Preclinical Toxicity Studies. Fourth Edition. Amsterdam, Academic Press/Elsevier: 180-206. [Google Scholar]
- 47.Greaves P., Barsoum N. ( 1990). Pathology of tumours in laboratory animals. Tumours of the rat. Tumours of soft tissues. IARC Scientific(99): 597-623. [PubMed]
- 48.Greaves P., Carlton W. W., Courtney C. L., Ernst H., Halm S., Isaacs K. R., Konishi Y., Krinke G. J., Mesfin S. G., Rinke M., Sandusky G.(2000). Proliferative and non-proliferative lesions of soft tissues and skeletal muscle in mice. Guides for Toxicologic Pathology. Washington DC, STP/ARP/AFIP. MSTM-1.
- 49.Greaves P, Faccini JM.Spontaneous fibrous histiocytic neoplasms in rats. Br J Cancer. 43: 402–411 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greaves P., Faccini J. M., Courtney C. L.(1992). Proliferative lesions of soft tissues and skeletal muscle in rats. Guides for Toxicologic Pathology. Washington DC, STP/ARP/AFIP. MST-1.
- 51.Greaves P., Seely J. C. (1996). Non-proliferative lesions of soft tissues and skeletal muscle in rats. Guides for Toxicologic Pathology. Washington DC, STP/ARP/AFIP. MST-1. [Google Scholar]
- 52.Hahn FF, Guilmette RA, Hoover MD.Implanted depleted uranium fragments cause soft tissue sarcomas in the muscles of rats. Environ Health Perspect. 110: 51–59 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haines DC, Chattopadhyay S, Ward JM.Pathology of aging B6;129 mice. Toxicol Pathol. 29: 653–661 2001 [DOI] [PubMed] [Google Scholar]
- 54.Hajdu SI. (1986). Histogenesis and classification. In: Differential Diagnosis of Soft Tissue and Bone Tumours. Philadelphia, Lea and Febiger: 3-34. [Google Scholar]
- 55.Hardisty JF, Elwell MR, Ernst H, Greaves P, Kolenda-Roberts H, Malarkey DE, Mann PC, Tellier PA.Histopathology of hemangiosarcomas in mice and hamsters and liposarcomas/fibrosarcomas in rats associated with PPAR agonists. Toxicol Pathol. 35: 928–941 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heider K, Eustis SL. (1994). Tumours of the soft tissues. Pathology of Tumours in Laboratory Animals. Tumours of the Mouse, 2nd Edition. V. S. Turusov and U. Mohr. Lyon, IARC Scientific Publ. 111. 2: 611-649. [PubMed] [Google Scholar]
- 57.Hinz B.The myofibroblast: paradigm for a mechanically active cell. J Biomech. 43: 146–155 2010 [DOI] [PubMed] [Google Scholar]
- 58.Hooson J, Grasso P, Gangolli SD.Injection site tumours and preceding pathological changes in rats treated subcutaneously with surfactants and carcinogens. Br J Cancer. 27: 230–244 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaweed MM, Alleva FR, Herbison GJ, Ditunno JF, Balazs T.Muscle atrophy and histopathology of the soleus in 6-mercaptopurine-treated rats. Exp Mol Pathol. 43: 74–81 1985 [DOI] [PubMed] [Google Scholar]
- 60.Kane AB.Animal models of malignant mesothelioma. Inhal Toxicol. 18: 1001–1004 2006 [DOI] [PubMed] [Google Scholar]
- 61.Keegan GM, Learmonth ID, Case CP.A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit Rev Toxicol. 38: 645–674 2008 [DOI] [PubMed] [Google Scholar]
- 62.Kempson RL, Fletcher CDM, Evans HL, Hendrickson MR, Sibley RK.(2001). Tumors of the Soft Tissues. Washington DC, Armed Forces Institute of Pathology. [Google Scholar]
- 63.Kirkpatrick CJ, Alves A, Köhler H, Kriegsmann J, Bittinger F, Otto M, Williams DF, Eloy R.Biomaterial-induced sarcoma: A novel model to study preneoplastic change. Am J Pathol. 156: 1455–1467 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konishi Y, Maruyama H, Mii Y, Miyauchi Y, Yokose Y, Masuhara K.Malignant fibrous histiocytomas induced by 4-(hydroxyamino)quinoline 1-oxide in rats. J Natl Cancer Inst. 68: 859–865 1982 [PubMed] [Google Scholar]
- 65.Kouchi M, Okimoto K, Matsumoto I, Michimae Y, Yamada T, Inoue T, Kimura T, Seki T, Yasuba M, Hino O.Postoperative fibromatosis-type fibromas in the Bhd gene mutant (Nihon) rat. Exp Toxicol Pathol. 59: 273–279 2008 [DOI] [PubMed] [Google Scholar]
