Abstract
Comparative Gene Identification-58 (CGI-58) is an α/β hydrolase-type protein that regulates lipid homeostasis and signaling in eukaryotes by interacting with and stimulating the activity of several different types of proteins, including a lipase in mammalian cells and a peroxisomal ABC transporter (PXA1) in plant cells. Here we show that plant CGI-58 also interacts with spermidine synthase 1 (SPDS1), an enzyme that plays a central role in polyamine metabolism by converting putrescine into spermidine. Analysis of polyamine contents in Arabidopsis thaliana plants revealed that spermidine levels were significantly reduced, and putrescine increased, in both cgi-58 and cgi-58/pxa1 mutant plants, relative to pxa1 mutant or wild-type plants. Evaluation of polyamine-related gene expression levels, however, revealed similar increases in transcript abundance in all mutants, including cgi-58, pxa1, and cgi-58/pxa1, in comparison to wild type. Taken together, the data support a model whereby CGI-58 and PXA1 contribute to the regulation of polyamine metabolism at the transcriptional level, perhaps through a shared lipid-signaling pathway, and that CGI-58 also acts independently of PXA1 to increase spermidine content at a post-transcriptional level, possibly through protein-protein interaction with SPDS1.
Keywords: Arabidopsis, CGI-58, Polyamines, PXA1, SPDS1, Spermidine, Spermidine Synthase
CGI-58 (Comparative Gene Identification-58) is an important regulator of lipid metabolism and signaling in both plants and animals, with a loss of CGI-58 activity resulting in the hyper-accumulation of triacylglycerols (TAGs) in cell types that normally do not store lipids, including the skin, liver, and muscle cells of animals and the mesophyll cells of plants.1,2 Despite this similarity in lipid-accumulation phenotypes, molecular analysis has revealed that CGI-58 carries out its biological function(s) by interacting with different types of proteins in plant and animal cells. In mammalian cells, for instance, CGI-58 interacts with a protein called perilipin, which binds and sequesters CGI-58 at the surface of lipid droplets, thereby regulating its availability for interacting with and stimulating the activity of an adipose TAG lipase.3-5 In plants, CGI-58 interacts with and stimulates the activity of peroxisomal ABC transporter 1 (PXA1), which is responsible for the uptake of fatty acids and lipophilic precursors of the jasmonate and auxin pathways into peroxisomes for their subsequent metabolism by β-oxidation (ref. 6 and references therein). Loss of CGI-58 in either system, therefore, results in reduced lipid turnover and an accumulation of TAG.
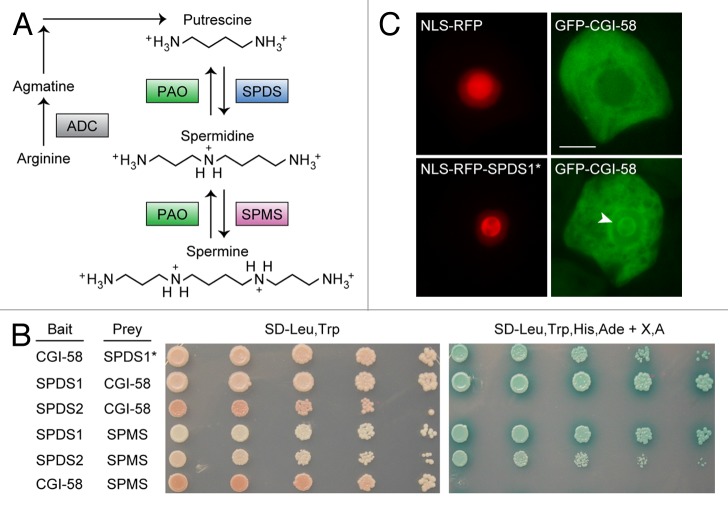
Previously, we searched for proteins that interacted with CGI-58 in plants by using the yeast 2-hybrid assay to screen an Arabidopsis thaliana cDNA library with CGI-58 as “bait.”6 Three strongly-interacting proteins were identified in the screen, including the C-terminal portion of the PXA1 transporter, which was subsequently shown to physically and genetically interact with CGI-58 to regulate both lipid metabolism and signaling in plants.6 For instance, loss of CGI-58 activity resulted in changes in lipid metabolism and lipid signaling that were similar to pxa1 mutant plants, particularly in vegetative tissues, and these alterations were no more severe in double mutant cgi-58/pxa1 plants.6 The other 2 CGI-58-interactors included portions of spermidine synthase 1 (SPDS1) and de-etiolated 3 (DET3), a subunit of the vacuolar H+-ATPase.7 While the functional significance of an interaction between CGI-58 and DET3 remains to be determined, SPDS1 was considered a particularly interesting candidate for further analysis because it is well known to play a key role in polyamine metabolism in plants (Fig. 1A).8,9 Polyamines are abundant aliphatic polycations that exist as either “free” compounds or conjugated to various small molecules,10 all of which are important for plant growth, development, and stress responses.11-13 As shown in Figure 1A, polyamine metabolism begins with arginine and includes 3 predominant polyamine species, namely putrescine, spermidine, and spermine, the ratios of which are maintained through a series of forward and reverse reactions.13-15 Given the importance of polyamines to plant physiology, in general, and the previous demonstration that enzymes of the polyamine pathway interact with each other and with other regulatory proteins,9,16 we sought to determine whether the interaction of CGI-58 and SPDS1 was functionally significant.
