Abstract
Although positive-strand RNA [(+)RNA] viruses have a limited coding capacity, they can replicate efficiently in host cells because of their ability to use host-derived proteins, membranes, lipids, and metabolites, and to rewire cellular trafficking pathways. Previously, we showed that a plant RNA virus, Red clover necrotic mosaic virus (RCNMV), hijacked Arf1 and Sar1, which are small GTPases that regulate the biogenesis of COPI and COPII vesicles, respectively, for viral RNA replication. These small GTPases are relocated from appropriate subcellular compartments to the viral RNA replication sites by p27 replication protein, which raises the possibility that RCNMV interferes with the cellular secretory pathway. Here, we examined this possibility by using green fluorescent protein-fused rice SCAMP1 and Arabidopsis LRR84A as secretory pathway marker proteins and showed that p27 inhibited the trafficking of these proteins. RCNMV-mediated inhibition of the host secretion pathway and its possible impact on plant–virus interaction are discussed.
Keywords: Arf1, COPI, COPII, ER, Sar1, golgi, intracellular membrane, membrane trafficking, plant RNA virus, replication protein
Exploiting Early Secretory Components for Viral RNA Replication
The secretory pathway in eukaryotic cells has essential roles in biogenesis and proper intracellular distribution of a wide range of proteins and lipids. Anterograde transport of newly synthesized proteins and lipids is initiated at the endoplasmic reticulum (ER). Therefore, ER-to-Golgi transport represents a vital gateway to the endomembrane system.1 Coat protein complex II (COPII) drives the anterograde pathway from the ER, whereas COPI regulates the retrograde trafficking from the Golgi.1 The interdependence of the antero- and retrograde trafficking pathways are generally conserved across eukaryotes.1 The trans-Golgi network (TGN) conducts final sorting steps to post-Golgi destinations such as plasma membrane (PM) and exchanges material with the endocytic pathway.2
Red clover necrotic mosaic virus (RCNMV) belongs to the genus dianthovirus in the family Tombusviridae. RCNMV encodes two replication proteins, an auxiliary replication protein p27, and RNA-dependent RNA polymerase p88pol. p27 has multiple functions during RNA replication and is an essential component of the RCNMV replicase complex, which assembles on the ER membranes and synthesizes progeny viral RNAs.3 p27 interacts with many partners such as p27 itself, p88pol, viral genomic RNAs, and host heat shock proteins, Hsp70 and Hsp90.4-7 Moreover, p27 induces ER membrane alternations.8,9 We previously showed that a host small GTPase, ADP ribosylation factor 1 (Arf1) plays an essential role during the replication of RCNMV.10 Arf1 is implicated in the formation of COPI vesicles on Golgi membranes.1 Arf1 function can be inhibited by brefeldin A (BFA) that is a well-known fungal metabolite.11 BFA inhibits the activation of Arf small GTPases by targeting BFA-sensitive guanine nucleotide-exchange factors (GEFs) via locking the abortive Arf–GDP–GEF complex, thereby blocking guanine nucleotide release.12-14 We found that downregulation of Arf1 expression by virus-induced gene silencing decreased viral RNA accumulation in leaves of Nicotiana benthamiana inoculated with the virus, and that BFA or expression of dominant-negative forms of Arf1 inhibited RCNMV RNA replication in protoplasts, indicating that Arf1 plays an essential role in RCNMV replication.10 Moreover, BFA inhibited the accumulation of viral replicase complexes and disrupted p27-induced ER remodeling, suggesting that Arf1 is involved in the formation of the membrane-bound RCNMV replicase complex. Direct interactions between p27 and Arf1 were shown by GST pull down assays in vitro and bimolecular fluorescent complementation assays in N. benthamiana epidermal cells. Consistent with this, p27 recruits Arf1 from the Golgi apparatus to the p27-positive perinuclear ER aggregated structures. From these findings, we concluded that RCNMV alters proper subcellular localization of Arf1 and actively utilizes it for viral multiplication.