- 66.Larade K, Jiang Z, Zhang Y, Wang W, Bonner-Weir S, Zhu H, Bunn HF.Loss of Ncb5or results in impaired fatty acid desaturation, lipoatrophy, and diabetes. J Biol Chem. 283: 29285–29291 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Calvez S, Perron-Lepage M-F, Burnett R.Subcutaneous microchip-associated tumours in B6C3F1 mice: a retrospective study to attempt to determine their histogenesis. Exp Toxicol Pathol. 57: 255–265 2006 [DOI] [PubMed] [Google Scholar]
- 68.Liu K, Tripp S, Layfield LJ.Heterotopic ossification: review of histologic findings and tissue distribution in a 10-year experience. Pathol Res Pract. 203: 633–640 2007 [DOI] [PubMed] [Google Scholar]
- 69.Liu ZJ, Zhuge Y, Velazquez OC.Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 106: 984–991 2009 [DOI] [PubMed] [Google Scholar]
- 70.Livingstone I, Johnson MA, Mastaglia FL.Effects of dexamethasone on fibre subtypes in rat muscle. Neuropathol Appl Neurobiol. 7: 381–398 1981 [DOI] [PubMed] [Google Scholar]
- 71.Long GG, Reynolds VL, Dochterman LW, Ryan TE.Neoplastic and non-neoplastic changes in F-344 rats treated with Naveglitazar, a gamma-dominant PPAR alpha/gamma agonist. Toxicol Pathol. 37: 741–753 2009 [DOI] [PubMed] [Google Scholar]
- 72.Majeed S. K.(1993). Survey on spontaneous systemic amyloidosis in aging mice. Arzneimittel-Forschung/Drug Research 43-1(2): 170-178. [PubMed]
- 73.Mann PC, Vahle J, Keenan CM, Baker JF, Bradley AE, Goodman DG, Harada T, Herbert R, Kaufmann W, Kellner R, Nolte T, Rittinghausen S, Tanaka T.(2012). International Harmonization of Toxicologic Pathology Nomenclature: An overview and review of basic principles. Pre-publication. [DOI] [PubMed] [Google Scholar]
- 74.Manor D, Sadeh M.Muscle fibre necrosis induced by intramuscular injection of drugs. Br J Exp Pathol. 70: 457–462 1989 [PMC free article] [PubMed] [Google Scholar]
- 75.Maronpot RR, Zeiger E, McConnell EE, Kolenda-Roberts H, Wall H, Friedman MA.Induction of tunica vaginalis mesotheliomas in rats by xenobiotics. Crit Rev Toxicol. 39: 512–537 2009 [DOI] [PubMed] [Google Scholar]
- 76.McClung JM, Mehl KA, Thompson RW, Lowe LL, Carson JA.Nandrolone decanoate modulates cell cycle regulation in functionally overloaded rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 288: R1543–R1552 2005 [DOI] [PubMed] [Google Scholar]
- 77.McConnell RF, Western HH, Ulland BM, Bosland MC, Ward JM. (1992). Proliferative lesions of the testis in rats with selected examples from mice. Guides for Toxicologic Pathology. Washington DC, STP/ARP/AFIP. [Google Scholar]
- 78.McNamara A, Williams DF.The response to the intramuscular implantation of pure metals. Biomaterials. 2: 33–40 1981 [DOI] [PubMed] [Google Scholar]
- 79.Miao CY, Li ZY.The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 165: 643–658 2012. > [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan RW, Elcock M.Artificial implants and soft tissue sarcomas. J Clin Epidemiol. 48: 545–549 1995 [DOI] [PubMed] [Google Scholar]
- 81.Munfus DL, Menke DM.Case of severe serous fat atrophy. Mayo Clin Proc. 84: 570 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mutsaers SE, Prêle CM, Lansley SM, Herrick SE.The origin of regenerating mesothelium: a historical perspective. Int J Artif Organs. 30: 484–494 2007. [DOI] [PubMed] [Google Scholar]
- 83.NTP NTP Historical Controls Report All Routes and Vehicles Rats. Research Triangle Park, National Toxicology Program. National Institute of Environmental Health Sciences. 2006 [Google Scholar]
- 84.Okada M, Inoue Y, Ube M, Sano F, Ikeda I, Sugimoto J, Takagi S.Skeletal muscle susceptibility to clofibrate induction of lesions in rats. Toxicol Pathol. 35: 517–520 2007 [DOI] [PubMed] [Google Scholar]
- 85.Okada M, Sano F, Ikeda I, Sugimoto J, Takagi S, Sakai H, Yanai T.Fenofibrate-induced muscular toxicity is associated with a metabolic shift limited to type-1 muscles in rats. Toxicol Pathol. 37: 517–520 2009 [DOI] [PubMed] [Google Scholar]
- 86.Oppenheimer BSC, Oppenheimer ET, Stout AP.Carcinogenic effect of imbedding various plastic films in rats and mice. Surg Forum. 4: 672–676 1953 [PubMed] [Google Scholar]
