Abstract
Rhamnogalacturonan-II (RG-II), a domain of plant cell wall pectins, is able to cross-link with other RG-II domains through borate diester bridges. Although it is known to affect mechanical properties of the cell wall, the biochemical requirements and lifecycle of this cross-linking remain unclear. We developed a PAGE methodology to allow separation of monomeric and dimeric RG-II and used this to study the dynamics of cross-linking in vitro and in vivo. Rosa cells grown in medium with no added boron contained no RG-II dimers, although these re-appeared after addition of boron to the medium. However, other Rosa cultures which were unable to synthesize new polysaccharides did not show dimer formation. We conclude that RG-II normally becomes cross-linked intraprotoplasmically or during secretion, but not post-secretion.
Keywords: rhamnogalacturonan-II, gel electrophoresis, pectin, boron, radiolabelling, cross-linking, cell wall, Rosa sp., Arabidopsis thaliana
Boron (B), available in soil as soluble boric acid, is an agriculturally important element for which plants have an absolute requirement.1,2 There is a narrow range of optimal concentrations and B deficiency or excess in soil are both problematic, with a range of symptoms such as shortened roots and stems, death of growing-points or roughening of the epidermis.3,4,5,6
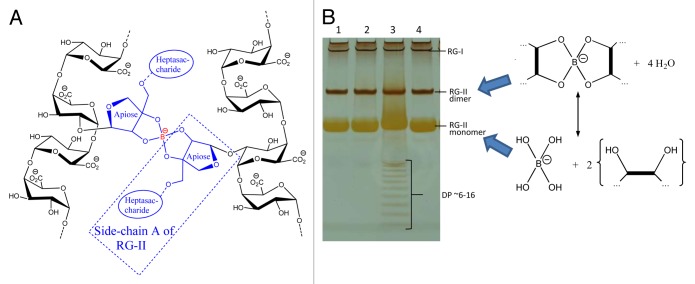
Early investigations into the effects of B deficiency revealed strong effects on the mechanical properties of plants, with tissues often feeling ‘brittle’,1,7 suggesting an important role for B in the structure of plant cell walls. Further studies found a correlation with pectin content,8 and it was hypothesized7 that pectic apiose residues, found in the rhamnogalacturonan-II (RG-II) component of pectin,9,10,11,12,13 bind B, resulting in the ability to form RG-II dimers cross-linked through B (Fig. 1A).
Figure 1. Boron-bridging of RG-II and separation by gel electrophoresis. (A) Schematic representation of boron-bridging of two pectin molecules (B) Gel electrophoresis separation of monomeric and dimeric RG-II. Sample 3 shows separation of oligomers with DP ~6–16 (hexasaccharide to hexadecasaccharide) resulting from the incomplete digestion of homogalacturonan.
We decided to investigate the formation of RG-II–(B−)–RG-II bridges and developed a new technique for the separation of RG-II monomers and dimers.14 Using standard gel electrophoresis equipment, we successfully separated these two compounds (which have similar charge:mass ratio) thanks to their difference in size (~5 and 10 kDa respectively), as is performed for protein SDS–PAGE or oligosaccharide PACE (Fig. 1B).15 This technique also allows fluorographic visualization of radioactive products, which were produced by radiolabelling RG-II preparations with NaB3H4. We demonstrated how this technique allows the rapid monitoring of cross-linking by successfully monomerising dimers of RG-II in vitro (by lowering pH with the addition of 0.1 M HCl) and dimerizing monomers (by adding 0.1–1.0 mM H3BO3, a process which was promoted by the addition of Pb2+).16
In order to study this cross-linking in vivo, we attempted to grow Rosa, Arabidopsis and Spinacia cell-suspension cultures with reduced H3BO3 concentrations in their respective media. The Arabidopsis and Spinacia cells did not survive in these conditions, but the Rosa cells continued to grow (and have been growing for > 2 y). After 8 wk in media with no added boron, only monomeric RG-II was detectable through gel electrophoresis. Re-addition of H3BO3 to the medium at the routine concentration (3.3 µM) led to the formation of small amounts of dimeric RG-II after 1 h, with the proportion increasing over 24 h. Interestingly, the quantities of monomeric RG-II did not decrease, suggesting that pre-formed monomeric domains were unable to dimerize and only newly formed RG-II formed dimers. To further test this hypothesis, we applied treatments (carbon starvation, respiratory inhibitors, anaerobiosis, freezing or boiling) to Rosa cells which decrease or prevent de-novo polysaccharide synthesis before re-supplying H3BO3. In all cases, negligible RG-II dimer formation was observed. Importantly, our data also showed that although boron bridges are important for the development of a cell, they are not essential for retaining pectin in the cell wall. Washing the zero-boron cells with Na2CO3 did not remove the pectins demonstrating they were an integral component of the cell wall. This is likely to be due to cross-linking with other cell wall components, possibly through Ca2+-bridges or glycosidic bonds.17,18 The mechanism by which Pb2+ promotes cross-linking is not understood16 and it is hypothesized that there may be other substances carrying a similar function in vivo. These could include enzymes, boron carriers or cationic RG-II chaperones, any of which may promote dimerization. We added monomeric RG-II and H3BO3 to spent cell culture medium and monitored dimerization by gel electrophoresis. Additionally, we used low concentrations of radiolabelled [3H]RG-II in case excess amounts of RG-II may be unfavorable to dimer formation. In neither case was dimer formation observed, supporting our hypothesis that cross-linking occurs intraprotoplasmically or during secretion. We also conducted in-vitro experiments to investigate the basis of B toxicity. Increasing B concentrations up to 2000-fold did not compromise the dimerization in vitro, but it would be of scientific interest to further investigate the toxicity problems by supplying excess B in vivo.