Figure 1. Interaction of CGI-58 and SPDS1. (A) Model of polyamine metabolism in plants (adapted from ref. 12). Key enzymes are highlighted in colored boxes, some of which are encoded by multiple genes. See text for additional details. Abbreviations include ADC, arginine decarboxylase; PAO, polyamine oxidase; SPDS, spermidine synthase; and SPMS, spermine synthase. (B) Yeast 2-hybrid analysis of protein-protein interactions between CGI-58 and various polyamine-related proteins, namely SPDS1, SPDS2 and SPMS. The indicated “bait” and “prey” proteins were co-expressed in yeast cells then similarly prepared serial (1:5) dilutions of yeast cultures were replica plated on either synthetic dextrose media lacking leucine and tryptophan (SD-Leu-Trp), which selects only for the presence of both “bait” and “prey” plasmids in the cells, or on media also lacking histidine, adenine, and containing X-α-Gal and Aureobasidin A (SD-Leu,Trp,His,Ade + X,A), which selects for 4 different reporter genes whose transcriptional activity is dependent on 2-hybrid protein interaction, as previously described.6 Note that the SPDS1* “prey” protein (top row) consists of only the C-terminal portion of SPDS1 (amino acids 131–334), whereas all other indicated “prey” (and “bait”) proteins are full length. (C) Protein-protein interaction analysis using the nuclear relocalization assay. Briefly, tobacco suspension-cultured (Bright Yellow-2) cells were transiently co-transformed (via biolistic bombardment; refer to ref. 6 for details) with plasmids expressing NLS-RFP and GFP-CGI-58 (upper panels), or a second set of plasmids expressing NLS-RFP N-terminally fused to the C-terminal half of SPDS1 (NLS-RFP-SPDS1*) and GFP-CGI-58 (bottom panels). Note in the bottom panels that a portion of the GFP-CGI-58 protein has been recruited from the cytosol into the nucleolus (refer to arrowhead) via its interaction with NLS-RFP-SPDS1*. Bar = 10 µm.
The SPDS1 “prey” protein recovered from the initial yeast 2-hybrid screen using CGI-58 as “bait” consisted of the C-terminal half of the protein, including amino acid residues 131–334 (refer to the top row in Figure 1B [CGI-58 and SPDS1*]). To test whether this region could also interact with CGI-58 in plant cells, the same C-terminal sequence of SPDS1 was fused to a red fluorescent protein (RFP) that also harbored a nuclear localization sequence (NLS), and the resulting fusion protein (i.e., NLS-RFP-SPDS1*) was co-expressed in tobacco suspension-cultured cells with GFP-CGI-58, as previously described.6 As a negative control, the NLS-RFP protein, without the SPDS1 C-terminal sequence, was also co-expressed with GFP-CGI-58 in tobacco cells. As shown in Figure 1C, expression of NLS-RFP resulted in localization of this protein to the nucleus, with prominent staining of the nucleoli, while GFP-CGI-58 was predominantly localized in the cytosol, as expected.1,6 By contrast, co-expression of NLS-RFP-SPDS1* and GFP-CGI-58 resulted in a portion of GFP-CGI-58 being relocalized to the nucleus, and also enriched in the nucleolus, thus confirming an interaction of CGI-58 with the C-terminal region of SPDS1. Co-expression of full-length SPDS1 and CGI-58 proteins in the yeast 2-hybrid system confirmed a strong interaction between the 2 proteins (Fig. 1B). Co-expression of the full-length proteins in the nuclear relocalization assay, however, revealed that full-length SPDS1 was not able to recruit CGI-58 to the nucleus (data not shown), suggesting that other factors, such as post-translational modification or the presence of other plant-specific proteins, might compete for interaction of SPDS1 and/or CGI-58 in plant cells. For instance, both proteins are known to interact with other proteins in plants, including spermidine synthase 2 (SPDS2) and spermine synthase (SPMS) for SPDS1,16 and PXA1 for CGI-58.6 Thus, there is likely some competition for various protein-binding partners under steady-state conditions, and the interaction of SPDS1 and CGI-58 in planta may be below the threshold required for the nuclear relocalization assay.