- 87.Oppenheimer BSC, Oppenheimer ET, Stout AP, Willhite M, Danishefsky I.The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 11: 204–213 1958 [DOI] [PubMed] [Google Scholar]
- 88.Oppenheimer ET, Willhite M, Stout AP, Danishefsky I, Fishman MM.A comparative study of the effects of imbedding cellophane and polystyrene films in rats. Cancer Res. 24: 379–382 1964 [PubMed] [Google Scholar]
- 89.Parrott E, Butterworth M, Green A, White INH, Greaves P.Adenomyosis—a result of disordered stromal differentiation. Am J Pathol. 159: 623–630 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Picha GJ, Goldstein JA, Stohr E.Natural-Y Même polyurethane versus smooth silicone: analysis of the soft-tissue interaction from 3 days to 1 year in the rat animal model. Plast Reconstr Surg. 85: 903–916 1990 [DOI] [PubMed] [Google Scholar]
- 91.Poteracki J, Walsh KM.Spontaneous neoplasms in control Wistar rats: a comparison of reviews. Toxicol Sci. 45: 1–8 1998 [DOI] [PubMed] [Google Scholar]
- 92.Prot M, Heripret L, Cardot-Leccia N, Perrin C, Aouadi M, Lavrut T, Garraffo R, Dellamonica P, Durant J, Le Marchand-Brustel Y, Binétruy B.Long-term treatment with lopinavir-ritonavir induces a reduction in peripheral adipose depots in mice. Antimicrob Agents Chemother. 50: 3998–4004 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prysor-Jones RA, Jenkins JS.Effect of excessive secretion of growth hormone on tissues of the rat, with particular reference to the heart and skeletal muscle. J Endocrinol. 85: 75–82 1980 [DOI] [PubMed] [Google Scholar]
- 94.Rittinghausen S, Ernst H, Muhle H, Mohr U.Atypical malignant mesotheliomas with osseous and cartilaginous differentiation after intraperitoneal injection of various types of mineral fibres in rats. Exp Toxicol Pathol. 44: 55–58 1992 [DOI] [PubMed] [Google Scholar]
- 95.Roe FJC, Carter RL.Iron-dextran carcinogenesis in rats: influence of dose on the number and types of neoplasm induced. Int J Cancer. 2: 370–380 1967 [DOI] [PubMed] [Google Scholar]
- 96.Sakamoto Y, Nakae D, Fukumori N, Tayama K, Maekawa A, Imai K, Hirose A, Nishimura T, Ohashi N, Ogata A.Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 34: 65–76 2009 [DOI] [PubMed] [Google Scholar]
- 97.Schaefer WH, Lawrence JW, Loughlin AF, Stoffregen DA, Mixson LA, Dean DC, Raab CE, Yu NX, Lankas GR, Frederick CB.Evaluation of ubiquinone concentration and mitochondrial function relative to cerivastatin-induced skeletal myopathy in rats. Toxicol Appl Pharmacol. 194: 10–23 2004 [DOI] [PubMed] [Google Scholar]
- 98.Schneider P, Busch U, Meister H, Qasem Q, Wünsch PH.Malignant fibrous histiocytoma (MFH). A comparison of MFH in man and animals. A critical review. Histology amd. Histopathol. 14: 845–860 1999 [DOI] [PubMed] [Google Scholar]
- 99.Schwartz LW, Hahn FF, Keenan CM, Brown HR, Mann PC. (1994). Proliferative lesions of the rat respiratory tract, R-1. Guides for Toxicologic Pathology. Washington DC, STP/ARP/AFIP. [Google Scholar]
- 100.Seiden D.Effects of colchicine on myofilament arrangement and the lysosomal system in skeletal muscle. Z Zellforsch Mikrosk Anat. 144: 467–483 1973 [DOI] [PubMed] [Google Scholar]
- 101.Sevastianova K, Sutinen J, Greco D, Sievers M, Salmenkivi K, Perttilä J, Olkkonen VM, Wågsäter D, Lidell ME, Enerbäck S, Eriksson P, Walker UA, Auvinen P, Ristola M, Yki-Järvinen H.Comparison of dorsocervical with abdominal subcutaneous adipose tissue in patients with and without antiretroviral therapy-associated lipodystrophy. Diabetes. 60: 1894–1900 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shoieb A, Allavena R, Swallow J, Debrue M.Peritoneal sarcomatosis associated with telemetry implants in Sprague Dawley CD rats: a review of eight cases. Toxicol Pathol. 40: 113–121 2012 [DOI] [PubMed] [Google Scholar]
- 103.Slotwiner P, Song SK, Anderson PJ.Spheromembranous degeneration of muscle induced by vincristine. Arch Neurol. 15: 172–176 1966 [DOI] [PubMed] [Google Scholar]
- 104.Stefanski SA, Elwell MR, Yoshitomi K.Malignant hibernoma in a Fischer 344 rat. Lab Anim Sci. 37: 347–350 1987 [PubMed] [Google Scholar]