RCNMV Interferes with the Cellular Secretory Pathway
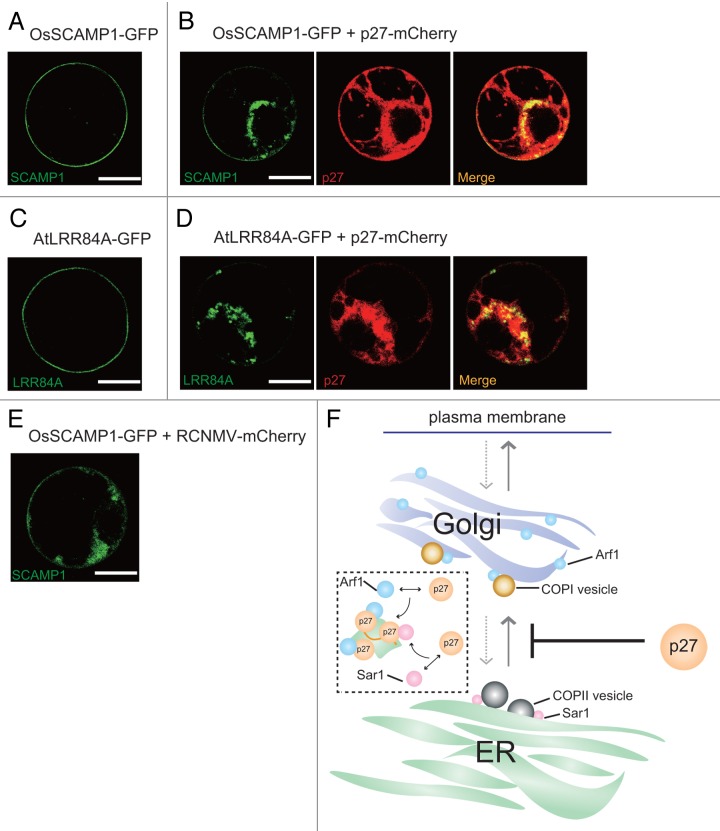
Sar1 (secretion-associated RAS-related 1), which is a small GTPase, is also required for RCNMV replication, and is relocalized with p27 in p27-induced large aggregate structures of ER membranes.10 Sar1 is implicated in the biogenesis of the COPII vesicles at ER exit sites. Our recent affinity purification and liquid chromatography-tandem mass spectrometry analysis revealed that Sar1 potentially interacts with p27 (unpublished data). Since Arf1 and Sar1 are essential factors in the biogenesis of COPI and COPII vesicles, respectively, we hypothesized that p27 affects the cellular secretory pathway. To address this hypothesis, we used two secretory marker proteins fused with green fluorescent protein (GFP); OsSCAMP1 (rice secretory carrier membrane protein 1), a tetraspan transmembrane protein, and Arabidopsis LRR84A, a type I integral membrane protein belonging to the leucine-rich repeat receptor-like kinase protein family, and tested whether p27 affects subcellular localization of these marker proteins. Both OsSCAMP1 and AtLRR84A can reach the PM via the conventional ER-Golgi-TGN pathway in tobacco BY-2 protoplasts.15,16 When transiently expressed in BY-2 protoplasts, both OsSCAMP1-GFP and AtLRR84A-GFP were found on the PM (Fig. 1A and 1C), as reported.15,16 However, when coexpressed with p27, the PM localization of these proteins was partially inhibited (Fig. 1B and 1D). Instead, a fraction of these proteins was found in the p27-containing ER aggregate structures (Fig. 1B and 1D). Moreover, in BY-2 protoplasts infected with a recombinant RCNMV in which the coat protein open reading frame was replaced by mCherry, the intracellular fluorescence of OsSCAMP1-GFP was observed (Fig. 1E). From these results, we propose that p27 interferes with the secretory pathway between the ER and the Golgi (Fig. 1F). The interference may be the result of p27-mediated sequestration of secretory pathway regulator proteins such as Arf1 and Sar1 from their original compartments.