The complete ‘career’ of an RG-II domain is still unknown, and boron cross-linking could occur at several different stages, such as during synthesis in the Golgi bodies, during section into the cell wall, or very soon after secretion into the wall. Further studies into the possibility of Pb2+ ‘mimics’, RG-II chaperones, or boron ‘donor substrates’ are required to understand this mechanism better, which may provide an insight into why B is such an essential element for plant cell wall development and why excess B is phytotoxic.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mr Tim Gregson and Mrs Janice Miller for technical assistance. This work was supported by a BBSRC (UK) grant (BB/H000690/1).
References
- 1.Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:481–500. doi: 10.1146/annurev.arplant.49.1.481. [DOI] [PubMed] [Google Scholar]
- 2.Goldbach HE, Wimmer MA. Boron in plants and animals: Is there a role beyond cell-wall structure? J Plant Nutr Soil Sci. 2007;170:39–48. doi: 10.1002/jpln.200625161. [DOI] [Google Scholar]
- 3.Warington K. The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot 1923; 37 (old series): 629-672.
- 4.Lehto T, Ruuhola T, Dell B. Boron in forest trees and forest ecosystems. For Ecol Manage. 2010;260:2053–69. doi: 10.1016/j.foreco.2010.09.028. [DOI] [Google Scholar]
- 5.Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff J, Arce-Johnson P. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant Cell Environ. 2012;35:719–34. doi: 10.1111/j.1365-3040.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- 6.Wimmer MA, Eichert T. Review: mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. 2013;203-204:25–32. doi: 10.1016/j.plantsci.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Loomis WD, Durst RW. Chemistry and biology of boron. Biofactors. 1992;3:229–39. [PubMed] [Google Scholar]
- 8.Hu H, Brown PH, Labavitch JM. Species variability in boron requirement is correlated with cell wall pectin. J Exp Bot. 1996;47:227–32. doi: 10.1093/jxb/47.2.227. [DOI] [Google Scholar]
- 9.Matoh T, Ishigaki K-i, Ohno K, Azuma J-i. Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol. 1993;34:639–42. [Google Scholar]
- 10.Kobayashi M, Matoh T, Azuma J. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–20. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matoh T, Kawaguchi S, Kobayashi M. Ubiquity of a borate rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 1996;37:636–40. doi: 10.1093/oxfordjournals.pcp.a028992. [DOI] [Google Scholar]
- 12.Ishii T, Matsunaga T, Hayashi N. Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol. 2001;126:1698–705. doi: 10.1104/pp.126.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen GJ, Bakx EJ, Verhoef RP, Schols HA, Voragen AGJ. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr Polym. 2007;70:224–35. doi: 10.1016/j.carbpol.2007.04.007. [DOI] [Google Scholar]
- 14.Chormova D, Messenger DJ, Fry SC. Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 2014;77:534–46. doi: 10.1111/tpj.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goubet F, Ström A, Quéméner B, Stephens E, Williams MAK, Dupree P. Resolution of the structural isomers of partially methylesterified oligogalacturonides by polysaccharide analysis using carbohydrate gel electrophoresis. Glycobiology. 2006;16:29–35. doi: 10.1093/glycob/cwj022. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J Biol Chem. 1996;271:22923–30. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- 17.Popper ZA, Fry SC. Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann Bot. 2005;96:91–9. doi: 10.1093/aob/mci153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mort A. (2013) Half of the xyloglucan in cell walls of tissue cultures is linked to pectin via a highly branched arabinan. In XIII Cell Wall Meeting Book of Abstracts, Nantes, France. [Google Scholar]