While CGI-58 could interact with full-length SPDS1 in the yeast 2-hybrid assay, CGI-58 did not interact with SPDS2 (Fig. 1B), which is the second (of 2) spermidine synthase isoforms in Arabidopsis and that possesses 75% polypeptide sequence identity with SPDS1. Notably, SPDS1 and SPDS2 are considered to be functionally redundant, although they do show somewhat different gene expression patterns and respond differently to certain hormones.8,17 Since prior studies16 have shown also that SPDS1 and SPDS2 interact with each other, as well as with the next enzyme in the polyamine biosynthetic pathway, namely spermine synthase (SPMS; Fig. 1A), we tested whether CGI-58 might also interact with SPMS. Although we could confirm that both SPDS1 and SPDS2 were capable of interacting with SPMS, as expected, CGI-58 did not interact with SPMS (Fig. 1B).
Based on the data described above, CGI-58 appears to interact specifically with SPDS1 and not SPDS2 or SPMS, and thus any potential regulation of polyamine metabolism by CGI-58 might be reflected in the relative amounts of putrescine and spermidine, the substrate and product of the SPDS1 enzyme, respectively. To test this possibility, we analyzed both free and conjugated forms of the 3 major polyamines in wild-type and various mutant Arabidopsis plants. As shown in Figure 2, cgi-58 mutant plants, compared with wild-type plants, possessed significantly elevated amounts of putrescine in both its free and conjugated forms, while the spermidine amounts were reduced. Notably, a similar increase in free putrescine and decrease in free spermidine was previously observed in spds1 mutant Arabidopsis plants, but not in spds2 mutants,18 suggesting that SPDS1 is predominantly involved in production of spermidine under basal conditions. We also observed similar changes in polyamine contents in cgi-58/pxa1 double mutant plants, but not in pxa1 single mutant plants, which were comparable to wild type (Fig. 2). Collectively, these data indicate that CGI-58 is required for the optimal production of spermidine under steady-state conditions in plants, and that this regulatory role for CGI-58 operates independently of PXA1.
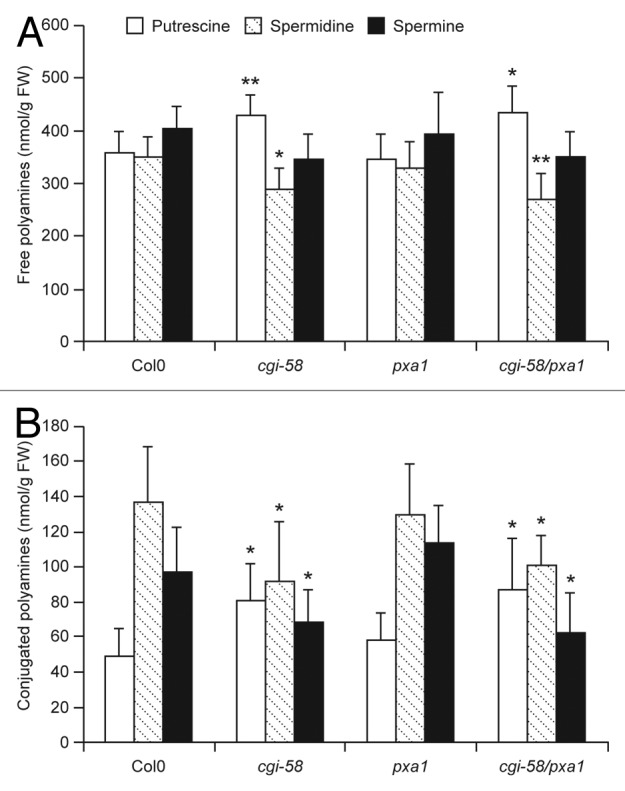
Figure 2. Analysis of polyamine contents in wild-type and mutant Arabidopsis plants. Polyamines in mature, approximately 40-day-old, wild-type or mutant Arabidopsis leaves were extracted, derivatized and analyzed (via HPLC) as described,8,20 with the exception that 1,7-diaminoheptane was used as an internal standard. Free polyamines are shown in (A), and acid-soluble, conjugated polyamines are shown in (B). Each data point represents the mean value of 4 independent analyses and error bars represent standard deviation. Plant lines are indicated along the bottom. Asterisks indicate values significantly different from the corresponding values of wild-type plants (Col0) determined using the student t-Test. *, P < 0.10; **, P < 0.05. Polyamines in the acid-insoluble pellet fraction were also analyzed but did not show any differences between any of the plant lines (data not shown).