Figure 1. Interference of protein trafficking mediated by dianthovirus p27 replication protein. A plasmid expressing OsSCAMP1-GFP (5 μg) (A and B) or AtLRR84A-GFP (5 μg) (C and D) was cotransfected with a plasmid expressing empty vector (12.5 μg) or p27-mCherry (12.5 μg) into tobacco BY-2 protoplasts. Images were taken at 20 h by confocal laser scanning microscopy. (E) A plasmid expressing OsSCAMP1-GFP (5 μg) was cotransfected with RNA1-mCherry, in which the coat protein open reading frame was replaced by mCherry, and RNA2 into tobacco BY-2 protoplasts. Images were taken at 24 h by confocal laser scanning microscopy. Scale bar = 10 μm. (F) Predicted model of the inhibition step of intracellular trafficking of AtLRR84A and OsSCAMP1 mediated by p27 replication protein. Appropriate trafficking of AtLRR84A and OsSCAMP1 (gray arrows) is likely to be inhibited by p27 at the ER-to-Golgi step. Arf1 and Sar1 are likely to be recruited to viral replication sites from their original compartments (as shown in the dashed-line square). Gray dashed-line arrows indicate retrograde trafficking route. ER, endoplasmic reticulum; Arf1, ADP ribosylation factor 1; Sar1, secretion-associated RAS-related 1; COPI, coat protein complex I, COPII, coat protein complex II.
The cellular secretory pathway is important for plant immunity for active defense against potential pathogens.17 By contrast, invasive pathogens have evolved a means to use these trafficking pathways for the suppression of plant defenses and for the benefit of microbial proliferation.17 For example, the Pseudomonas syringae pv tomato DC3000 effector HopM1 targets an Arf-GEF AtMIN7 that is required for both the pathogen-associated molecular pattern- and effector-triggered immunities.18,19 Moreover, Arf1 is required for both the nonhost resistance against a bacterial pathogen and N gene-mediated resistance against Tobacco mosaic virus in N. benthamiana.20 Therefore, it may be possible that p27-mediated interference of the cellular secretory pathway compromises plant immunity. It should be noted that the secretory pathway plays an important role not only in the delivery of antimicrobial molecules, but also in systemic acquired resistance, which provides broad-spectrum resistance against pathogens including viruses in plants.21-23 In animal viruses, enterovirus 3A protein binds to and inhibits the function of GBF1, a mammalian GEF for Arf1.24 This leads to inhibition of ER-to-Golgi transport, a function previously suggested to be important for viral suppression of immune responses.24 A virus carrying a 3A protein defective in inhibiting ER-to-Golgi transport is less virulent in mice.24 Hijacking of the host secretory pathway by RCNMV may be important not only for viral multiplication, but also for suppression of active defenses against viruses. Future studies will address this fascinating possibility.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Liwen Jiang (The Chinese University of Hong Kong) for kindly providing plasmids expressing OsSCAMP1-GFP and AtLRR84A-GFP. This work was supported by a Grant-in Aid for Scientific Research (A) (22248002) from the Japan Society for the Promotion of Science (JSPS). K. H. is a JSPS research fellow.
References
- 1.Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14:382–92. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes FC, Buono R, Otegui MS. Plant endosomal trafficking pathways. Curr Opin Plant Biol. 2011;14:666–73. doi: 10.1016/j.pbi.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Mine A, Takeda A, Taniguchi T, Taniguchi H, Kaido M, Mise K, Okuno T. Identification and characterization of the 480-kilodalton template-specific RNA-dependent RNA polymerase complex of red clover necrotic mosaic virus. J Virol. 2010;84:6070–81. doi: 10.1128/JVI.00054-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyodo K, Mine A, Iwakawa HO, Kaido M, Mise K, Okuno T. Identification of amino acids in auxiliary replicase protein p27 critical for its RNA-binding activity and the assembly of the replicase complex in Red clover necrotic mosaic virus. Virology. 2011;413:300–9. doi: 10.1016/j.virol.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Iwakawa HO, Mine A, Hyodo K, An M, Kaido M, Mise K, Okuno T. Template recognition mechanisms by replicase proteins differ between bipartite positive-strand genomic RNAs of a plant virus. J Virol. 