- 105.Stewart HL.Tumours of the soft tissues. Pathology of Tumours in Laboratory Animals. Tumours of the Mouse. V. S. Turusov. Lyon. IARC. 2: 487–525 1979 [PubMed] [Google Scholar]
- 106.Sundberg JP, Adkison DL, Bedigian HG.Skeletal muscle rhabdomyosarcomas in inbred laboratory mice. Vet Pathol. 28: 200–206 1991 [DOI] [PubMed] [Google Scholar]
- 107.Taguchi S, Kuriwaki K, Souda M, Funato M, Ninomiya K, Umekita Y, Yoshida H.Induction of sarcomas by a single subcutaneous injection of 7,12-dimethylbenz[a]anthracene into neonatal male Sprague-Dawley rats: histopathological and immunohistochemical analyses. Toxicol Pathol. 34: 336–347 2006 [DOI] [PubMed] [Google Scholar]
- 108.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J.Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 33: 105–116 2008 [DOI] [PubMed] [Google Scholar]
- 109.Thuilliez C, Dorso L, Howroyd P, Gould S, Chanut F, Burnett R.Histopathological lesions following intramuscular administration of saline in laboratory rodents and rabbits. Exp Toxicol Pathol. 61: 13–21 2009 [DOI] [PubMed] [Google Scholar]
- 110.Tillmann T, Kamino K, Dasenbrock C, Ernst H, Kohler M, Morawietz G, Campo E, Cardesa A, Tomatis L, Mohr U.Subcutaneous soft tissue tumours at the site of implanted microchips in mice. Exp Toxicol Pathol. 49: 197–200 1997 [DOI] [PubMed] [Google Scholar]
- 111.Dei Tos AP.Classification of pleomorphic sarcomas: where are we now? Histopathology. 48: 51–62 2006 [DOI] [PubMed] [Google Scholar]
- 112.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC.(2004). Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, IARC Press. [Google Scholar]
- 113.Tsuchiya T, Takahashi K, Takeya M, Hosokawa Y, Hattori T, Takagi K.Immunohistochemical, quantitative immunoelectron microscopic, and DNA cytofluorometric characterization of chemically induced rat malignant fibrous histiocytoma. Am J Pathol. 143: 431–445 1993 [PMC free article] [PubMed] [Google Scholar]
- 114.Valtieri M, Sorrentino A.The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 217: 296–300 2008 [DOI] [PubMed] [Google Scholar]
- 115.Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E.Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine. 23: 1359–1367 2005 [DOI] [PubMed] [Google Scholar]
- 116.von Ruhland CJ, Campbell L, Gumbleton M, Jasani B, Newman GR.Immunolocalization of caveolin-1 in rat and human mesothelium. J Histochem Cytochem. 52: 1415–1425 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waites CR, Dominick MA, Sanderson TP, Schilling BE.Nonclinical safety evaluation of muraglitazar, a novel PPARalpha/gamma agonist. Toxicol Sci. 100: 248–258 2007 [DOI] [PubMed] [Google Scholar]
- 118.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr.Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 112: 1796–1808 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wellen KE, Hotamisligil GS.Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 112: 1785–1788 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC.Statin-induced muscle necrosis in the rat: distribution, development, and fibre selectivity. Toxicol Pathol. 33: 246–257 2005 [DOI] [PubMed] [Google Scholar]
- 121.Westwood FR, Duffy PA, Malpass DA, Jones HB, Topham JC.Disturbance of macrophage and monocyte function in the dog by a thromboxane receptor antagonist: ICI 185,282. Toxicol Pathol. 23: 373–384 1995 [DOI] [PubMed] [Google Scholar]
- 122.Westwood FR, Scott RC, Marsden AM, Bigley A, Randall K.Rosuvastatin: characterization of induced myopathy in the rat. Toxicol Pathol. 36: 345–352 2008 [DOI] [PubMed] [Google Scholar]
- 123.Williams DF.Review. Tissue-biomaterial interactions. J Mater Sci. 22: 3421–3445 1987 [Google Scholar]
- 124.Wright JA, Goonetilleke URP, Waghe M, Horne M, Stewart MG.An immunohistochemical study of spontaneous histiocytic tumours in the rat. J Comp Pathol. 104: 223–232 1991 [DOI] [PubMed] [Google Scholar]
- 125.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H.Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 112: 1821–1830 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]