To gain further insight to how CGI-58 might regulate polyamine metabolism in plants, we measured gene expression levels for various polyamine-related genes in both wild-type and mutant Arabidopsis plants. As shown in Figure 3, the levels of transcripts encoding arginine decarboxylase 1 (ADC1), which catalyzes the conversion of arginine into agmatine (refer to Fig. 1A), SPDS1, SPDS2, and polyamine oxidase 2 (PAO2) were all significantly enhanced in cgi-58 mutant plants, revealing that CGI-58 is involved in regulating the polyamine pathway at the transcriptional level. The expression of these genes was also altered, however, in pxa1 mutant plants, which notably did not show significant changes in polyamine contents under steady-state conditions (Fig. 2). These observations suggest that polyamine content is predominantly regulated at the post-transcriptional level in the experiments described here. Moreover, since the increases in gene expression observed in either the cgi-58 or pxa1 mutant were not additive in the cgi-58/pxa1 double mutant (Fig. 3), these data suggest that CGI-58 and PXA1 operate in the same regulatory pathway to influence polyamine-related gene expression.
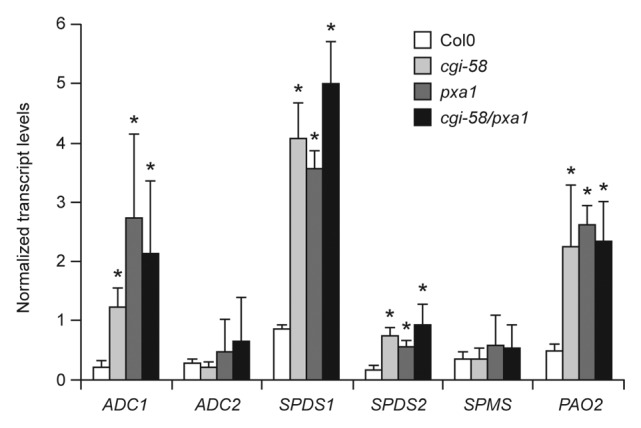
Figure 3. Gene expression profiles for various polyamine-related genes in wild-type and mutant Arabidopsis plants. Transcript levels present in mature, approximately 40-day-old, wild-type or mutant Arabidopsis leaves were determined for each of the indicated genes using primer sequences described in ref. 10 and qRT-PCR.6 Plant lines are indicated along the bottom. Transcript values were normalized based on rRNA and multiplied by 100,000. Each data point represents the mean value of 3 biological replicates and bars indicate standard deviation. Asterisks indicate values significantly different from the corresponding values of wild-type plants (Col0) determined using the student t-Test. *, P < 0.05.
Based on the results presented in this study and the prior demonstration that CGI-58 and PXA1 co-regulate both oxylipin and auxin signaling pathways,6 it is possible that CGI-58 and PXA1 participate in the regulation of polyamine metabolism at the transcriptional level through a shared lipid signaling pathway(s). Notably, polyamine-related genes are known to be responsive to both of these hormone classes, as well as to various stress conditions that include oxylipin signaling.12,13,17,19The results presented here also suggest that CGI-58 regulates polyamine production at the post-transcriptional level by interacting with and stimulating the activity of SPDS1. A similar scenario has been proposed previously for the regulation of SPDS2, which was shown to interact with a nematode effector protein whose presence in plants also resulted in alteration of polyamine-related gene expression.9 It will be interesting to further explore the functional connection between CGI-58 and SPDS1, which may provide new insight to the regulation and integration of 2 seemingly different metabolic pathways in plant cells, namely lipid and polyamine metabolism.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the US Department of Energy, BER Division, DE-FG02–09ER64812/DE-SC0000797. Support for this work was also provided by the USDA-ARS, CRIS Project No. 5347–21000–012–00D, the University of Guelph Research Chairs Program, and the Natural Sciences and Engineering Research Council of Canada (217291).