2011;85:497–509. doi: 10.1128/JVI.01754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mine A, Hyodo K, Takeda A, Kaido M, Mise K, Okuno T. Interactions between p27 and p88 replicase proteins of Red clover necrotic mosaic virus play an essential role in viral RNA replication and suppression of RNA silencing via the 480-kDa viral replicase complex assembly. Virology. 2010;407:213–24. doi: 10.1016/j.virol.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Mine A, Hyodo K, Tajima Y, Kusumanegara K, Taniguchi T, Kaido M, Mise K, Taniguchi H, Okuno T. Differential roles of Hsp70 and Hsp90 in the assembly of the replicase complex of a positive-strand RNA plant virus. J Virol. 2012;86:12091–104. doi: 10.1128/JVI.01659-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusumanegara K, Mine A, Hyodo K, Kaido M, Mise K, Okuno T. Identification of domains in p27 auxiliary replicase protein essential for its association with the endoplasmic reticulum membranes in Red clover necrotic mosaic virus. Virology. 2012;433:131–41. doi: 10.1016/j.virol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Turner KA, Sit TL, Callaway AS, Allen NS, Lommel SA. Red clover necrotic mosaic virus replication proteins accumulate at the endoplasmic reticulum. Virology. 2004;320:276–90. doi: 10.1016/j.virol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Hyodo K, Mine A, Taniguchi T, Kaido M, Mise K, Taniguchi H, Okuno T. ADP ribosylation factor 1 plays an essential role in the replication of a plant RNA virus. J Virol. 2013;87:163–76. doi: 10.1128/JVI.02383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–8. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–30. doi: 10.1016/S0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 13.Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12:1403–11. doi: 10.1016/S1097-2765(03)00475-1. [DOI] [PubMed] [Google Scholar]
- 14.Teh OK, Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–6. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Jia T, Lam SK, Ding Y, Gao C, San MW, Pimpl P, Jiang L. Multiple cytosolic and transmembrane determinants are required for the trafficking of SCAMP1 via an ER-Golgi-TGN-PM pathway. Plant J. 2011;65:882–96. doi: 10.1111/j.1365-313X.2010.04469.x. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Zhuang X, Wang J, Wang H, Lam SK, Gao C, Wang X, Jiang L. Vacuolar degradation of two integral plasma membrane proteins, AtLRR84A and OsSCAMP1, is cargo ubiquitination-independent and prevacuolar compartment-mediated in plant cells. Traffic. 2012;13:1023–40. doi: 10.1111/j.1600-0854.2012.01360.x. [DOI] [PubMed] [Google Scholar]
- 17.Frei dit Frey N, Robatzek S. Trafficking vesicles: pro or contra pathogens? Curr Opin Plant Biol. 2009;12:437–43. doi: 10.1016/j.pbi.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;313:220–3. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- 19.Nomura K, Mecey C, Lee YN, Imboden LA, Chang JH, He SY. Effector-triggered immunity blocks pathogen degradation of an immunity-associated vesicle traffic regulator in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:10774–9. doi: 10.1073/pnas.1103338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coemans B, Takahashi Y, Berberich T, Ito A, Kanzaki H, Matsumura H, Saitoh H, Tsuda S, Kamoun S, Sági L, et al. High-throughput in planta expression screening identifies an ADP-ribosylation factor (ARF1) involved in non-host resistance and R gene-mediated resistance. Mol Plant Pathol. 2008;9:25–36. doi: 10.1111/j.1364-3703.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–63. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Dong X. A highway for war and peace: the secretory pathway in plant-microbe interactions. Mol Plant. 2011;4:581–7. doi: 10.1093/mp/ssr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Weaver ND, Kesarwani M, Dong X. Induction of protein secretory pathway is required for systemic acquired resistance. Science. 2005;308:1036–40. doi: 10.1126/science.1108791. [DOI] [PubMed] [Google Scholar]
- 24.Wessels E, Duijsings D, Niu TK, Neumann S, Oorschot VM, de Lange F, Lanke KH, Klumperman J, Henke A, Jackson CL, et al. A viral protein that blocks Arf1-mediated COP-I assembly by inhibiting the guanine nucleotide exchange factor GBF1. Dev Cell. 2006;11:191–201. doi: 10.1016/j.devcel.2006.06.005. [DOI] [PubMed] [Google Scholar]