References
- 1.James CN, Horn PJ, Case CR, Gidda SK, Zhang D, Mullen RT, Dyer JM, Anderson RG, Chapman KD. Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci U S A. 2010;107:17833–8. doi: 10.1073/pnas.0911359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radner FP, Grond S, Haemmerle G, Lass A, Zechner R. Fat in the skin: Triacylglycerol metabolism in keratinocytes and its role in the development of neutral lipid storage disease. Dermatoendocrinol. 2011;3:77–83. doi: 10.4161/derm.3.2.15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem. 2004;279:30490–7. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 5.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–19. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Gidda SK, James CN, Horn PJ, Khuu N, Seay DC, Keereetaweep J, Chapman KD, Mullen RT, Dyer JM. The α/β hydrolase CGI-58 and peroxisomal transport protein PXA1 coregulate lipid homeostasis and signaling in Arabidopsis. Plant Cell. 2013;25:1726–39. doi: 10.1105/tpc.113.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H(+)-ATPase in plant growth and development. Genes Dev. 1999;13:3259–70. doi: 10.1101/gad.13.24.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai A, Matsuyama T, Hanzawa Y, Akiyama T, Tamaoki M, Saji H, Shirano Y, Kato T, Hayashi H, Shibata D, et al. Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol. 2004;135:1565–73. doi: 10.1104/pp.104.041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, Baum TJ. Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol. 2010;152:968–84. doi: 10.1104/pp.109.150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellenberg C, Ziegler J, Handrick V, Vogt T. Polyamine homeostasis in wild type and phenolamide deficient Arabidopsis thaliana stamens. Front Plant Sci. 2012;3:180. doi: 10.3389/fpls.2012.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusano T, Berberich T, Tateda C, Takahashi Y. Polyamines: essential factors for growth and survival. Planta. 2008;228:367–81. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 12.Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–49. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- 13.Wimalasekera R, Tebartz F, Scherer GFE. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011;181:593–603. doi: 10.1016/j.plantsci.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Moschou PN, Sanmartin M, Andriopoulou AH, Rojo E, Sanchez-Serrano JJ, Roubelakis-Angelakis KA. Bridging the gap between plant and mammalian polyamine catabolism: a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008;147:1845–57. doi: 10.1104/pp.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fincato P, Moschou PN, Spedaletti V, Tavazza R, Angelini R, Federico R, Roubelakis-Angelakis KA, Tavladoraki P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J Exp Bot. 2011;62:1155–68. doi: 10.1093/jxb/erq341. [DOI] [PubMed] [Google Scholar]
- 16.Panicot M, Minguet EG, Ferrando A, Alcázar R, Blázquez MA, Carbonell J, Altabella T, Koncz C, Tiburcio AF. A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell. 2002;14:2539–51. doi: 10.1105/tpc.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanzawa Y, Imai A, Michael AJ, Komeda Y, Takahashi T. Characterization of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett. 2002;527:176–80. doi: 10.1016/S0014-5793(02)03217-9. [DOI] [PubMed] [Google Scholar]
- 18.Alcázar R, Bitrián M, Bartels D, Koncz C, Altabella T, Tiburcio AF. Polyamine metabolic canalization in response to drought stress in Arabidopsis and the resurrection plant Craterostigma plantagineum. Plant Signal Behav. 2011;6:243–50. doi: 10.4161/psb.6.2.14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kausch KD, Sobolev AP, Goyal RK, Fatima T, Laila-Beevi R, Saftner RA, Handa AK, Mattoo AK. Methyl jasmonate deficiency alters cellular metabolome, including the aminome of tomato (Solanum lycopersicum L.) fruit. Amino Acids. 2012;42:843–56. doi: 10.1007/s00726-011-1000-5. [DOI] [PubMed] [Google Scholar]
- 20.Torrigiani P, Serafini-Fracassini D, Bagni N. Polyamine biosynthesis and effect of dicyclohexylamine during the cell cycle of Helianthus tuberosus tuber. Plant Physiol. 1987;84:148–52. doi: 10.1104/pp.84.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]